Formation of black nickel in leaching solution containing ammonia and chloride
ZHENG Guo-qu(郑国渠), ZHENG Li-feng(郑利峰), CAO Hua-zhen(曹华珍)
(College of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou 310014, China)
Abstract: The black nickel formation process in leaching solution containing ammonia and chloride was investigated in terms of cyclic voltammetric and galvanostatic reduction techniques. The structure of black nickel was examined by means of X-ray diffraction technique. The results show that in the scanning region, two oxidization current peaks are observed during the positive sweep, one of which is attributed to a valence state transformation of Ni(OH)2 to high valence nickel compound(black nickel), and the other is caused by nitrogen evolution. During the formation process of black nickel, γ-NiOOH probably tends to self-discharge with water to form α-Ni(OH)2. As a result, it is observed that Ipa/Ipc(Ipa—anodic peak current; Ipc—cathodic peak current) maintains at a constant with the scanning rate increasing. Two reduction current peaks in cyclic voltammogram and two potential plateaus in galvanostatic reduction curve for black nickel are ascribed to the reduction of various oxidization states of nickel oxide. The potential plateaus at about 0.75V reach the maximum in galvanostatic reduction curves. Black nickel presents amorphous structure.
Key words: ammonia complex; black nickel; NiOOH; cyclic voltammetry CLC number: TF813
Document code: A
1 INTRODUCTION
The cathodic process of metal electrowinning in ammonia complex system[1-4] has been investigated extensively, while the anodic process has not yet been done. In our previous research, it was identified that the anodic gas species were mainly composed of nitrogen[5] by vapor phase chromatograph. It was also demonstrated by means of linear sweep voltammetric technique that the main reaction on Ti-based IrO2 anodes was nitrogen evolution[6], which was ascribed to the electrochemical oxidization of ammonia at electrode potential lower than 1.1V or so. In addition, the anodic solid product, formed on the anode surface in the process of electrodepositon, was high valence nickel compound[7] in terms of X-ray diffractometry and chemical analysis. Moreover, the amount of solid product decreased with an increasing of ammonium chloride concentration by cyclic voltammetry analysis. Generally black nickel was obtained by electrolytic oxidization in alkaline solution to be applied in the separation of cobalt and nickel[8, 9]. In the field of electrochemical power source, NiOOH was also prepared by electrolytic oxidation in alkaline solution[10-13]. On the other hand, the electrochromic properties of the electrochemically deposited NiOxHy thin films on glass substrates coated with SnO2 thin film were investigated in mixed solution containing NiSO4 and NH4OH[14]. The redox of Ni(OH)2 was studied initially by means of cyclic voltammetric technique. However, the formation process of anodic solid product attracted little attention in the process of metal electrowinning in ammonia complex system.
In this papers, in order to provide theoretical base for nickel electrodeposition in leaching solution containing ammonia and chloride and the formation process of black nickel, cyclic voltammetric and galvanostatic reduction techniques, and X-ray diffraction technique were used.
2 EXPERIMENTAL
2.1 Electrochemical measurement
All the solutions were prepared using secondary distilled water and metal salts of analytical grade. The electrolytes were stored in a closed vessel to prevent from losses of ammonia vapor.
All cyclic voltammetric studies were carried out in a three-electrode system with saturated calomel electrode(SCE) using CHI650 electrochemical working station. A luggin probe was used to minimize resistance effects. The counter electrode was platinum, and the working electrode was RuO2/Ti electrode. The RuO2/Ti electrode was oxidized at a constant voltage of 0.90V, 0.95V, 1.00V, 1.05V, 1.10V for 5min in leaching solution containing ammonia and chloride, and then the curves of galvanostatic reduction of black nickel were measured by galvanostatic reduction technique using CHI660 electrochemical working station. The working electrode surface was soaked repeatedly in Na2SO3 and HCl solution respectively for 1min before each run, and its oil was removed using anhydrous ethanol. After that, it was rinsed in secondary distilled water. In this paper all potentials were referred to the saturated calomel electrode(SCE).
