黄药在黄铁矿及毒砂表面的氧化动力学
来源期刊:中南大学学报(自然科学版)1995年第5期
论文作者:杨家红 陈万雄
文章页码:600 - 604
关键词:电化学; 黄铁矿; 毒砂; 黄药; 氧化动力学
Key words:electrochemistry; pyrite; arsenopyrite; xanthate; oxidation kinetics
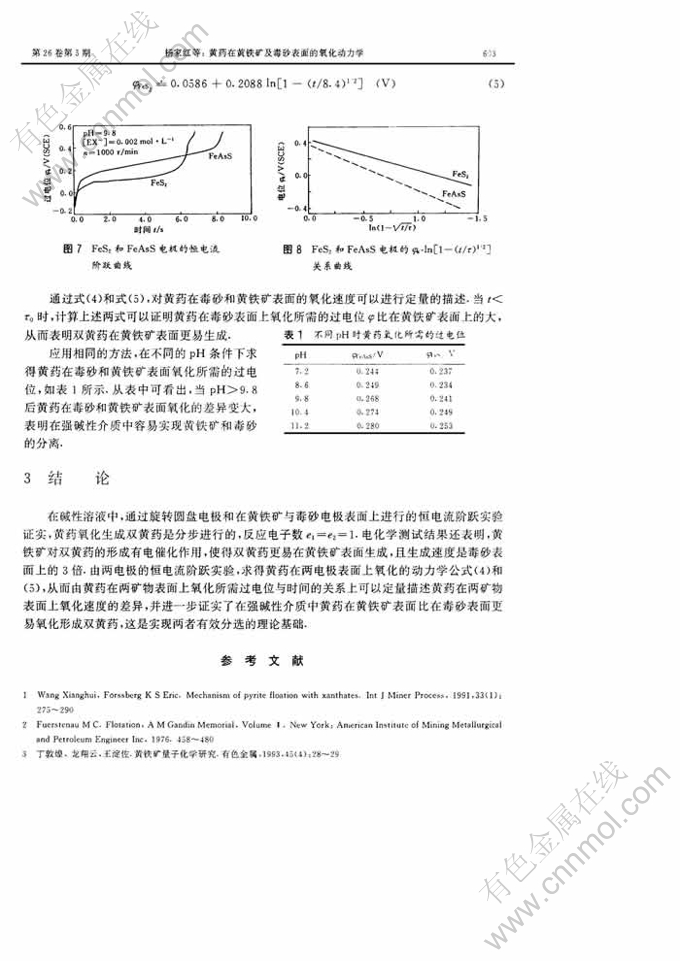
摘 要:采用旋转圆盘电极及多种电化学测试方法研究了黄药在黄铁矿和毒砂表面的氧化动力学。结果表明,在pH≥9.8的溶液中,首先发生黄药的电化学吸附然后化学吸附的一个黄原酸离子与另一个游离的黄原酸离子结合形成双黄药,反应电子数e1=e2=1.黄铁矿对黄药的氧化有电催化作用,采用恒电流阶跃法导出了黄药在这2种矿物表面氧化生成双黄药的动力学方程,可对其氧化过程进行定量描述。
Abstract: Kinetics of xanthate oxidation at the surface of pyrite and arsenopyrite was studied by using rotating-disc electrodes and various electrochemicaI test measures. Experimental re-sults demonstrate that the first step of oxidation is electrochemical adsorption of xanthate,and then one xanthogenic ion adsorbed integrates itself with another free xanthogenic ion andform dixanthogen in the solution of pH>9.8, the number of reaction electrons ei =e2=1.Pyrite has electrochemical catalyticaction on the oxidation of xanthate。 Kinetic equation ofxanthate oxidation and the producing of dixanthogen at the surfaces of two minerals was de-duced using the measure of potentiostatic step. The equation can quantitatively describe theoxidation processes.