超声强化纳米固体碱催化大豆油酯交换制备
生物柴油的动力学
任庆功1, 2,阎杰3,丘泰球2
(1. 常州大学 石油化工学院,江苏 常州,213164;
2. 华南理工大学 轻化工研究所,广东 广州,510640;
3. 仲恺农业工程学院 化学化工学院,广东 广州,510225)
摘要:以大豆油为原料,以纳米 KF/γ-Al2O3 为催化剂催化酯交换反应制备生物柴油;考察超声和机械搅拌对酯交换反应的影响,并在酯交换反应机理的基础上探讨超声强化纳米KF/γ-Al2O3催化酯交换反应动力学。研究结果表明:机械搅拌下纳米 KF/γ-Al2O3 催化大豆油酯交换反应在反应初始阶段是拟二级反应,而后为一级反应,后期转为零级反应,反应初期的反应活化能Ea为21.03 kJ/mol,指前因子K0为846.49 L/(mol·min);超声强化纳米 KF/γ-Al2O3催化酯交换反应动力学过程与常规法的动力学过程基本一致,反应初始阶段为拟二级反应,而后转为一级和零级,反应初期的K0为76.33 L/(mol·min),活化能Ea为12.99 kJ/mol,该活化能比机械搅拌下酯交换反应的略低,表明在超声作用下,酯交换反应更容易进行;超声强化酯交换反应速率常数比常规方法的高,从而反应能够短时间内达到平衡。
关键词:超声;反应动力学;氟化钾/γ-氧化铝;催化剂;大豆油;酯交换;生物柴油
中图分类号:TQ645.8 文献标志码:A 文章编号:1672-7207(2011)04-0903-06
Kinetics of nano solid base catalyzed transesterification of soybean oil for biodiesel production enhanced by ultrasonic irradiation
REN Qing-gong1, 2, YAN Jie3, QIU Tai-qiu2
(1. School of Petro Chemical Engineering, Changzhou University, Changzhou 213164, China;
2. Light Industry Institute, South China University of Technology, Guangzhou 510640, China;
3. College of Chemistry and Chemical Engineering, Zhongkai University of Agriculture and Engineering,
Guangzhou 510225, China)
Abstract: Transesterification of soybean oil and methanol to synthesis biodiesel with solid super base KF/γ-Al2O3 as the catalyst was studied. Effects of experimental parameters including ultrasonic and mechanical stirring on the transesterification of soybean oil and methanol were investigated. Based on the transesterification mechanism, the macro kinetic equation of triglycerides transesterification was obtained. The results show that the reaction of transesterification is the second grade reaction of increase stage at the beginning and finally reaches the one and zero grade reaction with the transesterification during mechanical stirring. The reactive activation energy is 21.03 kJ/mol and the pre-exponential factor is 846.49 L/(mol·min) of the transesterification using the nano KF/γ-Al2O3 as catalyst. The kinetics of the transesterification during ultrasonic irradiation is basically the same as that of mechanical stirring, and the second grade reaction of increase stage at the beginning and finally reaches the one and zero grade reaction with the transesterification. The reactive activation energy and pre-exponential factor of the transesterification during ultrasonic irradiation is 12.99 kJ/mol and 76.33 L/(mol·min), respectively. Compared with that of mechanical stirring, the reactive activation energy is lower, and the reaction rate is higher during ultrasonic irradiation, which means the transesterification reaction is easily progressed and reaches the reaction equilibrium in a short time.