2.2 X-ray diffraction species analysis
A RuO2/Ti electrode with 6.5cm×5.5cm in dimension was choosed as anode and the stainless steel with the same size as cathode. The oxidization at a constant voltage was performed for several hours. The black solid product formed on anode surface was scraped using a doctor blade, and it was examined by X-ray diffractometry(XRD) with CuKα radiation.
3 RESULTS AND DISCUSSION
3.1 Redox process of black nickel
Fig.1 shows cyclic voltammograms for RuO2/Ti electrode in leaching solution containing ammonia and chloride, which are measured at a scanning rate of 5mV/s. During anodic potential sweep, at about 0.89V, an anodic current peak A1 appears, and at about 0.93V, peak A2 appears, which corresponds to nitrogen evolution. One cathodic current peak C1 at about 0.75V is observed on the reverse sweep. The A1/C1 redox peaks are assigned to the Ni(Ⅱ)/Ni(Ⅲ) transition[15-17]. Equilibrium reaction (1) exists in leaching solution containing ammonia and chloride[18]:
Ni(NH3)2+n→Ni2++nNH3(1)
When the anodic potential is more than 0.8V, Ni(OH)2 is precipitated due to the increase in pH value at the electrode surface[19]:
Ni2++2OH- Ni(OH)2(2)
Ni(OH)2(2)
This Ni(OH)2 is oxidized to NiOOH at the electrode surface:
Ni(OH)2+OH- NiOOH+H2O+e(3)
NiOOH+H2O+e(3)
The redox reaction of A1/C1 may be represented by Eq.(4):
NiOOH+H++e Ni(OH)2(4)
Ni(OH)2(4)
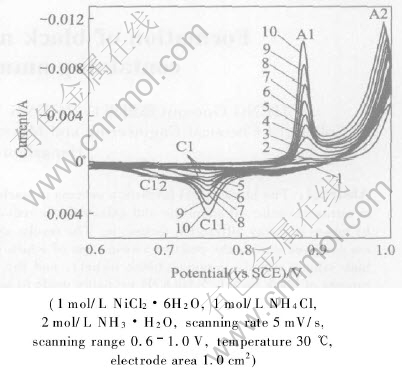
Fig.1 Cyclic voltammogram of anodic reaction in leaching solution containing ammonia and chloride
In the following anodic potential sweep, Ni(OH)2 species precipitated on electrode surface is oxidized continuously. As a result, the anodic current peak A1 and cathodic current peak C1 increase with the repeated scanning. Noteworthily, as shown in Fig.1, during the anodic potential sweep in the fifth cycle, anodic current peak A1 becomes sharp and this peak current increases greatly. On the reverse sweep, cathodic current peak at about 0.75V is obviously divided into two current peak C11 and C12. The C11 peak current increases greatly in the next sweep cycle, while the change of C12 peak current is not obvious.
As has been reported in Refs.[15-17, 20-22], nickel hydroxide may exist at least in two different crystallographic forms designated as α- Ni(OH)2 and β-Ni(OH)2, which are hydrous and anhydrous, respectively. In addition, the oxidization of nickel hydroxide gives two other varieties of oxyhydroxides β and γ, which can explain the existence of two divided cathodic reduction peaks during the negative sweep. The α-Ni(OH)2 is known to be unstable in alkaline medium and converts slowly and irreversibly to the β-Ni(OH)2, while on prolonged charging or at high potential, β-NiOOH converts to the γ-oxyhydroxide form.
Fig.2 shows cyclic voltammograms for RuO2/Ti electrode in solution in the absence of nickel ion(1mol/L NaCl, 1mol/L NH4Cl, 2mol/L NH3·H2O). It can be seen that there is no other current peak except the current peak A2 attributed to nitrogen evolution reaction at 0.90V. The disappearance of the current peak A1 and C1 (C11, C12) in the voltammogram recorded in the absence of nickel ion indicates that these peaks are associated with nickel ion.

