文章编号:1004-0609(2009)02-0292-06
H2O2在TiO2可见光催化反应中的作用机理
唐建军1,范小江2,邹 原1,邓爱华1,张 伟1,周康根2
(1. 深圳职业技术学院 建筑与环境工程学院,深圳 518055;
2. 中南大学 冶金科学与工程学院,长沙 410083)
摘 要:以锐钛矿、金红石及混晶TiO2作光催化剂,研究了H2O2在TiO2可见光催化反应过程中的作用机理。结果表明,H2O2在TiO2表面活性位吸附后可拓宽TiO2的光吸收范围至可见光区;通过对反应体系的荧光光谱分析显示,金红石型TiO2在H2O2存在条件下,经可见光激发可持续稳定产生羟基自由基?OH。光催化实验表明,往反应体系中加入H2O2后,3种光催化剂均能可见光催化降解苯酚,且金红石型TiO2显示出最高的催化活性,反应120 min对苯酚的降解率达80%;在TiO2可见光催化反应过程中,由锐钛矿型TiO2经一系列复杂反应产生H2O2,生成的H2O2虽只是一中间产物,但对污染物的可见光催化降解起决定性作用。
关键词:H2O2;TiO2;可见光催化
中图分类号:O 643 文献标识码: A
Effects of H2O2 on TiO2 photocatalysis under visible light irradiation
TANG Jian-jun1, FAN Xiao-jiang2, ZOU Yuan1, DENG Ai-hua1, ZHANG Wei1, ZHOU Kang-gen2
(1. School of Construction and Environmental Engineering, Shenzhen Polytechnic, Shenzhen 518055, China;
2. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The effects of H2O2 on TiO2 visible-light photocatalytic performance were studied, using anatase, rutile and mixing crystalline TiO2 as photocatalysts. The results indicate that the absorption of H2O2 on TiO2 surface to form active species extends the light absorption range of TiO2 into the visible light region. Fluorescence spectrum analysis indicates that the ?OH radical free from rutile TiO2 surface can be constantly produced under visible light irradiation with addition of H2O2. The photocatalytic tests indicate that phenol can be visible-light photocatalytic degraded with the addition of H2O2 by any one of the three photocatalysts, and the rutile form exhibits the best photocatalytic activity, its degradation ratio can be 80% after 120 min reaction. In the process of TiO2 visible-light photocatalysis, hydrogen peroxide is generated by anatase TiO2 from a series of complex reaction, and the generated hydrogen peroxide is only an intermediate, which is responsible for the degradation of organic pollutants under visible light irradiation.
Key words: hydrogen peroxide; titanium dioxide; visible-light photocatalytic