Key words: ultrasonic; kinetics; KF/γ-Al2O3; catalyst; soybean oil; transesterification; biodiesel
由于声化学自身具有低能耗、无污染等特点,使其可望成为21世纪的“绿色化学”[1]。目前,声化学技术在化学化工过程强化中已有较广泛的研究[2-4],超声强化酯交换反应制备生物柴油已有较多的报 道[5-10],如:Starvarache等[9]对超声强化NaOH和KOH均相催化酯交换反应制备生物柴油进行了研究,结果表明超声可以缩短反应时间,降低催化剂用量,并且反应条件温和。Hanh等[11]采用40 kHz功率超声强化KOH催化甘油三油酸酯与甲醇酯交换反应,并与传统方法进行了对比,结果也表明超声作用下催化剂用量减少,反应时间缩短。目前,德国Hielscher公司有不同规模的超声波生物柴油反应器产品[12]。Maeda等[13]在进行超声波连续法制备生物柴油时,加载的超声波功率密度为0.15 W/cm3,混合物料在反应系统中停留时间为10~20 min,反应规模已达19 L/h。南非Bio-Fuels ON公司正开发500 L/h的反应装置。超声波技术用于生物柴油生产的工业化前景较好。但是,已报道的研究大多集中在超声强化均相催化酯交换反应工艺参数方面,而对于超声强化酯交换反应的动力学及机理研究报道较少。Colucci等[14]在醇油比为6:1、温度为25~60 ℃条件下,建立了准级数二次动力学模型研究甘油二酯(DG)和甘油三酯(TG)的水解。TG和DG的反应级数在90%的置信区间内约为2,这与其他文献报道中的研究结果相近[15-16]。当温度为60 ℃时,反应速率常数KDG>KTG,且反应速率常数是文献报道中机械搅拌的3~5倍[15-16]。在该实验中,根据Arrhenius方程得到的TG活化能53.13 kJ/mol,DG活化能57.52 kJ/mol,均较文献[15-16]报道的小(67.25 kJ/mol和72.21 kJ/mol)。证明超声作用下酯交换反应更容易进行。目前,KF/Al2O3固体催化剂用于生物柴油制备也有较多文献报道[17-19]。由于纳米固体催化剂具有比表面大、催化活性高的特点,用于催化制备生物柴油与普通的固体催化剂比较,反应条件温和,反应速率快,酯交换反应产率高。胡圣扬等[20-21]以纳米γ-Al2O3为载体制备了纳米K2CO3/γ-Al2O3、纳米KF/γ-Al2O3酯交换催化剂,用于催化植物油制备生物柴油,取得较好效果。因此,纳米固体碱在生物柴油制备方面具有广阔的发展前景。但超声强化纳米固体碱 KF/γ-Al2O3 催化酯交换反应的动力学研究未见报道。为此,本文作者基于对超声强化纳米固体碱 KF/γ-Al2O3 催化酯交换反应系统的研究,探讨超声强化作用下酯交换反应的动力学参数,以便为超声反应器的研制和工艺放大提供参考。
1 实验材料与方法
1.1 实验材料
(1) 原料与试剂有:大豆油(福临门)(酸值1.13,皂化值191.8),中粮食品营销有限公司生产;纳米固体超强碱KF/γ-Al2O3(自制);色谱级甲醇,天津科密欧化学化剂有限公司生产;色谱级标样棕榈酸甲酯、硬脂酸甲酯、油酸甲酯、亚油酸甲酯和棕榈酸甲酯、内标物十一酸甲酯等,由Fluka公司生产。
(2) 实验仪器及设备有:DC-2020节能型智能恒温槽,宁波新芝生物科技股份有限公司制造;超声装置(40 kHz),昆山市超声仪器有限公司制造;101AS-1型不锈钢数显电热鼓风干燥箱,上海浦东荣丰科学仪器有限公司制造;RE-52A型旋转蒸发器,上海亚荣生化仪器厂制造;JB-90S型数显转速电动搅拌机,上海标本模型厂制造;Avanti 30型贝克曼高速冷离心机,美国贝克曼仪器公司制造;6890N型气相色谱仪,美国安捷伦公司制造。
1.2 实验方法
1.2.1 酯交换反应样品制备
(1) 机械搅拌强化酯交换反应样品制备。将132.4 g (0.15 mol)大豆油放入四直口圆底烧瓶(250 mL),预热。开启搅拌器调到预设转速600 r/min,将45.61 mL甲醇加入已恒温的反应器中,然后加入 1.99 g纳米催化剂KF/γ-Al2O3,反应计时并每隔一定时间取样,并所取样品立即放置到冰水中终止反应,然后将所取样品12 000 r/min,于4 ℃离心5 min分离催化剂,取离心后上层甲酯相1 mL于1.5 mL离心管中低温保存以备分析。反应体系温度采用DC-2020节能型智能恒温槽循环水浴控制,并维持循环水体积恒定。
