文章编号:1004-0609(2014)08-2137-08
包头稀土精矿的配合浸出及动力学
张晓伟1, 2,李 梅1, 2,柳召刚1,胡艳宏1, 2,王觅堂1,刘 佳1,阳建平1
(1. 内蒙古科技大学 材料与冶金学院,包头 014010;
2. 北京化工大学 材料科学与工程学院,北京 100029)
摘 要:采用氯化铝盐酸体系配合浸出包头混合稀土精矿,并对浸出过程动力学进行研究,浸出过程主要考察盐酸和氯化铝的浓度、液固比、搅拌速度、温度及反应时间对精矿浸出的影响。结果表明,随着盐酸和氯化铝的浓度和液固比的增大、反应时间的延长和反应温度的升高,精矿的浸出率逐渐增大,得到的优化浸出工艺条件如下:HCl和AlCl3浓度分别为4.0 mol/L和1.5 mol/L,液固比为20 mL/g,搅拌速度为300 r/min,温度为85 ℃,时间为90 min。SEM-EDS及动力学分析结果表明,精矿浸出过程符合一种受固体颗粒表面的界面交换和固膜扩散混合控制的新缩小核模型,表观活化能为35.3 kJ/mol,阿伦尼乌斯常数k0=419.95,反应级数a,b和c分别为1.265,1.208和1.22,通过计算推导出反应动力学方程。
关键词:氯化铝;盐酸;浸出;稀土精矿;动力学;活化能
中图分类号:TF803 文献标志码:A
Complex leaching and kinetics of Baotou mixed rare earth concentrate
ZHANG Xiao-wei1, 2, LI Mei1, 2, LIU Zhao-gang1, HU Yan-hong1, 2, WANG Mi-tang1, LIU Jia1, YANG Jian-ping1
(1. School of Materials and Metallurgy, Inner Mongolia Science and Technology University, Baotou 014010, China;
2. College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China)
Abstract: The complex leaching kinetics of Baotou mixed rare earth concentrate in HCl-AlCl3 solution was investigated. The effects of HCl concentration, AlCl3 concentration, liquid to solid ratio, stirring speed, temperature and reaction time were investigated. The results indicate that the concentrate leaching ratio increases with increasing the HCl and AlCl3 concentrations, liquid to solid ratio, temperature and reaction time. The optimal conditions are as follows: concentrations of HCl and AlCl3 are 4.0 mol/L and 1.5 mol/L, respectively, the liquid to solid ratio is 20 mL/g, the stirring speed is 300 r/min, temperature is 85 ℃, reaction time is 90 min. The SEM-EDS and kinetic analysis show that the concentrate leaching conforms with a new shrinking core model, in which both the interfacial transfer and diffusion through the product layer affect the reaction rate. The apparent activation energy is 35.3 kJ/mol, the Arrhenius constant k0 is 419.95, and the reaction series a, b, c are 1.27, 1.21 and 1.22, respectively. A kinetic equation was derived to describe the process.
