文章编号:1004-0609(2013)04-1114-05
黄铁矿表面黄药氧化还原反应的电极过程动力学
刘三军1, 2,覃文庆2,孙 伟2,衷水平1,陈 波1
(1. 低品位难处理黄金资源综合利用国家重点实验室,上杭 364200;
2. 中南大学 资源加工与生物工程学院,长沙 410083)
摘 要:利用单矿物电位调控浮选和循环伏安扫描方法研究浮选与电位的关系,考察黄铁矿的浮选电位范围(电极电位),并绘制黄铁矿在不同环境中的φ—pH—c图。结果表明:黄铁矿与黄药作用的起始电位为0.1 V左右;电位在0.1~0.3 V之间时,反应受表面电子转移步骤控制;当电位大于0.3 V时,反应受传质过程控制。黄铁矿对黄药氧化起电催化作用。黄药在黄铁矿表面的吸附过程是整个反应的控制步骤,磨矿和搅拌均会影响捕收剂与黄铁矿的作用。
关键词:黄铁矿;黄药;电极过程;动力学;电催化
中图分类号:TD91 文献标志码:A
Electrode redox reaction kinetics of xanthate on pyrite surface
LIU San-jun1, 2, QIN Wen-qing1, 2, SUN Wei1, 2, ZHONG Shui-ping1, CHEN Bo1
(1. The State Key Laboratory of Comprehensive Utilization of Low-Grade Ores, Shanghang 364200, China;
2. School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: The relationship between the flotation and potential of single mineral was studied by potential control flotation and cyclic voltammetry method, the range of pyrite flotation potential was measured, and the φ—pH—c diagram of pyrite in different environments was drawn. The results show that when the initial potential of the reaction between pyrite and xanthate is about 0.1 V;and when the electrical potential is between 0.1 and 0.3 V, the reaction is controlled by surface electron transfer step; yet when the electrical potential is over 0.3 V, the mass transfer process is the dominate step. Pyrite can electro-catalyze the oxidation of xanthate. The adsorption of xanthate on pyrite surface is the control step of the whole reaction, and grinding and mixing may affect the reaction between pyrite and collector.
Key words: pyrite; xanthate; electrode process; kinetics; electro-catalysis
基金项目:国家自然科学基金资助项目(51274255)
收稿日期:2012-03-15;修订日期:2012-08-20
通信作者:刘三军,讲师,博士;电话:0731-88832545;E-mail: lsj_115@126.com
黄铁矿,工业上又称硫铁矿,化学成分为FeS2,是地壳中分布最广的硫化物,几乎每一个硫化矿矿床中都或多或少含有黄铁矿,因此,黄铁矿的浮选和抑制一直是硫化矿浮选研究的热点。
SALAMY和NIXON[1]和CHANDER[2]用电化学测试方法,研究了滴汞电极表面黄药吸附的机理,发现不同电极电位下,黄药的吸附形式是不同的,并由此提出了黄药与硫化矿作用的电化学机理, 此后浮选电化学的发展也由此开始。
混合电位模型确立以前,有关捕收剂在矿物表面的吸附形式一直没有定论,1968年,NEERAJ[3]通过红外光谱研究硫化矿与黄药的作用机理,并认为硫化矿表面的捕收剂膜都是由双黄药和黄原酸盐混合而成,而1969年RAO[4]的工作却表明黄铁矿、方铅矿和闪锌矿表面产物的形式是截然不同的。