根据TiO2光催化的反应机理[1],TiO2只能在波长小于387 nm的紫外线激发下产生电子空穴对,再转化为羟基自由基(?OH)等活性物种而对污染物起降解作用。ZHAO等[2?3]则基于染料类物质可吸收可见光而形成激发态的特点,成功地开发了TiO2可见光催化反应,并提出了区别于紫外光催化的反应机理:

上述光敏化反应机理表明,在TiO2可见光催化反应过程中应生成中间产物H2O2,WU等[4]实验结果已证实这一点。H2O2是一种优良的电子俘获剂,有研究表明[5?6],在TiO2光催化反应中,加入适量H2O2可以抑制电子(e)与空穴(h+)的复合,从而获得更高的污染物降解效能,但现有研究一般只涉及紫外光催化反应体系。
LI等[7]以P25 TiO2为光催化剂,研究了可见光催化分解H2O2的特性,表明虽然P25 TiO2及H2O2均不能吸收可见光,但在两者共存时却能降解水杨酸,并认为H2O2可见光催化分解与其在光催化剂表面的吸附情况有关。OHNO等[8]研究了H2O2在TiO2表面的活性位吸附情况,表明H2O2在不同晶型TiO2表面将形成不同的吸附结构。
为此,本文作者以锐钛矿、金红石及混晶TiO2作光催化剂,以活性艳红X?3B及苯酚作模型污染物,研究H2O2在TiO2可见光催化反应过程中的作用机理,并提出对反应机理的新见解。
1 实验
1.1 测试和表征
TiO2样品的XRD分析采用X/Pent Pro型X射线衍射仪,室温,Cu Kα源,40 kV,40 mA,X射线波长λ=1.540 6 nm,根据谢东公式[9]计算平均粒径,并估算锐钛矿相及金红石相的含量;BET比表面积分析采用3H?2000型全自动氮吸附比表面仪;光吸收性能分析采用Hitachi U?3010型紫外?可见分光光度计(带积分球配件),以标准BaSO4为参比,扫描波长范围350 nm~500 nm;羟基自由基(?OH)的测试采用Hitachi F?7000型荧光分光光度计,以量浓度为3.0×10?3 mol/L的邻苯二甲酸作探针分子,溶液pH值为11,用312 nm波长光激发,以最大发射峰处的强度间接表示?OH的生成量。
1.2 光催化实验
激发光源采用金卤灯光纤照射装置(北师大光学仪器厂),功率200 W,波长范围380~800 nm,以规格400 nm的滤光片去掉近紫外部分,实验条件标记为Vis。催化剂采用自制的锐钛矿型TiO2(煅烧温度400 ℃)、金红石型TiO2(煅烧温度900 ℃)及混晶TiO2(Degussa产品),分别记为TIO-A、TIO-R及TIO-P25,根据XRD及BET分析结果,它们的特征参数见表1。模型污染物选用活性艳红X?3B(美国Sigma公司标准品)及苯酚(AR),起始质量浓度均为15 mg/L;当加入H2O2时,控制H2O2的起始量浓度为1.1×10?3 mol/L;溶液体积为50 mL,以NaOH及HNO3溶液调整pH至3,催化剂质量浓度为1.0 g/L。
表1 TiO2光催化剂的特征参数
Table 1 Characteristics of TiO2 photocatalysts
实验过程中,首先将含模型污染物及光催化剂的悬浊液在暗态下分散30 min,再置于反应器中进行实验;间隔一定时间取样,水样先经Hitachi CR22GⅡ型高速冷冻离心机分离,再由0.45 ?m的微孔滤膜过滤后用于浓度分析。
活性艳红X?3B浓度分析采用Hitachi U?3010型紫外?可见分光光度计(λmax=535 nm),苯酚浓度分析采用Waters 1525型高效液相色谱仪,其中进样量为20 ?L,流动相V(乙腈)?V(水)=60?40,流速为1.0 mL/min,分离柱为SYMMETRY C18,4.6×250 mm,采用2487高灵敏度双通道紫外检测器;H2O2浓度分析[10]采用N, N-二乙基对苯二胺/辣根过氧化物酶法测定。
2 结果与分析
2.1 TiO2可见光催化分解H2O2
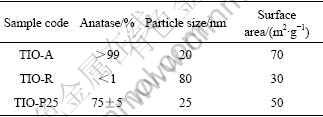
图1所示为H2O2处理对3种TiO2光催化剂的光吸收性能的影响,内附插图所示为TiO2的XRD谱。实验是将TiO2粉体用质量分数15%的H2O2浸泡处理30 min,其中固液比是1?25,再离心分离出TiO2粉体,用于光吸收性能分析。
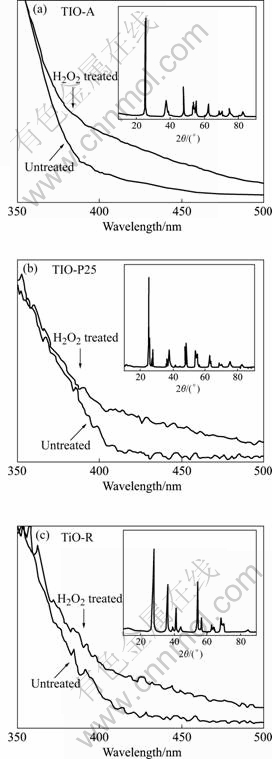
图1 H2O2处理对TiO2粉体光吸收性能的影响
Fig.1 Effects of H2O2 treatment on light absorption characteristics of TiO2 powders: (a) TIO-A; (b) TIO-P25; (c) TIO-R
由图1可知,H2O2处理后明显改善了TiO2对400 nm以上可见光的吸收效应;实验同时发现,3种TiO2光催化剂经H2O2浸泡处理后均显淡黄色,但经室内自然光照射后则逐渐褪色,并以TIO-R褪色快。