Fig.2 Cyclic voltammogram obtained in solution in absence of nickel ion
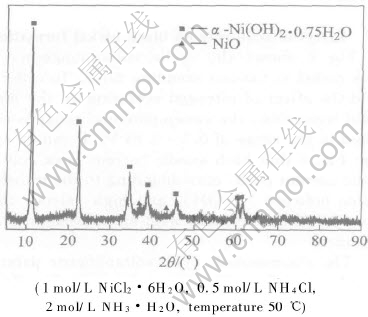
Fig.3 XRD pattern of black nickel obtained by oxidization at constant voltage
3.2 Structure of black nickel
Fig.3 shows the pattern of black nickel prepared by oxidization at constant voltage. It can be seen that the diffraction peaks obtained is in conformity with standard diffraction peaks of α-Ni(OH)2·0.75H2O and NiO, and the diffraction peaks of high valence nickel compound are not observed. The absence of diffraction peaks corresponding to high nickel compound indicates that it has an amorphous structure. As has been reported[23], in the XRD patterns of the nickel oxide prepared by cyclic voltammetry and potentiostatic techniques from the aqueous solution containing NiCl2, there were only diffraction peaks of graphite, and the diffraction peaks of high valence compound were not observed. It was suggested that the nickel oxides exhibit an amorphous structure. In the present research, α-Ni(OH)2·0.75H2O probably results from self-discharge of γ-NiOOH with H2O or in air, and NiO may be produced through dehydration of Ni(OH)2. When a small portion of black nickel are taken into diluted hydrochloric acid solution, a great deal of air bubble separate out and concomitant of pungent smell occurs. Subsequently colorless diluted hydrochloric acid solution turns into green one. This phenomenon indicates that a great deal of amorphous structure and strong oxidizing high valence nickel compound such as NiOOH still exist in black solid product obtained by oxidization at constant voltage although part of the products have been reduced in solution or in air. Therefore, Cl- in diluted hydrochloric acid solution can be oxidized into Cl2, and high valence nickel ion can be reduced into Ni2+, which makes the solution turn green.
3.3 Effect of sweep potential region on cyclic voltammograms for black nickel formation
Fig.4 shows the cyclic voltammograms for black nickel formation at various potential regions. It can be seen that anodic current peak A1 greatly increases with sweep potential region from 0.6-0.94V to 0.6-1.12V, so does the cathodic current peak C11, and anodic peak potentials shift toward more positive potentials. Noteworthily, cathodic current peak C12 at about 0.65V undergoes complex change, such as from nonexistence to appearance to disappearance. Anodic peak potentials shifting toward more positive potential is probably due to an increase in sweep potential region, which results in an increase in gas evolution, and the black nickel precipitated on electrode surface presents porous structure, which plays an important role on redox of nickel oxide. As electrolyte diffusion velocity decreases under the effect of porous structure of nickel oxide, the polarization of black nickel formation reaction increases greatly. As a result, anodic peak potentials shift toward more
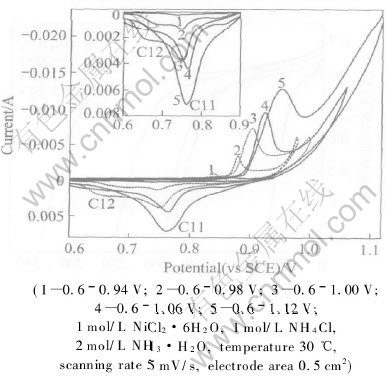
Fig.4 Seventh cyclic voltammogram for black nickel formation in various sweep potential regions
positive potentials. It can be observed obviously that peak currents of A1 and C11 increase remarkably as sweep potential region shifts to more positive potential. Probably due to an increasing gas evolution with an increase in sweep potential region, the active surface of nickel oxides redox increases. Ni(OH)2 is likely to be oxidized fully during the anodic potential sweep, and NiOOH may be reduced completely during the cathodic potential sweep. On the other hand, owing to sweep potential region shifting to more positive potential, it is in favor of γ-NiOOH formation during the andic potential sweep, and a small portion of γ-NiOOH self-discharge to form Ni(OH)2 in leaching solution containing ammonia and chloride, which contributes to increment of reactant and results in oxidization peak current increasing of black nickel.