1972年,ALLISON等[5-6]通过提取不同静电位下不同硫化矿与黄药作用的产物进行检测,从而得出了系统的结论。其结果如表1所列。
表1 几种硫化矿在黄药溶液中的静电位以及表面产物
Table 1 Electric potential and surface products of various sulfide minerals in xanthate solution
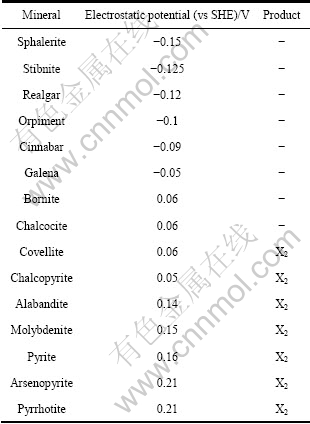
ALLISON等[5-6]认为,如果矿物的静电位高于捕收剂氧化的可逆电位,则在矿物表面生成捕收剂的双聚物,这种类型的矿物有黄铁矿、毒砂和铜蓝等,反之则生成捕收剂盐,这种类型的矿物有方铅矿和斑铜矿等。RICHARDSON等[7]用乙硫氮和黑药进行了类 似的研究,得出了相似的结论。
混合电位模型可以从热力学方面很好地解释硫化矿表面捕收剂的吸附形式,但对影响硫化矿表面反应的动力学问题却很少涉及,本文作者旨在探索影响表面电极反应速度的基本因素,以及硫化矿与浮选药剂的表面反应机制,从而为针对不同的硫化矿改变电极反应方向和控制电极反应的速度提供依据和参考,达到分选矿物的目的。
1 实验
1.1 电极
试验采用矿物电极、碳棒电极和AgCl电极,其中碳棒电极和AgCl电极为Model 273电化学测试仪自带的电极,矿物电极按以下方法制备。
挑选结晶良好的PbS和FeS2,切割后,用不同粒级的砂轮逐级打磨,制成直径1 cm的圆片,再用3~6号砂纸逐级打磨,用丙酮除油后,放入特制的电极套中以备测试。
1.2 电化学测试
电化学测试使用的硬件包括美国普林斯顿EG&G PARC公司的 Model 273电化学测量系统、阻抗测试系统、686旋转电极装置以及自行设计的机械电化学装置,使用软件包括270、388和52测试软件。
测量方法包括循环伏安扫描和Tafel曲线测试。结果分析采用仪器自带软件结合Origin6.1完成。
2 结果与讨论
2.1 黄铁矿对黄药氧化的电催化反应电子数和速度常数
图1所示为黄铁矿与黄药在不同旋转速率下的单向伏安扫描曲线。由图1可以看出,在所有旋转速率
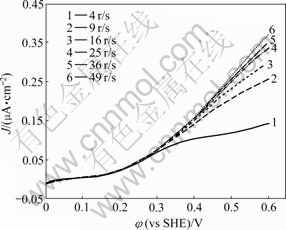
图1 不同旋转速率下黄铁矿表面黄药氧化的单向伏安曲线
Fig. 1 Unidirection CV curves of xanthate oxidation onto pyrite at different rotation rates (Scanning rate: 0.1 mV/s; KNO3: 0.1 mol/L; butyl xanthate: 2×10-4 mol/L; pH=7)
下,伏安曲线均表现为一个明显的单氧化波,由于受到一些干扰因素的影响,氧化波没有明显的平顶极限电流,在此,极限扩散电流取各氧化波中电流的最大值。由图1可知,黄铁矿与黄药作用的起始电位为0.1 V左右,当电位在0.1~0.3 V之间时,反应电流与旋转速率基本无关,根据电化学原理,此时反应受表面电子转移步骤控制,当电位大于0.3 V时电流随旋转速率的增大而增大,反应受传质过程控制。
根据Levich方程和扩散定律[8-13]进行推导,旋转圆盘电极表面可逆与不可逆反应的电流与旋转速率关系式分别如下:
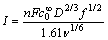 (1)
(1)
 (2)
(2)
式中: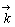 为正向反应速度常数;
为正向反应速度常数;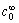 为反应物浓度(mol/L);
为反应物浓度(mol/L);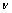 为溶液运动黏度,对水溶液来说为1×10-2 cm2/s;D为离子扩散系数(cm2/s);f为旋转速率(r/s);F为法拉第常数。
为溶液运动黏度,对水溶液来说为1×10-2 cm2/s;D为离子扩散系数(cm2/s);f为旋转速率(r/s);F为法拉第常数。
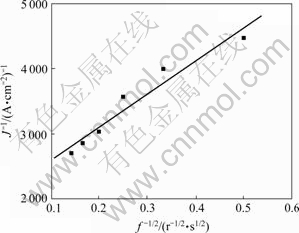
图2 黄铁矿与黄药作用 相对于
相对于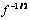 标绘
标绘
Fig. 2 Linear relationship between  and
and 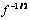 (J is current density of reaction between pyrite and xanthate, f is rotation rate)