Key words: aluminium chloride; hydrochloric acid; leaching; mixed rare earth concentrate; kinetics; activation energy
位于内蒙古包头市白云鄂博地区的稀土矿是由氟碳铈矿和独居石组成的混合型稀土矿物,占世界已探明稀土储量的43%以上[1],是非常重要的稀土资源。目前,工业生产所使用的稀土精矿是选铁后的矿渣经磁选和浮选后所得的氧化稀土含量在50%以上的精矿,其中氟碳铈矿与独居石的质量比在9:1~6:4之间[2-4]。
目前,工业上对于分解包头混合型稀土矿物广泛采用的工艺主要有浓硫酸强化焙烧法和烧碱法[5],特别是浓硫酸强化焙烧法应用更加普遍,因为该方法对精矿品位要求低,生产成本低,处理流程短,分解效率高。但是不论是浓硫酸强化焙烧法还是烧碱法,都要求首先对混合稀土精矿进行焙烧,焙烧过程中氟碳铈矿会分解,氟元素以HF气体的形式溢出,同时也溢出大量的含硫气体,严重污染环境。近些年来,为了降低环境污染,提高生产效率,本领域的科研工作者也在不断研究新的分解工艺,例如,张丽清等[6]采用加碳氯化氧化反应方法分解混合稀土精矿,于秀兰等[7]采用AlCl3脱氟-碳热氯化法从混合稀土精矿中提取稀土,这些方法都取得了一定的分解效果,但是目前都处于实验室研究阶段,还无法进行工业化生产。
在湿法冶金领域有一种络合浸出的方法[8-10],可在低温条件下有效去除化合物中铁元素及氟化钙等杂质,效果非常显著,这些都是利用了铁离子或者氟离子与某些阴离子具有极强的络合作用。由于氟与铝也具有极强的络合作用,稳定常数[11]为6.9×1019,极易形成[AlF6]3+。所以,本文作者根据以上原理研究了HCl-ACl3体系分解包头混合稀土精矿中的氟碳铈矿,并对分解过程的动力学进行了详细研究。此方法在氟碳铈矿分解的同时将氟碳铈矿与独居石进行了分离,精矿不需要高温焙烧,通过湿法冶金的方法在低温条件下将矿物分解,并对浸出过程动力学进行研究。
1 实验
1.1 原料
实验中所用的混合稀土精矿来自于包钢稀土高科股份有限公司,粒度小于0.04 mm,颜色为浅灰色,其稀土精矿中的化学成分及稀土配分由包头稀土研究院分析室测定,结果见表1和表2。
实验中所用盐酸和结晶氯化铝等试剂均为市售分析纯试剂。
表1 包头稀土精矿的化学成分
Table 1 Chemical contents of Baotou rare earth concentrate (mass fraction, %)

表2 包头稀土精矿中稀土组分含量
Table 2 Rare earth contents of Baotou rare earth concentrate (mass fraction, %)

1.2 实验方法及步骤
浸出实验在带有循环冷凝装置的400 mL三颈烧瓶中进行,用HH-4恒温数显水浴锅来控制反应的温度,用金坛JJ-1型精密恒速搅拌器控制搅拌速度。所有实验中每次都称取10 g的稀土精矿样品。首先按要求配制一定浓度的HCl-AlCl3体系溶液加入烧瓶中,再将烧瓶放在设定好温度的水浴锅中,用温度计监测烧瓶中温度,待烧瓶中液体温度与水浴锅温度相同后,将称好的稀土精矿缓慢加入到烧瓶中,打开搅拌器调好转速后开始计时,到达计划时间后迅速进行热过滤,用蒸馏水洗涤滤渣3次,放入DZF-6090真空干燥箱中在120 ℃干燥2 h后用BS2202S电子天平称量滤渣的质量,采用QUANDTA 400型扫描电子显微镜对原矿和浸出渣进行微观及能谱分析。
