
Rapid synthesis of Mo5Si3-Al2O3 nanocomposite powders by mechanochemical reduction method
CHEN Hui, MA Qin, SONG Qiu-xiang
State Key Laboratory of Gansu Advanced Non-ferrous Metal Materials, School of Materials Science and Engineering, Lanzhou University of Technology, Lanzhou 730050, China
Received 16 August 2010; accepted 24 November 2010
Abstract: Mo5Si3-20%Al2O3 (mass fraction) nanocomposite was synthesized by mechanical alloying (MA) of mixture of MoO3, Mo, Si and Al powders. The structural evolutions of powder particles during mechanical alloying were studied by X-ray diffractometry (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and differential thermal analysis (DTA). Results show that Mo5Si3-20%Al2O3 was obtained after 10 h of milling. The spontaneous reaction of powders takes place in an explosive mode. The crystallite sizes of Mo5Si3 and Al2O3 after milling for 30 h were 36.3 nm and 21.9 nm, respectively. With longer milling time, the intensities of Mo5Si3 and Al2O3 peaks decreased and became broad due to the decrease in crystallite size. Thermal analysis results and XRD analysis results show that the Mo5Si3-Al2O3 nanocomposite powders are very stable during milling (up to 30 h) and heating (up to 1 000 °C) and no transformation takes place.
Key words: Mo5Si3-Al2O3; nanocrystalline; mechanical alloying; intermetallic
1 Introduction
The Mo-Si ternary system materials have been the focus of significant research and development effort during the last ten years [1-5]. This intermetallic compounds possess an attractive combination of the high melting point, high corrosion resistance and low density [6]. Some recent studies indicate Mo5Si3 and Mo5Si3-based composites(such as Mo5Si3-Al2O3) as a promising creep resistant material with good high temperature oxidation resistance [7]. Further, Mo5Si3 and Mo5Si3-based composites have superior creep resistance as compared with monolithic MoSi2 in the temperature range of 1 100-1400 °C. Many studies on the MoSi2 have been reported. But the synthesis of Mo5Si3 and Mo5Si3-based composites has hardly attempted.
Mechanical alloying (MA) technique is one of the important methods in powder metallurgy because of its high flexibility, sample control of process parameters and ability to produce a wide spectrum of materials [8]. The easy control and ability to produce nanocrystalline make this method attractive for the powder metallurgy of nanoscale materials. It has been used to synthesize nanostructured alloys, oxide-dispersion strengthened alloys, various intermetallics compounds, etc [9-11]. The mechanical alloying method has already been efficiently applied to synthesis of pure MoSi2 and MoSi2-based composites with different second phases such as, ZrO2, SiC, Si3N4, TiB2 and Al2O3 [12-15].
In the present work, we used MoO3, Mo and Si powders as materials and Al as reducing agents for the synthesis of Mo5Si3-20%Al2O3 nanostructured composite powders by mechanical alloying. MoO3, Mo and Si powders were milled and then the morphology, particle size and phase constitution of powders were investigated with SEM and XRD methods, respectively. The solid state reactions occurring during the MA process as well as during subsequent annealing process were investigated.
2 Experimental
MoO3(99.5% purity and particle size 3-5 μm), Mo(99.5% purity and particle size <8 μm), Al(99.5% purity and particle size 10-35 μm), Si(99.9% purity and particle size <3 μm) elemental powders were used as starting materials. Mechanical alloying was performed in a planetary ball milling at a rotation speed of 200 r/min and nominally at room temperature under air atmosphere. The vials are stainless steel containers with a capacity of 120 mL. The mass ratio of ball to powder (BPR) was 15:1 and diameter of ball was 8 mm in the MA process. A total of 20 g powder without process control agent was used in all runs. Samples for analysis were removed by interrupting the ball mill at various intervals. The surface temperature of the vial was measured with a digital thermometer at fixed intervals. When an obvious and abrupt temperature increase was observed, as reported by ATZMON [16], the time was considered to be the critical ball milling time of the self-propagating reaction during mechanical alloying.
Phase transformation and crystallite size evolution during milling were determined by X-ray diffractometer (D/Dmax-2400, Rigaku) with Cu Kα radiation (λ= 0.154 18 nm). Crystallite size was evaluated using the Williamson-Hall method. Scanning electron microscope (SEM, JSM-6700F, JEOL) and transmission electron microscope (TEM, JEM-2010) were employed to observe particulate morphology and electron diffraction pattern. The thermal properties were examined using differential thermal analyzer (DTA, STA/449C, NETZSCH). The procedure was performed under a flow of nitrogen atmosphere and the heating rate was 10 °C/min.