图2所示为3种TiO2光催化剂在可见光作用下分解H2O2的特性,其中H2O2的初始浓度为5.5×10?4 mol/L,溶液pH为3,图中阴影部分表示暗态吸附时间段。由图可知,3种不同晶型组成的TiO2光催化剂对H2O2均有一定的吸附性能;单纯可见光照射下H2O2的浓度变化很小,而在加入TiO2光催化剂时,则H2O2浓度显著减小,说明H2O2能被TiO2可见光催化分解。
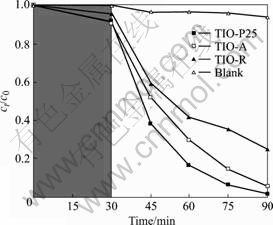
图2 TiO2可见光催化分解H2O2的实验结果
Fig.2 H2O2 decomposition results by TiO2 visible-light photocatalysis
2.2 ?OH的检测
图3所示为TIO-R在Vis/H2O2条件下,不同反应时间溶液的荧光谱,其中实验使用420 nm的滤光片以保证激发光源波长不低于420 nm,H2O2的浓度是2.2×10?3 mol/L。

图3 TIO-R在Vis/H2O2条件下不同反应时间的荧光光谱
Fig.3 Fluorescence spectra at various irradiation periods obtained from irradiated TIO-R under condition of Vis/H2O2
由图3可知,在波长312 nm光激发下,422 nm发射处有一很强的荧光峰,且随反应时间由10 min延长至40 min,422 nm发射处荧光峰明显增强;对比实验结果表明,当在Vis/O2条件下,荧光峰是非常弱的,如图4所示。
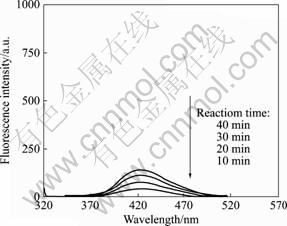
图4 TIO-R在Vis/O2条件下不同反应时间的荧光光谱
Fig.4 Fluorescence spectra at various irradiation periods obtained from irradiated TIO-R under condition of Vis/O2
羟基自由基(?OH)是一种活性物种,但邻苯二甲酸可与其作用生成荧光物质羟基邻苯二甲酸,因此,可通过荧光峰及其强度来判断反应体系是否产生强氧化
性的活性物种?OH。如图3和4所示,当以TIO-R作光催化剂,在Vis/O
2条件下,?OH的生成量微弱,而往反应体系中加入H
2O
2后,则?OH的生成量非常明显。
图5所示为3种不同晶型组成的TiO2光催化剂在Vis/H2O2条件下产生?OH的对比,其中?OH的生成量以422 nm发射处荧光峰的强度间接反映。可以看出,?OH的生成量随反应时间呈线性增大关系;在相同反应时间里,当以TIO-R作光催化剂时,?OH生成量最大,TIO-P25次之,而当以TIO-A作光催化剂时,则基本没检测到?OH的生成。
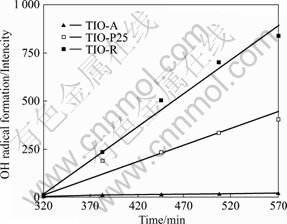
图5 3种TiO2光催化剂在Vis/H2O2条件下的?OH生成量对比
Fig.5 Comparison among three TiO2 photocatalysts by ?OH formation amount under condition of Vis/H2O2
2.3 可见光催化实验
图6所示为3种TiO2光催化剂在Vis/O2条件下降解活性艳红X?3B的实验结果。表明单纯可见光照射对活性艳红X?3B无降解作用,当以TIO-A及TIO-P25作光催化剂时,则对X?3B有明显的降解作用,反应75 min后降解率已达100%,但TIO-R的催化活性则非常微弱,反应90 min后降解率仅15%。实验同时发现,在Vis/O2条件下,3种TiO2光催化剂对苯酚均无降解效果。
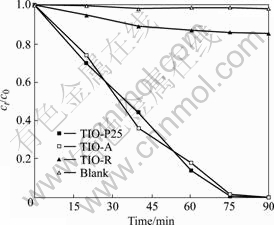
图6 Vis/O2条件下活性艳红X?3B的光催化降解实验结果
Fig.6 Photocatalytic degradation results of reactive red X?3B under condition of Vis/O2
图7所示为3种TiO2光催化剂在Vis/H2O2条件下降解活性艳红X?3B的实验结果。表明当在Vis/H2O2条件下时,不仅TIO-A及TIO-P25对活性艳红X?3B有明显的降解作用,TIO-R的催化活性也非常明显,反应90 min后降解率已达100%。

图7 Vis/H2O2条件下活性艳红X?3B的光催化降解实验 结果
Fig.7 Photocatalytic degradation results of reactive red X?3B under condition of Vis/H2O2