稀土精矿浸出率的计算方法如下:
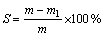 (1)
(1)
式中:S为稀土精矿浸出率(%);m1为滤渣质量(g);m为稀土精矿质量(g)。
2 结果与讨论
2.1 盐酸浓度对精矿浸出的影响
图1所示为盐酸浓度对精矿浸出的影响。由图1可以看出,当盐酸浓度由0增至6 mol/L时,精矿的浸出率逐渐增加,当反应进行到60 min,盐酸浓度由4 mol/L增至6 mol/L时,精矿的浸出率变化不大,这是因为当盐酸浓度增大到一定程度时,矿物颗粒表面的H+浓度处于饱和状态,碳酸稀土与H+充分接触,导致化学反应达到了准平衡,所以4 mol/L的盐酸浓度可以作为浸出的优化条件。
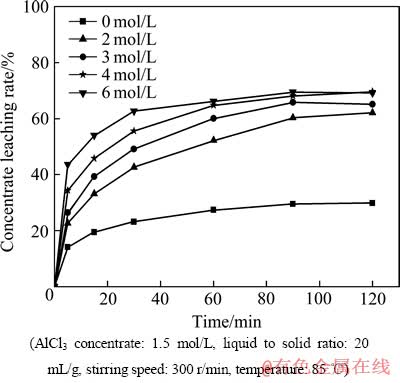
图1 盐酸浓度对精矿浸出的影响
Fig. 1 Effect of HCl concentration on concentrate leaching rate
当盐酸浓度为0时,精矿仍有部分浸出,最大浸出率达到29.82%,这是由于发生以下反应:
2REF3 + Al3+ = [AlF6]3- + 2RE3+,△GΘ = -140.439 kJ/mol (2)
3CaF2 + Al3+ = [AlF6]3- + 3Ca2+ ,△GΘ = -66.579 kJ/mol (3)
部分氟化稀土及氟化合物可直接与AlCl3发生络合反应,由此可见,Al3+对F-确实具有极强的络合作用,Al3+与颗粒表面的氟化物发生化学反应导致了部分精矿的溶解。
2.2 氯化铝浓度对精矿浸出的影响
图2所示为精矿浸出率随AlCl3浓度变化的曲线。由图2中看出,随着AlCl3浓度的增加,精矿的浸出率逐渐增加,当反应进行到90 min后达到准平衡状态;当AlCl3的浓度为1.5和2 mol/L,反应进行到90 min时,精矿浸出率分别为71.16%和72.55%,仅相差1.39%,所以选择1.5 mol/L的AlCl3浓度为浸出优化条件。
当ACl3浓度为0时,仍然有部分的精矿浸出,这主要是由于以下反应的发生:
RE2(CO3)3 +6H+ = 2RE3+ +3CO2↑ + 3H2O,△GΘ = -180.255 kJ/mol (4)
精矿中的部分碳酸稀土与HCl反应,最大的浸出率达到34.89%。
根据以上两个因素对精矿浸出的影响可以看出,在本研究中氟碳铈矿的完全分解必须通过HCl和AlCl3的共同作用才能实现,这是因为氟碳铈矿是氟化稀土与碳酸稀土的复合化合物。
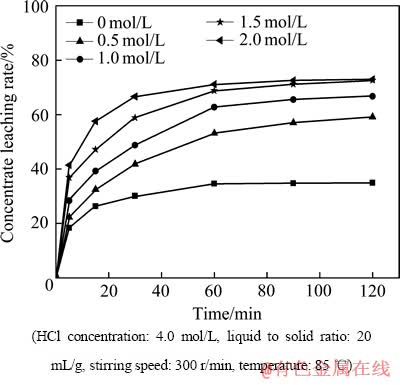
图2 AlCl3浓度对精矿浸出率的影响
Fig. 2 Effect of AlCl3 concentration on concentrate leaching rate
2.3 液固比对精矿浸出的影响