3.4 Effect of oxidization potential on curves of galvanostatic reduction for black nickel
Fig.5 shows the curves of galvanostatic reduction for black nickel obtained by oxidization at various potentials. Different characteristics are observed for various galvanostatic reduction curves of black nickel. All galvanostatic reduction curves for black nickel have a potential plateau at about 0.75V, which is corresponded to cathodic current peaks at 0.75V in cyclic voltammograms. However, the length of potential plateaus is really different. Firstly, potential plateau extends with an increase in oxidization potential, and it reaches the maximum at 1.0V. Then potential plateau shrinks as oxidization potential gets more positive. The maximum of potential plateau indicates that the amount of black nickel reaches a maximum. It is probablydue to the lower oxidization current at lower ano-
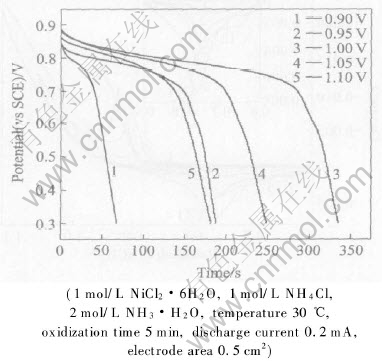
Fig.5 Curves of galvanostatic reduction for black nickel obtained by oxidization at various potentials
dic potential, which is not enough to get a great deal of black nickel, and oxidization current increases with an increase in anodic potential, however, an increasing nitrogen evolution gets dominant as anodic potential goes further.
Besides the potential plateaus at about 0.75V in galvanostatic reduction curves for black nickel by oxidization at 0.95V, 1.0V and 1.05V, weak potential plateau at 0.65V is observed, which corresponds to cathodic current peak at 0.65V in cyclic voltammograms. The weak potential plateau undergoes complex change, which is from non-existence to appearance to disappearance. It was suggested by HU and CHENG[23] that the galvanostatic reduction curves for nickel oxide are significantly influenced by the thickness and/or structure, which is a function of the electrochemical oxidization methods and oxidization potentials. Therefore, it is proposed that the potential plateaus are ascribed to the reduction of various nickel oxides.
3.5 Effect of scan rate on black nickel formation
Fig.6 shows the cyclic voltammograms for black nickel at various scanning rates. In order to avoid the effect of nitrogen evolution on the black nickel formation, the sweep potential range is controlled in the range of 0.5-0.94V. It can be seen from Fig.6 that both anodic current peak and cathodic current peak, corresponding to the transformation between Ni(OH)2 and high valence compound NiOOH, decrease with an increase in scanning rate.
The characteristic cylic voltammetric parameters obtained form Fig.6 are shown in Fig.7 and Fig.8 as a function of scanning rate. As seen from Fig.7, as the voltage scan rate increases, the
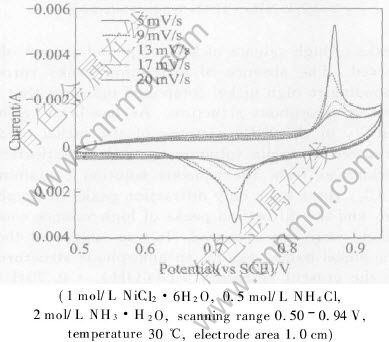
Fig.6 Fifth cyclic voltammograms at various scanning rate
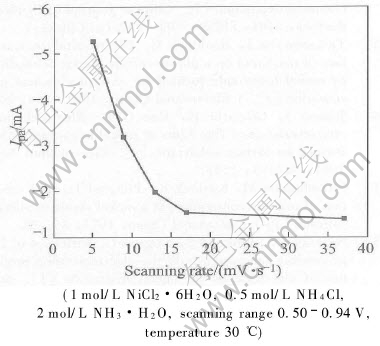
Fig.7 Change of anodic peak current with scanning rate
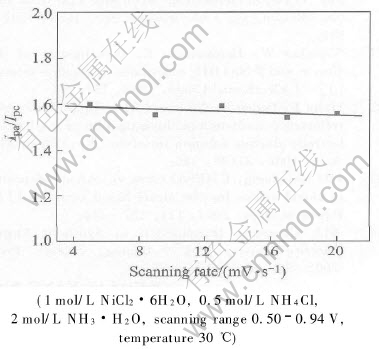
Fig.8 Plot of Ipa/Ipc vs scanning rate