3 Results and discussion
3.1 Mechanical alloying
As-received MoO3, Mo, Al and Si powders were mixed on stoichiometric ratio (Mo5Si3-20%Al2O3) according to following reaction:
MoO3+2Al→Mo+Al2O3 (1)
Mo+Si→Mo3Si+Mo5Si3 (2)
or
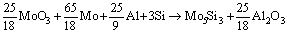 (3)
(3)
Figure 1 shows the XRD patterns of powder mixture after different milling time. After 0.5 h of milling, the powders are mixture of the starting reactant materials, characterized by sharp Bragg peaks of MoO3, Mo, Al and Si. Increasing the MA time (0.5-5 h) leads to a decrease in peak intensity and broadening of the Bragg reflection. This phenomenon indicates the refinement of crystalline size of the powders and an increase in atomic level strains. After 10 h of MA the XRD peaks of the raw materials virtually disappeared due to the reaction of MoO3, Mo, Al and Si to give Mo5Si3 and Al2O3 phase upon reaction (3). This suggests that the reaction of MoO3, Mo, Al and Si takes place in an explosive mode, analogous to self-propagating high temperature synthesis reaction. Typically, ?H/C>2 000 K is required for the propagation of a self-sustaining reaction [17]. This value for reaction (1) is 4 285 K [18]. Consequently, the propagating of the reaction takes place during milling of MoO3, Mo, Al and Si powder mixture. The change of vial temperature during milling of powder mixture is shown in Fig. 2. It is clear that, after 7 h of milling, the temperature of vial shows a sudden increase, suggesting that the exothermic combustion occurs among MoO3, Mo, Al and Si powders. This can be promoted by the dynamically maintained high reaction interface areas, as well as the short-circuit diffusion path provided by the large number of defects such as dislocations and grain boundaries induced during ball milling.
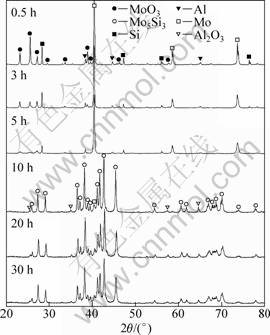
Fig. 1 XRD patterns of powder mixtures after different milling time

Fig. 2 Variation of vial temperature versus time during milling of powder mixture
The apparent sizes of MoO3, Mo, Al, Si, Al2O3 and Mo5Si3 crystallites were obtained from XRD profile analysis by the Williamson-Hall method and are listed in Table 1. The figures show the crystallite size of the starting powders as a function of the mechanical- activation time. There is a remarkable decrease in the crystallite size during the initial 5 h of mechanical activation. Further increase in the milling time led to a state reaction rapidly. In the final stage of milling, Al2O3 and Mo5Si3 had a crystallite size of about 21.9 nm and 36.3 nm, respectively.
Figure 3 shows SEM images of the milled powders. It can be seen from the morphologies that the powder particles are uneven and irregular after 0.5 h of MA (Fig. 3(a)). With increasing milling time to 5 h the size and irregularity of powders decrease but still there is a distribution of agglomerate sizes (Fig. 3(b)). High magnification image reveals that some small particles attached tightly to the rough surface of the relatively large particles. After 30 h of milling powder particles become spherical in shape. In this stage the particles and agglomerates are very small (Fig. 3(d)). The average size of powder particles after 10 h is 1-3 μm.
Figures 4(a) and (b) present a bright-field TEM image and selected area diffraction pattern (SADP) of powder milled for 10 h, respectively. It is obvious that the particle (about 200 nm in size) is composed of some smaller grains. The crystallite size of obtained Mo5Si3-20%Al2O3 after milling for 30 h is very fine. The measured crystallite sizes of Mo5Si3 and Al2O3 according to XRD pattern of 30 h-sample were 36.3 nm and 21.9 nm, respectively. Figure 4(b) shows the corresponding selected area diffraction pattern of the particle. The selected area diffraction rings clearly demonstrate that the particles are nanocrystalline. The EDS pattern of particles (Fig. 4(c)) suggest that the particles consist of the fine Mo5Si3 and Al2O3 grains.
Table 1 Grain size evolution of powders during milling

Fig. 3 SEM images of powders milled for different time: (a) 0.5 h; (b) 5 h; (c) 10 h; (d) 30 h

Fig. 4 Bright-field TEM image (a), selected area diffraction pattern (b) and EDS pattern (c) of powder mixtures milled for 10 h
3.2 Thermal analysis
In order to study the reaction mechanism of powders and the effect of milling on this reaction, thermal analysis of the as-blended and ball milled powders were carried out. The DSC patterns of MoO3, Mo and Si powders milled with Al are presented in Fig. 5. All samples were heated to 1 000 °C in Ar atmosphere at a heating rate of 10 °C/min and cooled to room temperature.