在液固反应中,液固比与矿浆浓度成反比,它对于液固反应有着非常重要的影响。图3所示为液固比对精矿浸出率的影响。由图3可以看出,在60 min前,随着液固比由5 mL/g增加至30 mL/g,精矿浸出率迅速增加,60 min后精矿浸出率增加缓慢。这是因为当液固比增大时,矿浆浓度就会随之减小,而溶液中离子的扩散动能增大,所以有利于化学反应方程(2),(3)和(4)向右进行。反应进行到90 min后基本达到了化学反应准平衡,且液固比为20和30 mL/g时精矿的浸出率基本相同,所以选择20 mL/g为优化工艺条件。
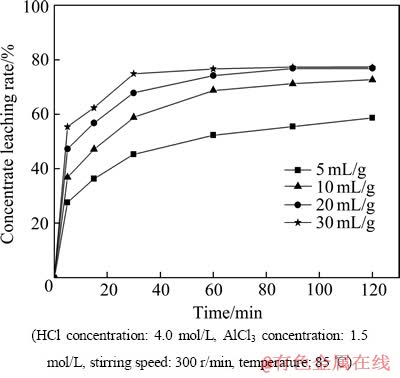
图3 液固比对精矿浸出率的影响
Fig. 3 Effect of liquid to solid rate on concentrate leaching ratio
2.4 搅拌速度对精矿浸出的影响
实验研究了搅拌速度对稀土精矿浸出率的影响。搅拌对于液固反应过程中的化学反应及离子扩散具有非常重要的作用。图4所示为搅拌速度对精矿浸出率的影响。由图4可知,当搅拌速度达到200 r/min时,烧瓶中的固体颗粒在溶液中已经达到了均匀的悬浮状态。当搅拌速度由100 r/min增加到300 r/min时,精矿的浸出率逐渐增加,但是当搅拌速度大于300 r/min以后,精矿的浸出率开始下降。这是因为当固体颗粒与溶液发生反应时,固体颗粒表面首先要吸附离子,然后发生化学反应,如果搅拌速度过大,必然会降低固体颗粒表面的吸附反应,所以精矿的浸出率就会下降,因此,300 r/min为最佳的搅拌速度。
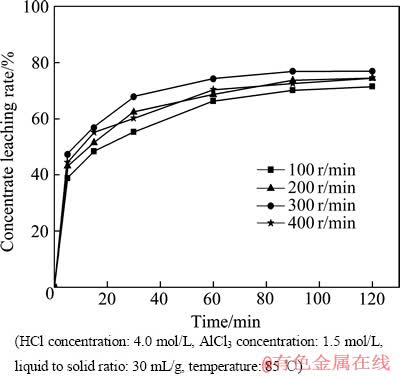
图4 搅拌速度对精矿浸出率的影响
Fig. 4 Effect of stirring speed on concentrate leaching rate
2.5 温度对精矿浸出的影响
温度是化学反应过程中的最大的影响因素。图5所示为温度对精矿浸出率的影响。由图5可以看出,在反应进行到5 min后,温度由45 ℃增加至100 ℃时,精矿的浸出率迅速地由22.8%增加至55.36%。在100 ℃,当反应进行到30 min时就已达到了化学反应准平衡。在85 ℃,反应进行到60~90 min之间达到了化学反应准平衡,可见温度越高,精矿的浸出速率越快,但是实验过程中温度越高,实验操作的难度就越大,因为高温条件下,溶液中水分及盐酸的挥发速度会相应的增大,容器内压力随之增大,冷凝水的流速就必须增大,在本研究中选择85 ℃为优化浸出温度,该优化条件下,90 min时的浸出率达到76.83%,理论推算矿物的最大溶解率为77.97%。,由此判断混合稀土精矿中的氟碳铈矿几乎全部分解。
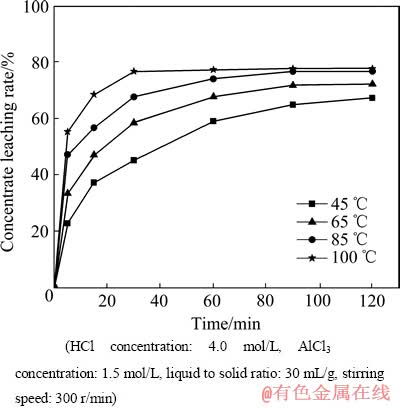
图5 温度对稀土精矿浸出率的关系
Fig. 5 Effect of temperature on rare earth concentrate leaching rate
2.6 精矿及浸出渣的SEM-EDS分析
通过SEM-EDS测试了精矿浸出前后的表面结构及成分。精矿的SEM像如图6所示。由图6(a)和(b)可以看出,精矿颗粒表面光滑并且有很多白色物质分散于颗粒表面,对比浸出渣的SEM像(见图7(a)和(b))可见,精矿经过酸浸以后,表面变得凹凸不平,表面的白色物质消失,颗粒变得均匀且粒径变小。在颗粒的表面没有发现新的固体产物层的包裹。对比精矿浸出前后的EDS能谱(见图6(c)和(d),7(c)和(d))发现,矿物中的F元素消失,在浸渣中P元素的峰强明显增大,Si和Fe的峰强减弱但仍然存在,说明仍然有部分Si和Fe的化合物没有溶解。