考虑到活性艳红X?3B和苯酚对400 nm以上可见光吸收效应的差异性,再在Vis/H2O2条件下进行苯酚的降解实验,结果如图8所示。由图可知,在Vis/H2O2条件下,3种光催化剂对苯酚均有明显的降解作用;虽然TIO-R粒径最大,比表面积最小,在Vis/O2条件下显示微弱的催化活性,在Vis/H2O2条件下降解活性艳红X?3B时催化活性较TIO-A和TIO-P25弱,但在Vis/H2O2条件下降解苯酚时却显示最高的活性,如反应120 min后,TIO-A、TIO-P25和TIO-R对苯酚的降解率分别约48%、68%和80%。
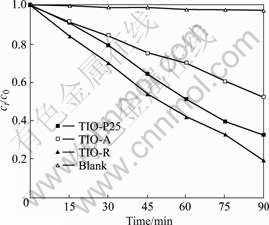
图8 Vis/H2O2条件下苯酚的光催化降解实验结果
Fig.8 Photocatalytic degradation results of phenol under condition of Vis/H2O2
3 讨论
活性艳红X?3B是一种染料类物质,其最大吸收波长处约535 nm。如反应(1)~(5)所示,吸附于TiO2表面的活性艳红X?3B分子可吸收400 nm以上的可见光形成激发态,再向TiO2的导带注入一电子,从而经一系列的复杂反应使活性艳红X?3B获得降解。由于不同晶型TiO2对O2、H2O及OH?的吸附性能差异大[11],产生H2O2的能力区别大,如GOTO等[12]认为H2O2是由锐钛矿型TiO2产生的;金红石型TiO2对O2的吸附能力差,限制了反应(3)和反应(4)的发生,不能生成中间产物H2O2,TIO-R的催化活性也因此非常微弱。
苯酚仅对波长小于300 nm的紫外线有吸收作用,不能吸收400 nm以上的可见光形成激发态,限制了反应(1)的发生,因此不能生成中间产物H2O2;TiO2光催化剂本身也不能吸收400 nm以上的可见光,导致在Vis/O2条件下3种光催化剂对苯酚均无降解作用。当在Vis/H2O2条件下,虽然苯酚并不能吸收400 nm以上的可见光而形成激发态,H2O2也不能被可见光激发分解产生?OH,但由于H2O2能在TiO2表面吸附形成复合 物[7?8],从而拓宽TiO2的光吸收范围至可见光区,导致在H2O2存在条件下苯酚的可见光催化降解。
在Vis/H2O2条件下,以TIO-R作光催化剂时?OH浓度最高,而以TIO-A做光催化剂时,本研究基本检测不到?OH的生成,但TIO-A在降解苯酚时也显示较好的催化活性。其原因是H2O2吸附于锐钛矿型TiO2光催化剂表面形成on-top或μ-peroxide结构,而吸附于金红石型TiO2表面则形成 η2-peroxide结构;η2-peroxide结构在光照下生成游离自由基?OH,而on-top或μ-peroxide结构则在光照下生成表面自由基?OH及超氧自由基O2?●[8],表面自由基?OH紧紧吸附于催化剂的表面,最终反应生成OH?或H2O,此时O2?●是起氧化作用的主要活性物种[13]。荧光分光光度法只能测试进入溶液体系,并与探针分子邻苯二甲酸反应生成荧光物质羟基邻苯二甲酸的?OH[14],因此以TIO-A作光催化剂时不能检测到?OH的生成。
一般认为,光催化剂TiO2在染料类有机物的可见光催化反应过程中只起到电子转移的作用[2?3, 15]。我们则认为,锐钛矿型TiO2经染料的敏化作用下产生H2O2,再在TiO2表面形成活性位吸附生成复合物,从而拓宽TiO2的光吸收范围至可见光区,导致污染物在可见光作用下获得降解;反应过程中生成的H2O2虽只是一瞬时产物,但对污染物的降解起决定性作用;TiO2可见光催化的反应过程如下:① 染料分子吸收可见光形成激发态;② 激发态的染料分子向TiO2的导带注入电子;③ 被注入电子的锐钛矿型TiO2与其表面吸附的O2或H2O反应生成H2O2;④ H2O2在TiO2(锐钛矿或金红石型)表面形成活性位吸附;⑤ 吸附于TiO2表面的H2O2形成特殊的吸附结构,再在400 nm以上可见光的作用下产生?OH或O2?●等活性物种。
4 结论
1) 不同晶型TiO2在H2O2存在条件下有不同的催化活性及?OH产生能力,以金红石型TiO2作光催化剂,在Vis/H2O2条件下降解苯酚时,经120 min时间对苯酚的降解率达80%。
2) 在TiO2可见光催化反应中,由锐钛矿型TiO2经一系列复杂反应生成中间产物H2O2,产生的H2O2虽只是一瞬时产物,但对污染物的降解起决定性作用。
3) 下步工作的重点是揭示H2O2在TiO2表面形成的吸附结构与其光催化活性及反应机理的内在关系。
REFERENCES
[1] 谢立进, 马俊峰, 赵忠强, 田 华, 周 军. 半导体光催化剂的研究现状及展望[J]. 硅酸盐通报, 2005, 24(6): 80?84.