(2) 超声强化酯交换反应样品制备。将132.4 g (0.15 mol)的大豆油和45.61 mL的甲醇加入到250 mL四口烧瓶中,并加入1.99 g(大豆油质量的 1.5%)纳米KF/γ-Al2O3催化剂,开启超声装置(40 kHz)到设定功率密度54 W/L。反应计时并在一定时间取样,并所取样品立即放置到冰水中终止反应,然后将所取样品12 000 r/min,于4 ℃离心5 min分离催化剂,取离心后上层甲酯相1 mL于1.5 mL离心管中低温保存以备分析。反应体系温度采用 DC-2020节能型智能恒温槽循环水浴控制,并维持循环水体积恒定。
1.2.2 生物柴油样品分析
反应体系中脂肪酸甲酯的含量通过气相色谱内标法测定。取样品0.200 0 g,加入一定量的内标物十一酸甲酯,用苯溶解并定容到10 mL。采用美国HP公司的AgilentGC6890N气相色谱仪分析样品,其工作参数为:毛细管柱(DB-FFAP);FID 检测器。柱升温程序为:初温150 ℃,保持2 min,然后以10 ℃/min升至230 ℃,保持8 min;汽化室温度为250 ℃;N2(高纯)流量为25 mL/min,H2流量为40 mL/min,空气流量为450 mL/min,检测器温度:300 ℃;进样量1 μL,分流进样,分流比为48.9:1.0。脂肪酸甲酯含量wME由以下公式得出:
 (1)
(1)
式中:mi为内标物质量;ms为样品质量;Ai为内标物峰面积;fs为相对校正因子;As为脂肪酸甲酯峰面积。
2 结果与讨论
2.1 反应动力学模型建立
甘油三酯TG 和甲醇酯交换反应方程式为:
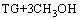
 GL+3ME (2)
GL+3ME (2)
式中:GL为甘油;ME为脂肪酸甲酯。因而可用1个动力学模型描述该过程。已知宏观反应速率方程为:
 (3)
(3)
由于反应初始阶段甲醇大大过量,所以,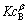 为常数,则方程转化为:
为常数,则方程转化为:
 (4)
(4)
式中: ,将式(4)线性化:
,将式(4)线性化:
 (5)
(5)
利用式(5)和同一反应中不同组分的反应速率间服从的化学计量学关系:
 ;
; (6)
(6)
整理可得:
 (7)
(7)
式中:cA为时刻t时的甘油三酯浓度,mol/L;cA0=0.788 2 mol/L;cp为脂肪酸甲酯浓度,cp=2.078wME,mol/L。
因此,只要通过试验测定酯交换反应过程中不同时间脂肪酸甲酯含量wME,确定浓度与反应时间的变化关系,即可确定动力学参数。
2.2 常规方法动力学参数确定
在搅拌速度为600 r/min,纳米KF/γ-Al2O3用量为大豆油质量1.5%,醇与油的物质的量比为7.5:1.0,酯交换反应温度分别为298,308,318和333 K的条件下进行酯交换反应制备生物柴油。每个实验温度采样15次,结果见图1。

图1 反应温度和时间对甲酯含量的影响
Fig.1 Effect of temperature and time on methyl ester concentration
利用Origin软件拟合图1 不同反应温度下甲酯含量变化曲线由开始到拐弯点这一段数据,得到拟合方程见表1。
表1 不同温度下拟合方程
Table 1 Curve fitting equations at different temperatures

将方程转化为微分形式即得速率方程,如表2 所示。
将计算得到的不同反应时间脂肪酸甲酯浓度cp和反应速率rp代入式(7),利用Matlab软件包最小二乘法可确定式(7)中的各常数,结果见表3。
由表3结果可知:常规方法即机械搅拌下,酯交换反应动力学在反应起始阶段为拟二级,然后转为一级或零级反应。这与邬国英等[22-23]的均相催化酯交换反应的动力学过程类似。反应速率常数与单因素的试验结果表现趋势一致,随着反应温度的升高而逐渐增加。因此,常规方法强化酯交换反应制备生物柴油时,适当提高温度有助于加快反应速度。
表2 不同温度下速率方程
Table 2 Reaction rate equations at different temperatures

表3 搅拌速度为600 r/min时酯交换反应的速率常数和反应级数
Table 3 Rate constants and reaction orders of transesterification reaction at mechanical stirring of 600 r/min

根据Arrhenius方程:
 (8)
(8)