2.7 浸出动力学分析
精矿浸出过程的总反应方程式如下:
2REF3·RE2(CO3)3 + AlCl3 + 12HCl =5RECl3 + H3AlF6+ 6H2O +6CO2↑ (5)
根据SEM-EDS图像分析,认为该浸出反应过程应该属于缩小核模型,利用传统的动力学模型[12-15]对该浸出过程进行动力学分析发现,线性相关系数R2均小于0.95,所以传统的动力学模型不适合用来描述该浸出反应的动力学过程。根据DICKINSON等[16]和DEHGHAND等[17]的研究给出了一种新的缩小核模型,他们认为这种浸出过程是受固体颗粒表面的界面交换和固膜扩散共同控制的缩小核模型,动力学方程如下:
 (6)
(6)
式中: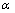 为精矿的浸出率;Km为反应表观速率常数;t为反应时间。
为精矿的浸出率;Km为反应表观速率常数;t为反应时间。
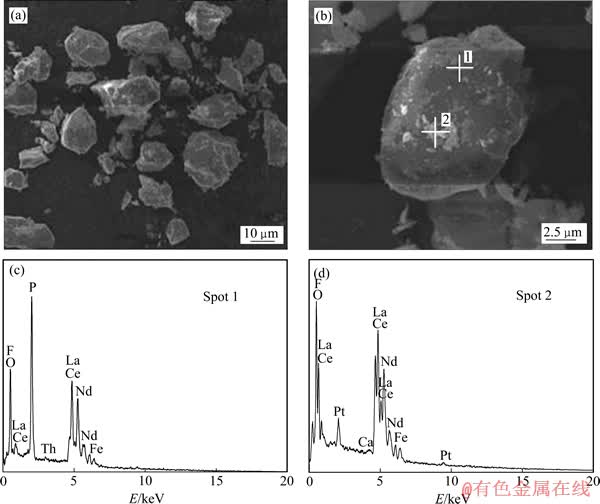
图6 精矿的SEM像及相应的EDS谱
Fig. 6 SEM images ((a), (b)) and EDS patterns ((c), (d)) of rare earth concentrate
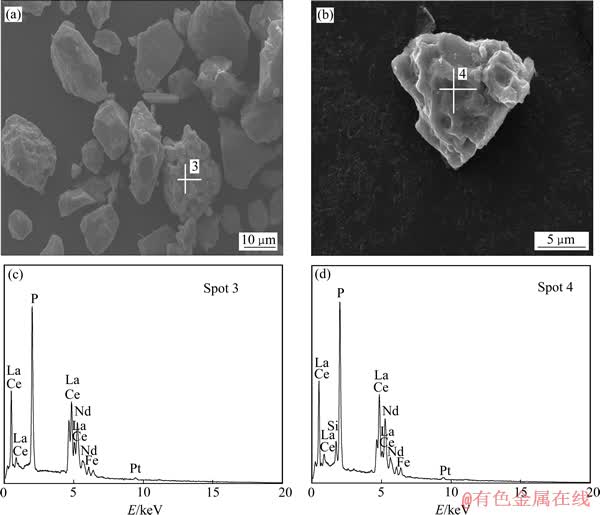
图7 浸出渣的SEM像及相应的EDS谱
Fig. 7 SEM images ((a), (b)) and EDS patterns ((c), (d)) of leaching residue
将图5得到的精矿浸出率的结果代入式(6)中,得到了在不同的反应温度下 与时间t的直线,见图8。
与时间t的直线,见图8。
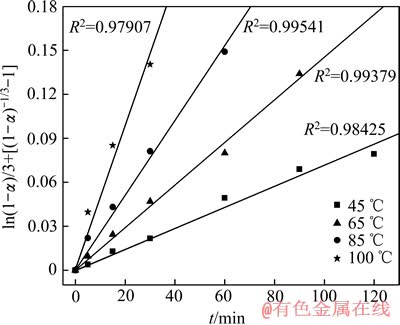
图8 在不同温度下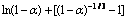 与时间t的关系
与时间t的关系
Fig. 8 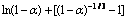 vs t at different temperatures
vs t at different temperatures
由图8可以看出,在各温度条件下得到的反应相关系数R2均大于0.97,所以具有很好的线性相关性,本研究中精矿浸出过程符合这种动力学模型。根据Arrhenius公式[18-19]K=Aexp[-Ea/(RT)]两边取对数可得:lg K=lg A-Ea/(2.303RT),用lg K-1/T作图9,求得活化能Ea=35.3 kJ/mol。
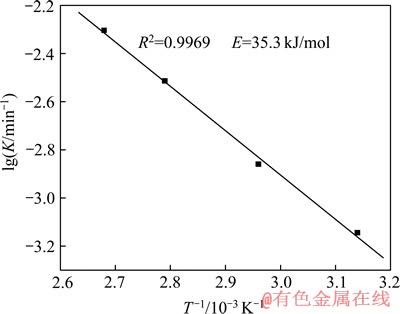
图9 精矿浸出的Arrhenius图
Fig. 9 Arrhenius plot of concentrate leaching
在本研究的液固反应中,温度、时间、液固比、盐酸和氯化铝的浓度对浸出反应都有很大的影响,因此,新的缩小核模型的变体表观反应速率常数可以写成与各影响因素的关系如下:
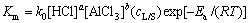 (7)