XIE Li-jin, MA Jun-feng, ZHAO Zhong-qiang, TIAN Hua, ZHOU Jun. Prospect and current status in the semiconductor photocatalyst [J]. Bulletin of the Chinese Ceramic Society, 2005, 24(6): 80?84.
[2] WU T X, LIN T, ZHAO J C, HIDAKA H, SERPONE N. TiO2-assisted photodegradation of dyes. 9. photooxidation of a squarylium cyanine dye in aqueous dispersions under visible light Irradiation[J]. Environ Sci Technol, 1999, 33: 1379?1387.
[3] 刘光明, 张天永, 吴太兴, 赵进才, 王 慧. 可见光照射下染料茜素红的光催化降解机理[J]. 催化学报, 1999, 20(3): 359?361.
LIU Guang-ming, ZHANG Tian-yong, WU Tai-xing, ZHAO Jin-cai. Mechanism of photocatalytic degradation of dye polluent-alizarin red under visible light radiation[J]. Chinese Journal of Catalysis, 1999, 20(3): 359?361.
[4] WU T X, LIU G M, ZHAO J C. Evidence for H2O2 generation during the TiO2 assisted photodegradation of dyes in aqueous dispersions under visible light illumination[J]. J Phly Chem B: 1999, 103: 4862?4867.
[5] BENJAMIN J C, LINDA A L, PETER K R. Hydrogen peroxide enhanced photocatalytic oxidation of microystin-LR using titanium dioxide[J]. Applied Catalysis B: Environmental, 2000, 25(1): 59?67.
[6] 李青松, 高乃云, 马晓雁, 汪 力, 赵建夫, 林乐生. TiO2光催化降解水中内分泌干扰物17β-雌二醇[J]. 环境科学, 2007, 28(1): 120?125.
LI Qing-song, GAO Nai-yun, MA Xiao-yan, WANG Li, ZHAO Jian-fu, LIN Le-sheng. Photocatalytic fndocrine disruptor 17β-Estradiol(E2) in drinking water by nano titanium suspended system[J]. Environmental Science, 2007, 28(1): 120?125.
[7] LI X Z, CHEN C C, ZHAO J C. Mechanism of photodecomposition of H2O2 on TiO2 surfaces under visible light irradiation[J]. Langmuir, 2001, 17: 4118?4122.
[8] OHNO T, MASAKI Y, HIRAYAMA S, MATSUMURA M. TiO2 photocatalyzed epoxidation of 1-decene by H2O2 under visible light[J]. J Catal, 2001, 204: 163?168.
[9] 张立德, 牟其美. 纳米材料学[M]. 沈阳: 辽宁科学技术出版社, 1994: 95.
ZHANG Li-de, MOU Qi-mei. Nano materials technology[M]. Shenyang: Liaoning Scientific and Technology Press, 1994: 95.
[10] BADER H, STURZENEGGER V, HOIGNE J. Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of N, N-diethyl-p- Phenylenediamine (DPD)[J]. Wat Res, 1988, 22(9): 101?109.
[11] 唐玉朝, 李 薇, 胡 春, 王怡中. TiO2形态结构与光催化活性关系的研究[J]. 化工进展, 2003, 15(5): 379?384.
TANG Yu-chao, LI Wei, HU Chun, WANG Yi-zhong. Studies on morphologica structure and photoactivity of TiO2 heterogeneous photocatalysts[J]. Progress in Chemistry, 2003, 15(5): 379?384.
[12] GOTO H, HANADA Y, OHNO T, MATSUMURA M. Quantitative analysis of superoxide ion and hydrogen peroxide produced from molecular oxygen on photoirradiated TiO2 particles[J]. Catal, 2004, 225: 223?229.
[13] HIRAKAWA T, YAWATA K, NOSAKA Y. Photocatalytic reactivity for O2?● and OH? radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition[J]. J Applied Catalysis A: General, 2007, 325: 105?111.
[14] HIRAKAWA T, NOSAKA Y. Properties of O2·-and OH· formed in TiO2 aqueous suspensions by photocatalytic reaction and the influence of H2O2 and some ions[J]. Langmuir, 2002,18: 3247?3254.
[15] 王 侃, 陈英旭, 叶芬霞. 不同光源对TiO2光催化降解染料污染物的影响[J]. 太阳能学报, 2005, 26(1): 39?43.
WANG Kan, CHEN Ying-xu, YE Fen-xia. Photodegradation of dye pollutants over TiO2 particles under UV-Vis light irradiation[J]. Acta Energiae Solaris Sinica, 2005, 26(1): 39?43.
(编辑 何学锋)