(J is current density of reaction between pyrite and xanthate, f is rotation rate)
取图1中的极限电流密度(电流密度最大值)Jmax,将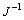 相对于旋转速率的
相对于旋转速率的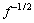 作图,结果如图2所示。由图2看出,
作图,结果如图2所示。由图2看出,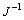 相对于
相对于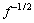 作图表现为一条截距不等于0的直线,表明黄铁矿表面黄药氧化为不可逆过程,直线斜率为3 344.41,根据式(2)可知,斜率
作图表现为一条截距不等于0的直线,表明黄铁矿表面黄药氧化为不可逆过程,直线斜率为3 344.41,根据式(2)可知,斜率
为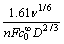 ,黄药的扩散系数为7.46×10-6 cm2/s[14],
,黄药的扩散系数为7.46×10-6 cm2/s[14],
由此可以求得,n=2.38,事实上,黄药在黄铁矿表面的反应应该是整数电子反应,因此可以认为黄药在黄铁矿表面的氧化过程为一个双电子反应过程,n=2,出现偏差的原因可能是由于在所试验的电位区间内还存在其他氧化反应(黄铁矿的氧化)。直线截距为1 575.93,由截距和n还可以求出黄药氧化的正向反应速度常数为1.219 9 cm/s。
2.2 黄铁矿对黄药氧化的电催化反应机制
研究表明[15],在黄药水溶液中,黄药分子在体相溶液中与氧气的作用是一个非常缓慢的过程,以至于对浮选结果没有太大影响,而图1表明,黄药在黄铁矿表面的氧化却很快可以发生(在黄铁矿的腐蚀电位附近即可达到可观的反应电流),这意味着黄铁矿对黄药的氧化有着某种催化作用,也就是说黄铁矿不仅为黄药氧化提供了反应位置和基底,而且加快了反应的速度,在电化学领域,这种现象被称为电催化。电催化的原理示意如图3所示。
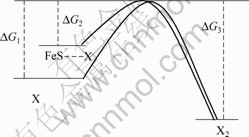
图3 黄铁矿催化黄药氧化示意图
Fig. 3 Schematic diagram showing xanthate catalyzed by pyrite
图3表明,黄药自身氧化需要较高的活化能(?G1),而在黄铁矿的存在下,黄铁矿对黄药的特性吸附使得黄药氧化的活化能降低(?G2),进而加快了氧化的速度,达到催化的效果。
黄药在硫化矿表面的氧化为双电子反应,可以表示如下:
2X-→X2,ads+2e (3)
可以假设存在以下两种反应途径:
1) FeS2+X-→FeS2…Xadsorp+e (4)
FeS2…Xads+FeS2…Xads→2FeS2…X2 (5)
2) FeS2+X-→FeS2…Xadsorp+e (6)
FeS2…X+X-→FeS2-X+e (7)
以下用A、B、C分别代表反应(6)、(5)和(7)。
当反应A为控制步骤时,反应速度可以表示如下:
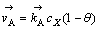 (8)
(8)
式中: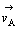 和
和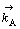 分别表示正方向A反应的反应速度和速度常数,1-θ表示未吸附黄药分子可用于反应的表面分数,cX为X-的浓度,如果式(5)或式(7)相对于式(6)总是快的,则1-θ→1,于是:
分别表示正方向A反应的反应速度和速度常数,1-θ表示未吸附黄药分子可用于反应的表面分数,cX为X-的浓度,如果式(5)或式(7)相对于式(6)总是快的,则1-θ→1,于是:
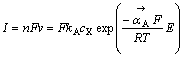 (9)
(9)
式中:F表示法拉第常数,E表示电化学反应的平衡电位。对式(9)两边取对数,可得:
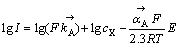 (10)
(10)
式中: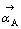 表示反应A为正方向时X-的活度,一般取0.5,
表示反应A为正方向时X-的活度,一般取0.5,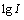 相对于
相对于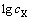 斜率为1,表明反应相对于黄药来说是一级反应,Tafel斜率为