采用线性拟合的方法,将表3中数据代入式(8)计算即可得到指前因子K0及活化能Ea,结果见图2。

图2 1/T与ln K1的关系
Fig.2 Relationship between 1/T and ln K1
通过拟合图2 得到拟合方程为ln K1=-2 528.9/T+ 6.741 1,拟合系数R2为0.956 5,因而求得K0为846.49 L/(mol·min),活化能Ea为21.03 kJ/mol。活化能与Freedam等[15, 24]研究结果(反应活化能Ea为33~83.7 kJ/mol)相比略低,说明纳米KF/γ-Al2O3催化酯交换反应较容易进行,催化剂催化活性较高。
2.3 超声强化酯交换反应动力学参数确定
在超声功率密度为54 W/L,纳米KF/γ-Al2O3用量为大豆油质量1.5%,醇与油的物质的量比为7.5:1.0,酯交换反应温度分别为298,308,318和333 K的条件下进行酯交换反应制备生物柴油。每个实验温度采样15次,结果见图3。
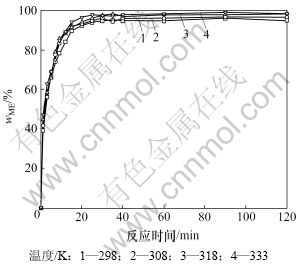
图3 反应温度和时间对甲酯含量的影响
Fig.3 Effect of reaction temperature and time on reaction products
采用与2.1中同样的方法,利用Origin软件拟合图3不同反应温度下甲酯含量变化曲线由开始到拐弯点这一段数据,得到拟合方程见表4。
表4 不同温度下拟合方程
Table 4 Curve fitting equations at different temperatures

将方程转化为微分形式即得速率方程,如表5 所示。
将计算得到的不同反应时间脂肪酸甲酯浓度cp和反应速率rp代入式(7),利用Matlab软件包最小二乘法可确定式(7)中的各常数,结果见表6。
由表6 结果可知:超声强化酯交换反应动力学与常规方法下基本一致,在反应起始阶段为拟二级,然后转为一级或零级反应。与机械搅拌强化酯交换反应过程相比,在超声作用下,反应速率常数随着温度的升高而增加的幅度较小,但速率常数显著高于机械搅拌强化酯交换反应的速率常数。表明超声强化酯交换反应在较低的温度条件下反应也比较迅速,也可以获得较高酯交换率。
表5 不同温度下速率方程
Table 5 Reaction rate equations at different temperatures

表6 超声功率密度为54 W/L时酯交换反应的速率常数和反应级数
Table 6 Rate constants and reaction orders of transesterification reaction at ultrasonic irradiation of 54 W/L

根据式(8),采用线性拟合的方法,计算即可得到指前因子K0及活化能Ea,结果见图4。
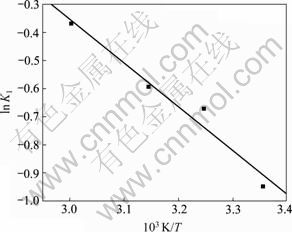
图4 1/T与ln K1的关系
Fig.4 Relationship between 1/T and ln K1
通过拟合图4 得到拟合方程为ln K1=-1 562.5/T+ 4.335 1,R2为0.963 9,求得反应起始阶段的K0为 76.33 L/(mol·min),活化能Ea为12.99 kJ/mol。活化能比机械搅拌下酯交换反应的略低,表明超声作用下,纳米 KF/γ-Al2O3 催化酯交换反应更容易进行。
3 结论
(1) 纳米KF/γ-Al2O3催化大豆油酯交换反应在反应初始阶段是拟二级反应,而后为一级反应,后期转为零级反应,反应初期的反应活化能Ea为21.03 kJ/mol,K0为846.49 L/(mol·min)。
(2) 超声强化非均相纳米KF/γ-Al2O3 催化酯交换反应动力学过程基本与常规法的动力学过程一致,反应初始阶段为拟二级反应,而后转后一级和零级。反应初期的K0为76.33 L/(mol·min),活化能Ea为12.99 kJ/mol。活化能比机械搅拌下酯交换反应的略低,表明超声作用下,酯交换反应更容易进行;超声强化酯交换反应速率常数比常规方法的高,从而反应能够在短时间内达到平衡。
参考文献:
[1] Mason T J. Sonochemistry and the environment—Providing a green link between chemistry, physics and engineering[J]. Ultrasonics Sonochemistry, 2007, 14(4): 476-483.