(7)
式中:k0为阿伦尼乌斯常数;a、b和c为反应级数;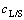 为液固比。
为液固比。
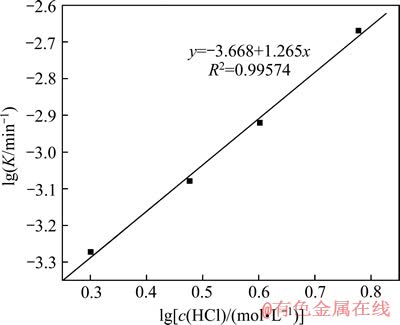
图10 精矿浸出的 的关系
的关系
Fig. 10 Plot of  for concentrate leaching
for concentrate leaching
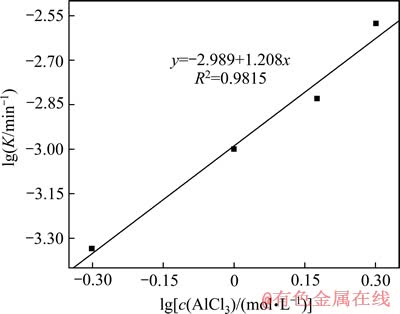
图11 精矿浸出的 的关系
的关系
Fig. 11 Plot of  for concentrate leaching
for concentrate leaching
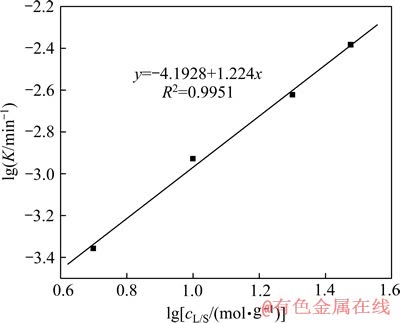
图12 精矿浸出的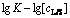 的关系
的关系
Fig. 12 Plot of 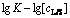 for concentrate leaching
for concentrate leaching
式(6)可以写成下面的形式:
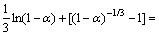
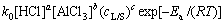 (8)
(8)
根据图9求得该反应的活化能Ea为35.3 kJ/mol,由截距求得阿伦尼乌斯常数k0为419.95。反应级数a、b和c可以分别由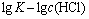 、
、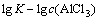 、
、 作图分别求出,见图10,11和12。
作图分别求出,见图10,11和12。
由图10、11和12分别得出反应级数a=1.27、b=1.21和c=1.22,分别代入式(7)得出该精矿浸出过程的动力学方程式如下:
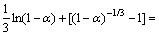
 (9)
(9)
3 结论
1) 各因素对包头混合稀土精矿络合浸出的研究结果表明,精矿的浸出率随着盐酸和氯化铝的浓度、液固比、温度和反应时间的增大而逐渐增大,得到的优化浸出工艺条件如下:HCl和AlCl3浓度分别为4.0 mol/L和1.5 mol/L,液固比为20 mL/g,温度为85 ℃,时间为90 min,搅拌速度为300 r/min。
2) 精矿及浸出渣的SEM-EDS图像表明,精矿浸出属于缩小核反应,但浸渣表面没有新的固体产物层产生,一个新的缩小核模型可很好地用于描述精矿浸出过程的动力学,该反应过程受固体颗粒表面的界面交换和固膜扩散混合控制。
3) 通过计算及分析得出反应的表观活化能为Ea=35.3 kJ/mol,阿伦尼乌斯常数k0=419.95,反应级数a、b和c分别为1.27、1.21和1.22,然后,得到矿物浸出动力学方程。
REFERENCES
[1] 吴志颖, 吴文远, 孙树臣, 边 雪, 涂赣峰. 混合稀土精矿氧化焙烧过程中氟的逸出规律研究[J]. 稀土, 2009, 30(60): 18-21.