As observed in Fig. 5(a), three peaks appear in DSC curve. A sharp endothermic peak appears at about 659 °C (point A), due to the melting of Al in the starting material. Then this melt reacted with MoO3 powder and an exothermic peak (point B) appears at about 778 °C. The XRD analysis results show that the sample after 30 h of MA mainly consists of Mo5Si3 and Al2O3. Therefore, the exothermic peak (point C) should be attributed to the reaction of Mo and Si to give Mo5Si3. After 30 h of MA there is no significant peak in the alloy powders, confirming that the reaction has been completed after 30 h (Fig. 5(b)). Therefore, with respect to the thermal analysis results and XRD analysis results mentioned in the previous section, the process has the appearance of just blending Mo5Si3 and Al2O3 powders with increasing the MA time.
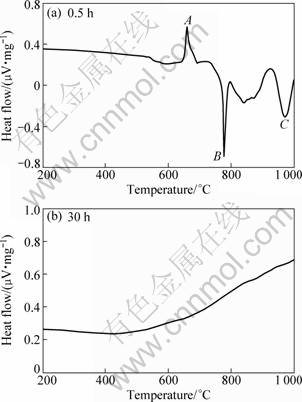
Fig. 5 DSC patterns of mixture powders milled for different time: (a) 0.5 h; (b) 30 h
3.3 Heat treatment
Thermal stability of Mo5Si3-Al2O3 composite structure was investigated by annealing of milled powders at 1 000 °C for 1 h. Figure 6 shows the XRD patterns of powder milled for 30 h and then annealed at 1 000 °C for 1 h. The powder includes Mo5Si3 and Al2O3 and no reaction takes place after being annealed at 1 000 °C. The width values of XRD peaks of Mo5Si3 and Al2O3 decrease and their intensities increase due to the reduction of lattice strain as well as grain growth. The grain size of Mo5Si3 increases from 36.3 nm to 65 nm and the lattice strain decreases from 2.56% to 0.12%. Thermal analysis results and XRD analysis results show that nanocomposite powder is stable during heating from room temperature to 1 000 °C.
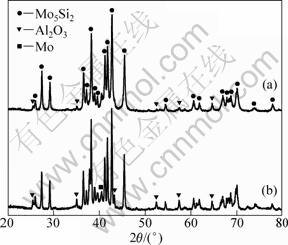
Fig. 6 XRD patterns of powder mixtures milled for 30 h (a) and then annealed at 1 000 °C (b)
4 Conclusions
1) Mo5Si3-20%Al2O3 nanocomposite was successfully synthesized by ball milling of mixture of MoO3, Mo, Al and Si powders. It is found that Mo5Si3- Al2O3 nanocomposite formed with high rate.
2) Reaction mechanism is a rapid self-sustaining combustion reaction and reaction completes in less than 10 h. This synthesis method does not require heating and starting materials easy to obtain; thus, it may be used for large scale production of this composite.
3) In the final stage of milling, Al2O3 and Mo5Si3 have a crystallite size of about 21.9 nm and 36.3 nm, respectively.
4) Thermal analysis results and XRD analysis results show that the Mo5Si3-Al2O3 nanocomposite powder is very stable during milling (up to 30 h) and heating (up to 1 000 °C) and no transformation takes place.
References
[1] WANG Fang, SHAN Ai-dang, DONG Xian-ping, WU Jian-sheng. Oxidation behavior of multiphase Mo5SiB2(T2)-based alloys at high temperatures [J]. Transactions of Nonferrous Metals Society of China, 2007, 17: 1242-1247.
[2] LIU L, CUI K. Mechanical alloying of refractory metal-silicon systems [J]. Journal of Materials Processing Technology, 2003, 138(1-3): 394-398.
[3] YEN B K, IZAWA T, KIHARA J. Synthesis and formation mechanisms of molybdenum silicides by mechanical alloying [J]. Materials Science and Engineering A, 1996, 220(1-2): 8-14.
[4] KANG Peng-chao, YIN Zhong-da, OKOYE C. Effect of milling time on phase transition and grain growth during the annealing process of MA powders [J]. Materials Science and Engineering A, 2005, 395(1-2): 167-172.