[2] 闫向宏, 张亚萍. 功率超声对稠油流变性影响的研究[J]. 声学技术, 1996, 15(4): 43-44.
YAN Xiang-hong, ZHANG Ya-ping. Studies on effect of power ultrasound on the the rheological behavior of heavy oil[J]. Technical Acoustics, 1996, 15(4): 43-44.
[3] 郭孝武. 超声波提取和回流提取对益母草总碱提出率的影响[J]. 声学术, 1996, 15(4): 44-46.
GUO Xiao-wu. Effect of ultrasound and reflux on the extraction yield of total alkaloids from leonurus[J]. Technical Acoustics, 1996, 15(4): 44-46.
[4] 林勤保, 高大维. 超声波对酶反应的影响[J]. 声学技术, 1997, 16(1): 27-29.
LIN Qin-bao, GAO Da-wei. Effect of ultrasound on enzyme catalysed reaction[J]. Technical Acoustics, 1997, 16(1): 27-29.
[5] 阎杰, 丘泰球, 任庆功. 声化学技术在强碱催化制备生物柴油中的应用[J]. 声学技术, 2008, 27(5): 690-695.
YAN Jie, QIU Tai-qiu, REN Qing-gong. Application of sonochemistry in the production of bio-diesel catalyzed by alkali[J]. Technical Acoustics, 2008, 27(5): 690-695.
[6] Stavarache C, Vinatoru M, Maeda Y. Aspects of ultrasonically assisted transesterification of various vegetable oils with methanol[J]. Ultrasonics Sonochemistry, 2007, 14(4): 380-386.
[7] Deshmane V G, Gogate P R, Pandit A B. Ultrasound assisted synthesis of isopropyl esters from palm fatty acid distillate[J]. Ultrasonics Sonochemistry, 2009, 16(3): 345-350.
[8] 林琳, 董英, 徐自明, 等. 超声波辅助制备米糠生物柴油及其燃料排放特性[J]. 农业工程学报, 2008, 24(8): 202-205.
LIN Lin, DONG Ying, XU Zi-ming, et al. Biodiesel production from rice bran oil by means of ultrasonic energy and emission properties as engine fuel[J]. Transactions of the CSAE, 2008, 24(8): 202-205.
[9] Stavarache C, Vinatoru M, Nishimura R, et al. Fatty acids methyl esters from vegetable oil by means of ultrasonic energy[J]. Ultrasonics Sonochemistry, 2005, 12(5): 367-372.
[10] JI Jian-bing, WANG Jian-li, LI Yong-chao, et al. Preparation of biodiesel with the help of ultrasonic and hydrodynamic cavitation[J]. Ultrasonics, 2006, 44: e411-e414.
[11] Hanh H D, Dong N T, Starvarache C, et al. Methanolysis of triolein by low frequency ultrasonic irradiation[J]. Energy Conversion and Management, 2008, 49(2): 276-280.
[12] 毕良武, 赵振东, 陈元平, 等. 超声波生物炼制技术综述[J]. 现代化工, 2008, 28(z2): 1-6.