WU Zhi-ying, WU Wen-yuan, SUN Shun-chen, BIAN Xue, TU Gan-feng. Study of fluorine escaping in calcining process of mixed rare earth concentrate[J]. Journal of Rare Earths, 2009, 30(60): 18-21.
[2]  Effect of thiourea on sulphuric acid leaching of bastnaesite[J]. Hydrometallurgy, 2003, 68(1/3): 195-202.
Effect of thiourea on sulphuric acid leaching of bastnaesite[J]. Hydrometallurgy, 2003, 68(1/3): 195-202.
[3] PRADIP, FUERSTENAU D W. The role of organic and inorganic reagents in the flotation separation of the rare earth ores[J]. International Journal of Mineral Processing, 1991, 32(1/2): 1-22.
[4] KANAZAWA Y, KAMITANI M. Rare earth minerals and resources in the world[J]. Journal of Alloys and Compounds, 2006, 408/412: 1339-1343.
[5] 黄小卫, 李红卫, 薛向欣, 张国成. 我国稀土湿法冶金发展状况及研究进展[J]. 中国稀土学报, 2006, 24(2): 129-133.
HUANG Xiao-wei, LI Hong-wei, XUE Xiang-xin, ZHANG Guo-cheng. Development status and research progress in rare earth hydrometallurgy in china[J]. Journal of Rare Earths, 2006, 24(2): 129-133.
[6] 张丽清, 张凤春, 姚淑华, 姜琳琳, 王小欢. 加碳氯化-氧化反应方法从氟碳铈矿-独居石混合精矿中提取稀土[J]. 过程工程学报, 2007, 7(1): 75-78.
ZHANG Li-qing, ZHANG Feng-chun, YAO Shu-hua, JIANG Lin-lin, WANG Xiao-huan. Rare earth extraction from mixed bastnaesite-monazite concentrate by carbochlorination- oxidation[J]. The Chinese Journal of Process Engineering, 2007, 7(1): 75-78.
[7] 于秀兰, 王之昌, 王 勇, 董德千, 刘 嘉. 采用 AlCl3脱氟-碳热氯化法从混合稀土精矿中提取稀土[J]. 过程工程学报, 2008, 8(2): 258-262.
YU Xiu-lan, WANG Zhi-chang, WANG Yong, DONG De-qian, LIU Jia. Extraction of rare earths from mixed bastnaesite- monazite concentrate by carbochlorination reaction with AlCl3 as defluorinating agent[J]. The Chinese Journal of Process Engineering, 2008, 8(2): 258-262.
[8] LEE S O, TRAN T, PARK Y Y, KIM S J, KIM M J. Study on the kinetic of iron oxide leaching by oxalic acid[J]. International Journal of Mineral Processing, 2006, 80(2/4): 144-152.