anodic peak current I decreases. However, as the scanning rate increases above 17mV/s, the anodic peak current approximately tends to be a constant. It indicates that reactant Ni(OH)2 provided according to reaction (1) and reaction (2) is incapable of supplying abundant Ni(OH)2 for oxidization. As mentioned above, a valence state transformation of Ni(OH)2 to high valence compound NiOOH is involved in chemical-electrochemical reaction(CE)[24]. Owing to no existence of pre-inhibition in NiOOH reduction to Ni(OH)2 during the negative sweep, the cathodic peak current Ipc is not affected remarkably, and it can be proposed that Ipa/Ipc should be lower as the scanning rate increases. However, the change tendency of Ipa/Ipc is not obvious as expected. It is probably due to Ni(OH)2 transformation to a kind of unstable, high valence compound γ-NiOOH during the oxidization process, and γ-NiOOH tends to self-discharge with water to form α-Ni(OH)2 simultaneously, just as the following reaction:
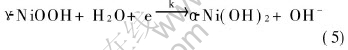
Therefore, a catalytic reaction is proposed based on the combination of reaction(3) and reaction(5). A small portion of γ-NiOOH transformation into Ni(OH)2 during positive sweep results in decrease of reactant concentration during negative sweep, concomitant decrease of cathodic reduction current and increase of anodic oxidization current. As a result, it is observed that Ipa/Ipc maintains at a constant.
4 CONCLUSIONS
1) In the scanning region, two oxidization current peaks are observed during the positive sweep. One is attributed to a valence state transformation of Ni(OH)2 to high valence nickel compound, and the other is caused by nitrogen evolution. The oxidization current peak increases with scanning rate increasing, so does the corresponding reduction current peak. Black nickel presents amorphous structure.
2) Two reduction current peaks in cyclic voltammogram and two potential plateaus in galvanostatic reduction curve for black nickel are ascribed to the reduction of various oxidization states of nickel oxide. The potential plateaus at about 0.75V reach the maximum in galvanostatic reduction curves.
3) During the formation process of black nickel, γ-NiOOH probably tends to self-discharge with water to form α-Ni(OH)2 in the oxidization process. As as result, it is observed that Ipa/Ipc maintains at a constant as the scanning rate increases.
REFERENCES
[1]Darchen A, Drissi-Daoudi R. Electrochemical investigation of copper etching by Cu(NH3)4Cl2 in ammoniacal solutions [J]. J Appl Electrochem, 1997, 27: 448-454 .
[2]TAND Mo-tang, YANG Sheng-hai. Electrowinning zinc in the system of Zn(Ⅱ)-NH3-NH4Cl-H2O and mechanism of anodic reaction [J]. Journal of South Center University of Technology, 1999, 30(2): 153-156.(in Chinese)
[3]Gonzalez I, Scharifker B R. Silver electrocrystallization from a nonpolluting aqueous leaching solution containing ammonia and chloride [J]. J Appl Electrochem, 1996, 26: 451-457.
[4]Trejo G, Gil A F, Gonzalez I. Electrodeposition of gold in ammoniacal medium: influence of substrate and temperature [J]. J Appl Electrochem, 1996, 26: 1287-1294.
[5]ZHENG Guo-qu, ZHENG Li-feng, CAO Hua-zhen, et al. Nickel electrodeposition from leaching solution containing ammonia and chloride [J]. Trans Nonferrous Met Soc China, 2003, 13(1): 217-220.
[6]ZHENG Guo-qu, ZHENG Li-feng, CAO Hua-zhen, et al, Investigation on nitrogen evolution of Ti based IrO2 anodes in leaching solution containing ammonia and chloride [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(1): 84-88.( in Chinese )
[7]ZHENG Guo-qu, GAO Zhi-feng, CAO Hua-zhen, et al. Anodic process of nickel electrowinning from leaching solution containing ammonia and chloride [J]. Nonferrous Metals, 2003, 55(2): 31.(in Chinese)
[8]ZHAN Hui-fang. Application and study on preparation of black nickel by electro-oxidization [J]. Nonferrous Metals(Metallurgy part), 1995, 3: 14-16.