斜率为1,表明反应相对于黄药来说是一级反应,Tafel斜率为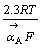 ,其值为120 mV。
,其值为120 mV。
当反应B为控制步骤时,反应速度可以表示为
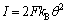 (11)
(11)
式中:kB为B反应的反应速度常数。
根据物质平衡,有:
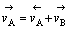 (12)
(12)
由于反应B为控制步骤,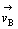 ≤
≤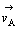 ,因此可以认为反应A处于平衡,则有:
,因此可以认为反应A处于平衡,则有:
 (13)
(13)
式中: 表示反方向反应A的速度常数。
表示反方向反应A的速度常数。
由式(13)求得:
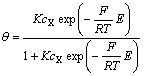 (14)
(14)
式中: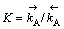 ,将式(14)代入式(11),有:
,将式(14)代入式(11),有:
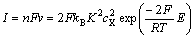 (15)
(15)
 (16)
(16)
这一机理的Tafel斜率为 ,其值为30 mV,
,其值为30 mV,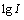 相对于
相对于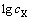 斜率为2,表明反应相对于黄药来说为2级反应。
斜率为2,表明反应相对于黄药来说为2级反应。
同理,当式(7)为控制步骤时,在低过电位下,可以求得:
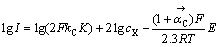 (17)
(17)
式中:kC为反应C的反应速度常数;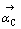 为反应C的正方向时的活度。
为反应C的正方向时的活度。
Tafel斜率为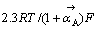 ,其值为40 mV,相对于黄药反应级数为2。3种反应机理的动力学参数 如表2所列。
,其值为40 mV,相对于黄药反应级数为2。3种反应机理的动力学参数 如表2所列。
表2 3种反应机理的动力学参数
Table 2 Kinetic parameters of three reaction mechanisms

上述分析表明,无论那种反应机理,其反应电流都与kA有关,kA的大小与矿物表面活性存在联系,表面活性越高,吸附步骤式(4)进行得越快,kA就越大。
将图1中极化电位处于0.1~0.3 V时的电流取对数(在这个电位区间内,反应受电化学步骤控制),对电位作图,结果如图4所示。
由图4求得Tafel斜率为120 mV,根据前面的分析可知,黄药在黄铁矿表面的氧化机理是按照第一种机理进行,反应式(4)为控制步骤。即说黄药在黄铁矿
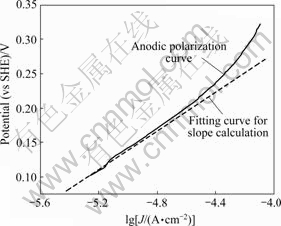
图4 黄铁矿与黄药作用的阳极极化曲线
Fig. 4 Anodic polarization curves showing reaction between pyrite and xanthate
表面的吸附过程是整个反应的控制步骤,吸附过程与粒子碰撞几率、温度、表面活性点数目密切相关,这些因素又受磨矿和搅拌的制约,表明磨矿和搅拌可以影响捕收剂与黄铁矿的作用。
3 结论
1) 黄铁矿与黄药作用的起始电位为0.1 V左右,其中在0.1~0.3 V之间,反应电流与旋转速率基本无关,根据电化学原理,此时反应受表面电子转移步骤控制,当电位大于0.3 V时电流随旋转速率的增大而增大,反应受传质过程控制。
2) 黄药在黄铁矿表面的氧化可以很快发生,表明黄铁矿对黄药的氧化电催化作用,即黄铁矿不仅为黄药氧化提供了反应位置和基底,而且加快了反应的 速度。
3) 黄药在黄铁矿表面的吸附过程是整个反应的控制步骤,吸附过程与粒子碰撞几率、温度、表面活性点数目密切相关,这些因素又受到磨矿和搅拌的制约,表明磨矿和搅拌可以影响捕收剂与黄铁矿的作用。
REFERENCES
[1] SALAMY S G, NIXON J C. Recent developments in mineral dressing[J]. Institution of Mining and Metallurgy, 1953: 503-516.