[9] 边 雪, 尹少华, 张丰云, 吴文远, 涂赣峰. 柠檬酸配合浸出分离稀土氧化物与氟化钙[J]. 材料与冶金学报, 2011, 10(4): 244-248.
BIAN Xue, YIN Shao-hua, ZHANG Feng-yun, WU Wen-yuan, TU Gan-feng. The separation of rare earth oxide and celeium fluoride with the method of citric acid complex leaching[J]. Journal of Materials and Metallurgy, 2011, 10(4): 244-248.
[10] 边 雪, 吴文远, 罗 瑶, 尹少华, 张 博. HCl-AlCl3溶液络合浸出稀土氧化物和氟化钙的研究[J]. 中国稀土学报, 2010, 28(3): 322-329.
BIAN Xue, WU Wen-yuan, LUO Yao, YIN Shao-hua, ZHANG Bo. Coordination leaching of rare earths oxide and CaF2 by HC-lAlC l3[J]. Journal of Rare Earths, 2010, 28(3): 322-329.
[11] 杭州大学化学系分析化学教研室. 分析化学手册(第一分册)[M]. 2版. 北京: 化学工业出版社, 2003: 153-175.
Analytical Chemistry Teaching and Research Section of Chemistry Department Hangzhou University. Handbook of analytical chmuistry (first fascicule)[M]. 2nd ed. Beijing: Chemical Industry Press, 2003: 153-175.
[12] PUENTE-SILLER D M, FUENTES-ACEITUNO J C, NAVA-ALONSO F. A kinetic–thermodynamic study of silver leaching in thiosulfate-copper-ammonia-EDTA solutions[J]. Hydrometallurgy, 2013, 134/135: 124-131.
[13] GHARABAGHI M, IRANNAJAD M, AZADMEHR A R. Leaching kinetics of nickel extraction from hazardous waste by sulphuric acid and optimization dissolution conditions[J]. Hydrometallurgy, 2013, 91(2): 325-331.
[14] PARHI P K, PARK K H, SENANAYAKE G. A kinetic study on hydrochloric acid leaching of nickel from Ni-Al2O3 spent catalyst[J]. Hydrometallurgy, 2013, 19(2): 589-594.
[15] LIU Kui, CHEN Qi-yuan, YIN Zhou-lan, HU Hui-ping, DING Zhi-ying. Kinetics of leaching of a Chinese laterite containing maghemite and magnetite in sulfuric acid solutions[J]. Hydrometallurgy, 2012, 125/126: 125-136.
[16] DICKINSON C F, HEAL G. R. Solid-liquid diffusion controlled rate equations[J]. Thermochimica Acta, 1999, 340/341: 89-103.
[17] DEHGHAN R, NOAPARAST M, KOLAHDOOZAN M. Leaching and kinetic modilling of low-grade calcareous sphalerite in acidic ferric chloride solution[J]. Hydrometallurgy, 2009, 96(4): 275-282.
[18] 覃文庆, 唐双华, 厉 超. 高硅低品位氧化锌矿的酸浸动力学[J]. 矿冶工程, 2008, 28(1): 62-65.
QIN Wen-qing, TANG Shuang-hua, LI Chao. Kinetics of sulfuric acid leaching of high silica low-grade zinc oxide ore[J]. Mining and Metallurgical Engineering, 2008, 28(1): 62-65.
[19] LI Min-ting, WEI Chang, QIU Shuang, ZHOU Xue-jiao, LI Cun-xiong, DENG Zhi-gang. Kinetics of vanadium dissolution from black shale in pressure acid leaching[J]. Hydrometallurgy, 2010, 104(2): 193-200.
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51174115);国家杰出青年基金资助项目(51025416);国家重点基础研究发展计划前期研究专项(2011CB411911);国家“十二五”科技支撑计划资助项目(2012BAE01B01);国家高技术研究发展计划资助项目(2012AA061904)
收稿日期:2013-09-28;修订日期:2014-06-14
通信作者:李 梅,教授,博士;电话:0472-5954390;E-mail: limei@imust.cn