[9]Kuznetsova, Kuznetsov Vv, Zayakina Lv. Electrodeposition of black nickel [J]. Journal of Applied Chemistry of the USSR, 1979, 52(5): 1134-1135.
[10]XIA Xi, PAN Ren, GUO Zai-ping. Studies on β-NiOOH(Ⅱ) reaction mechanism of charge/discharge [J]. Chinese Journal of Power Sources, 2001, 25(3): 203-205.(in Chinese)
[11]YAN Jie, ZHOU Zhen, WANG Xian-you, et al. Studies on structural changes of spherical nickel hydroxide during charge/discharge [J]. Chinese Journal of Power Sources, 1998, 22(4): 152-154.(in Chinese)
[12]Kim M S, Hwang T S, Kim K B. A study of the electrochemical redox behavior of electrochemically precipitated nickel hydroxides using electrochemical quartz microbalance [J]. J Electrochem Soc, 1997, 144(5): 1537-1543.
[13]WANG X Y, YAN J, ZHANG Y S, et al. Cyclic voltammetric studies of pasted nickel hydroxide electrode microencapsulated by cobalt [J]. J Appl Electrochem, 1998, 28: 1377-1382.
[14]FENG Bo-xue, XIE Liang, CAI Xing-min, et al. Electrochromic performance and mechanism of noncrystalline NiOxHy thin films fabricated by electrochemical deposition [J]. Chinese Journal of Semiconductors, 2001, 22(2): 193-197.(in Chinese).
[15]Taraszewska J, Roslonek G. Electrocatalytic oxidation of methanol on a glassy carbon electrode modified by nickel hydroxide formed by ex situ chemical precipitation [J]. J Electroanal Chem, 1994, 364: 209.
[16]Joseph J, Gomathi H, Rao G P. Electrochemical characteristics of thin films of nickel hexacyanoferrate formed on carbon substrates [J]. Electrochem Acta, 1991, 36(10): 1537.
[17]Fleischmann M, Korinek K, Pletcher D. The oxidation of organic compounds at a nickel anode in alkaline solution [J] J Electroanal Chem, 1971, 31: 39.
[18]Natarajan C, Ohkubo S, Nogami G. Influence of film processing temperature on the electrochromic properties of electrodeposited nickel hydroxide [J]. Solid State Ionics, 1996, 86-88: 949-954.
[19]Chen Y W D, Noufi R N. Electrodepositon of nickel and cobalt oxides onto platinum and graphite electrodes for alkaline water electrolysis [J]. J Electrochem Soc, 1984, 131(6): 1447.
[20]Tench D, Warren L F. Electrodeposition of conducting transition metal oxide/hydroxide film from aqueous solution [J]. J Electrochem Soc, 1983, 130(4): 869.
[21]Visscher W, Barendrecht E. Investigation of thin-film α- and β-Ni(OH)2 electrodes in alkaline solutions [J] . J Electroanal Chem, 1983, 154: 69.
[22]Hahn F, Beden B, Croissant M J. In situ UV visible reflectance spectroscopic investigation of the nickel electrode-alkaline solution interface [J] . Electrochem Acta, 1986, 31(3): 335.
[23]HU Chi-chang, CHENG Chen-yi. Anodic deposition of nickel oxides for the nickel-based batteries [J]. J Power Sources, 2002, 111: 137-144.
[24]MA Chun-an. Introduction to Synthetic Organic Electrochemistry [M]. Beijing: Science Press, 2002. 90-92.(in Chinese)
(Edited by YANG Bing)
Foundation item: Project(50004005) supported by the National Natural Science Foundation of China
Received date: 2004-12-26; Accepted date: 2005-02-21
Correspondence: ZHENG Guo-qu, Associate Professor, PhD; Tel: +86-571-88320429; E-mail: ZGQ003047@163.com