[2] CHANDER S. Oxidation/reduction effects in depression of sulfide minerals[J]. Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers, 1985, 278(8): 26-35.
[3] NEERAJ K M. Kinetic studies of sulfide mineral oxidation and xanthate adsorption[D]. Virginia Polytechnic Institute and State University, 2000: 15-20.
[4] RAO S R. Surface forces in flotation[J]. Minerals Science and Engineering, 1974, 6(1): 45-53.
[5] ALLISON S A, GOOLD L A, NICOL M J, GRANVILLE A. Determination of the products of reaction between various sulfide minerals and aqueous xanthate solution, and a correlation of the products with electrode rest potential[J]. Metallurgical Transactions, 1972, 3(5): 2613-2618.
[6] ALLISON S A, FINKELSTEIN N P. Products of reaction between galena and aqueous xanthate solutions[J]. Transactions of Institution of Mining and Metallurgy, 1969, 178(4): 181-184.
[7] RICHARDSON P E, GEBHARDT J E, RICE D A, YOON R H. Electrochemical control of sulfide flotation circuits[J]. Symposium on Emerging Computer Techniques for the Minerals Industry, 1993, 18(4): 344-346.
[8] 沈慕昭. 电化学基本原理及其应用[M]. 北京: 北京师范大学出版社, 1987.
SHEN Mu-zhao. The basic principle of electrochemistry and its application[M]. Beijing: Beijing Normal University Press, 1987.
[9] RAND D A. Oxygen reduction on sulfide minerals. Part III. Comparison of activity of various copper, iron, lead, nickle mineral electrodes[J]. Electrochemistry and Interfacial Electrochemistry, 1975, 60: 265-275.
[10] MISHRA K. Preparation and structural properties of mononuclear and binuclear ruthenium(Ⅱ) cyclopentadienyl complexes with 4-cyanopyridine and 4-cyanopyridine 1-oxide[J]. Journal of the Electrochemical Society, 1992, 139(6): 749-752.
[11] BUCKLEY A N, Woods M R. X-ray photoelectron spectroscopic and electrochemical studies of the interaction of xanthate with galena in relation to the mechanism[J]. International Journal of Mineral Processing, 1990, 28: 301-311.
[12] SALVADOR P, TAFALLA D. Reaction mechanism at n-FeS2/I interface[J]. Journal of the Electrochemical Society, 1991, 132(6): 1350-1356.
[13] 留永辉. 电化学测试技术[M]. 上海: 上海科学技术出版社, 1985.
LIU Yong-hui. The measuring technology of electrochemistry [M]. Shanghai: Shanghai Scientific and Technical Press, 1985.
[14] 覃文庆. 硫化矿物颗粒的电化学行为和电位调控浮选技术[D]. 长沙: 中南工业大学, 1998: 98-109.
QIN Wen-qing. Electrochemistry mechanics of sulfide particle and potential-control technique[D]. Changsha: Central South University of Technology, 1998: 98-109.
[15] GUY P J, TRAHAV W J. The influence of grinding and flotation environments on the batch flotation of galena[J]. International Journal of Mineral Processing, 1984, 12: 15-38.
(编辑 龙怀中)