[5] YAMAUCHI A, YOSHIMI K, KUROKAWA K, HANADA S. Synthesis of Mo-Si-B in situ composites by mechanical alloying [J]. Journal of Alloys and Compounds, 2007, 434-435: 420-423.
[6] FUJIWARA H, UEDA Y. Thermodynamic properties of molybdenum silicides by molten electrolyte EMF measurements [J]. Journal of Alloys and Compounds, 2007, 441(1-2): 168-173.
[7] MISRA A, PETROVIC J J, MITCHELL T E. Microstructures and mechanical properties of a Mo3Si-Mo5Si3 composite [J]. Scripta Materialia, 1998, 40(2): 191-196.
[8] KRAKHMALEV P V. Preparation of Mo(Si,Al)2-ZrO2 nanocomposite powders by mechanical alloying [J]. International Journal of Refractory Metals and Hard Materials, 2004, 22(5): 205-209.
[9] KOCH C C. Intermetallic matrix composites prepared by mechanical alloying [J]. Materials Science and Engineering A, 1998, 244(1): 39-48.
[10] SURYANARAYANA C. Mechanical alloying and milling[J]. Progress in Materials Science, 2001, 46(1-2): 1-184.
[11] ZAKERI M, YAZDANI-RAD R, ENAYATI M H, RAHIMIPOOR M R. Synthesis of MoSi2-Al2O3 nanocomposite by mechanical alloying [J]. Materials Science and Engineering A, 2006, 430: 185-188.
[12] ZAKERI M, YAZDANI-RAD R, ENAYATI M H, RAHIMIPOUR M R, MOBASHERPOUR I. Mechanochemical reduction of MoO3/SiO2 powder mixtures by Al and carbon for the synthesis of nanocrystalline MoSi2 [J]. Journal of Alloys and Compounds, 2007, 430: 170-174.
[13] PATEL M, SUBRAMANYUAM J, BHANU PRASAD V V. Synthesis and mechanical properties of nanocrystalline MoSi2-SiC composite [J]. Scripta Materialia, 2008, 58: 211-214.
[14] YEH C L, CHEN W H. Combustion synthesis of MoSi2 and MoSi2-Mo5Si3 composites [J]. Journal of Alloys and Compounds, 2007, 438: 165-170.
[15] GUO Zhi-quan, BLUGAN G, GRAULE T, REECE M, KUEBLER J. The effect of different sintering additives on the electrical and oxidation properties of Si3N4-MoSi2 composites [J]. Journal of the European Ceramic Society, 2007, 27: 2153-2161.
[16] ATZMON M. In situ thermal observation of explosive compound formation reaction during mechanical alloying [J]. Physical Review Letters, 1990, 64(4): 487-490.
[17] MUNIR Z A, ANSELMI-TAMBURINI U. Self-propagating exothermic reactions: The synthesis of high- temperature materials by combustion [J]. Materials Science Reports, 1989, 3(7-8): 277-365.
[18] HU Qiao-dan, LUO Peng, QIAO Da, YAN You-wei. Self-propagation high-temperature synthesis and casting of Cu-MoSi2 composite [J]. Journal of Alloys and Compounds, 2008, 464(2): 157-161.
机械化学还原法快速制备Mo5Si3-Al2O3纳米复合粉体
陈 辉,马 勤,宋秋香
兰州理工大学 甘肃省有色金属新材料省部共建国家重点实验室,兰州 730050
摘 要:以MoO3粉、Mo粉、Si粉及Al粉为原料,采用机械合金化法合成了纳米Mo5Si3-20%Al2O3(质量分数)复合粉体。采用XRD、SEM、TEM和DTA等对复合粉体在球磨过程中结构变化进行了研究。结果表明:球磨10 h后合成的Mo5Si3-20%Al2O3复合粉体,反应以爆炸模式进行。球磨30 h后,Mo5Si3和Al2O3的晶粒尺寸分别为36.3 nm和21.9 nm。随着球磨时间的延长,Mo5Si3和Al2O3的晶粒尺寸变小,衍射峰宽化程度降低。DTA和XRD分析结果表明,复合粉体具有好的热稳定性,球磨30 h后再在1 000 °C退火1 h后复合粉体没有发生物相转变。
关键词:Mo5Si3-Al2O3;纳米晶;机械合金化;金属间化合物
(Edited by YANG Hua)
Foundation item: Project(3ZS061-A25-038) supported by the Natural Science Foundation of Gansu Province, China
Corresponding author: CHEN Hui; Tel: +86-931-2806859; E-mail: chenhui002@qq.com
DOI: 10.1016/S1003-6326(11)60896-0