BI Liang-wu, ZHAO Zhen-dong, CHEN Yuan-ping. Review on ultrasonic technology for biorefinery[J]. Modern Chemical Industry, 2008, 28(z2): 1-6.
[13] Maeda Y, Vinatoru M, Stavarache C, et al. Method for producing fatty acid alcohol ester: US, 2004159537A1[P]. 2004-08-19.
[14] Colucci J A, Borrero, E E, Alape F. Biodiesel from an alkaline transesterification reaction of soybean oil using ultrasonic mixing[J]. J Am Oil Chem Soc, 2005, 82(7): 525-530.
[15] Freedman B, Butterfield R O, Pryde E H. Transesterification kinetics of soybean oil[J]. J Am Oil Chem Soc, 1986, 63(10): 1375-1380.
[16] Darnoko D, Cheryan M. Kinetics of palm oil transesterification in a batch reactor[J]. J Am Oil Chem Soc, 2000, 77(12): 1263-1267.
[17] XIE Wen-lei, LI Hai-tao. Alumina-supported potassium iodide as a heterogeneous catalyst for biodiesel production from soybean oil[J]. Journal of Molecular Catalysis A: Chemical, 2006, 255(1/2): 1-9.
[18] Ebiura T, Echizen T, Ishikawa A, et al. Selective transesterification of triolein with methanol to methyl oleate and glycerol using alumina loaded with alkali metal salt as a solid-base catalyst[J]. Applied Catalysis A: General, 2005, 283(1/2): 111-116.
[19] LIU Xue-jun, HE Hua-yang, WANG Yu-jun, et al. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst[J]. Fuel, 2008, 87(2): 216-221.
[20] 胡圣扬, 王运, 韩鹤友, 等. 纳米K2CO3/γ-Al2O3催化剂的制备及其用于菜籽油制备生物柴油的研究[J]. 生物质化学工程, 2008, 42(1): 6-10.
HU Sheng-yang, WANG Yun, HAN He-you, et al. Preparation of nano K2CO3 /γ-Al2O3 catalyst and its application for synthesis of biodiesel from rapeseed oil[J]. Biomass Chemical Engineering, 2008, 42(1): 6-10.
[21] 卞庆贵, 胡明敏, 田建利, 等. 纳米催化剂KF/γ-Al2O3的制备及催化乌桕籽油制备生物柴油[J]. 应用化工, 2007, 36(12): 1197-1200.
BIAN Qing-gui, HU Ming-min, TIAN Jian-li, et al. Preparation of KF/γ-Al2O3 nanocatalyst and its catalysis in the preparation of biodiesel from stillingia oil[J]. Applied Chemical Industry, 2007, 36(12): 1197-1200.
[22] 邬国英, 林西平, 巫淼鑫, 等. 棉籽油间歇式酯交换反应动力学研究[J]. 高校化学工程学报, 2003, 17(3): 314-318.
WU Guo-ying, LIN Xi-ping, WU Miao-xin, et al. Kinetics of cotton seed oil transesterification in a batch reactor[J]. Journal of Chemical Engineering of Chinese Universities, 2003, 17(3): 314-318.
[23] 马利, 洪建兵, 甘孟瑜, 等. 酯化-酯交换两步法制备生物柴油的动力学[J]. 化工学报, 2008, 59(3): 708-712.
MA Li, HONG Jian-bing, GAN Meng-yu, et al. Kinetics of esterification and transesterification for biodiesel production in two-step process[J]. Journal of Chemical Industry and Engineering, 2008, 59(3): 708-712.
[24] Noureddini H, Zhu D. Kinetics of transesterification of soybean oil[J]. JAOCS, 1997, 74(11): 1457-1463.
(编辑 杨幼平)
收稿日期:2010-02-07;修回日期:2010-05-10
基金项目:广州市科技计划项目(2007J1-C0451);常州大学博士启动基金资助项目(ZMF10020013)
通信作者:丘泰球(1941-),男,广东梅州人,教授,从事天然产物与声化工技术研究;电话:020-87113308;E-mail: tqqiu@scut.edu.cn