Process for recycle of spent lithium iron phosphate battery via a selective leaching-precipitation method
来源期刊:中南大学学报(英文版)2020年第11期
论文作者:叶华 李昊昱 孙明藏 陈武杰
文章页码:3239 - 3248
Key words:lithium iron phosphate batteries; selective leaching; recovery; sodium persulfate; lithium carbonate
Abstract: Applying spent lithium iron phosphate battery as raw material, valuable metals in spent lithium ion battery were effectively recovered through separation of active material, selective leaching, and stepwise chemical precipitation. Using stoichiometric Na2S2O8 as an oxidant and adding low-concentration H2SO4 as a leaching agent was proposed. This route was totally different from the conventional methods of dissolving all of the elements into solution by using excess mineral acid. When experiments were done under optimal conditions (Na2S2O8-to-Li molar ratio 0.45,0.30 mol/L H2SO4, 60 °C, 1.5 h), leaching efficiencies of 97.53% for Li+, 1.39% for Fe3+, and 2.58% for PO43- were recorded. FePO4 was then recovered by a precipitation method from the leachate while maintaining the pH at 2.0. The mother liquor was concentrated and maintained at a temperature of approximately 100 °C, and then a saturated sodium carbonate solution was added to precipitate Li2CO3. The lithium recovery yield was close to 80%.
Cite this article as: LI Hao-yu, YE Hua, SUN Ming-cang, CHEN Wu-jie. Process for recycle of spent lithium iron phosphate battery via a selective leaching-precipitation method [J]. Journal of Central South University, 2020, 27(11): 3239-3248. DOI: https://doi.org/10.1007/s11771-020-4543-3.

J. Cent. South Univ. (2020) 27: 3239-3248
DOI: https://doi.org/10.1007/s11771-020-4543-3
LI Hao-yu(李昊昱), YE Hua(叶华), SUN Ming-cang(孙明藏), CHEN Wu-jie(陈武杰)
College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: Applying spent lithium iron phosphate battery as raw material, valuable metals in spent lithium ion battery were effectively recovered through separation of active material, selective leaching, and stepwise chemical precipitation. Using stoichiometric Na2S2O8 as an oxidant and adding low-concentration H2SO4 as a leaching agent was proposed. This route was totally different from the conventional methods of dissolving all of the elements into solution by using excess mineral acid. When experiments were done under optimal conditions (Na2S2O8-to-Li molar ratio 0.45,0.30 mol/L H2SO4, 60 °C, 1.5 h), leaching efficiencies of 97.53% for Li+, 1.39% for Fe3+, and 2.58% for PO43- were recorded. FePO4 was then recovered by a precipitation method from the leachate while maintaining the pH at 2.0. The mother liquor was concentrated and maintained at a temperature of approximately 100 °C, and then a saturated sodium carbonate solution was added to precipitate Li2CO3. The lithium recovery yield was close to 80%.
Key words: lithium iron phosphate batteries; selective leaching; recovery; sodium persulfate; lithium carbonate
Cite this article as: LI Hao-yu, YE Hua, SUN Ming-cang, CHEN Wu-jie. Process for recycle of spent lithium iron phosphate battery via a selective leaching-precipitation method [J]. Journal of Central South University, 2020, 27(11): 3239-3248. DOI: https://doi.org/10.1007/s11771-020-4543-3.
1 Introduction
Lithium-ion batteries (LIBs) are important energy storage devices for lightweight and mobile commerce applications compared to ordinary batteries [1]. Owing to their high energy density, inflated energy capacity, higher battery voltage, wide operating temperature range, and high stability in charge-discharge cycles, LIBs have been widely applied in numerous electronic devices, such as computers, digital camcorders, and mobile telephones, and also as energy-storage systems in power sources for electric cars [2-4] . The growing energy demand for electric vehicles (EVs) and consumer electronics (CE) has boosted the proliferation of discarded batteries and increased environmental pollution. Approximately 13000 t of LiFePO4 materials were spent worldwide in making LiFePO4 batteries in 2014. China’s LiFePO4 exportation was close to 33000 t, accounting for 65% of the global market in 2015. Meanwhile, the LiFePO4 markets are expected to expand continuously and the average growth rate from 2016 to 2020 is estimated at 20% [5]. LIB manufacturers will be forced to face the difficulties of waste disposal when their service life ends. Therefore, it is imperative for researchers to find an effective method of disposal that avoids environmental pollution and wasting of resources [6]. The recycling of LIBs can improve the efficiency of natural resource utilization and greatly reduce their production costs [7]. The major element components’ content of LIBs is certainly higher than that of ores under certain circumstances [8, 9]. The LIBs for these cathode material mostly consist of 5%-7% Li, 5%-10% Ni, 5%-20% Co, 15% organics, and 7% plastic (this composition may vary slightly owing to different manufacturing processes) [7, 10]. Therefore, the recycling and reuse of waste LiFePO4 batteries will face unprecedented opportunities and challenges.
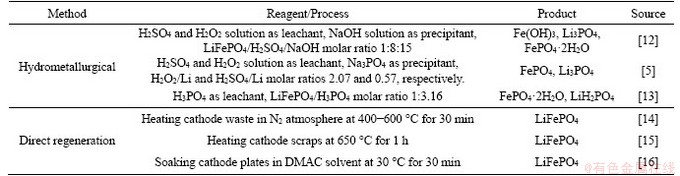
LIBs usually include four main parts: the anode, cathode, electrolyte, and membrane. The anode is a typical Cu sheet wrapped by a compound of graphite, conductor, binder polyvinylidene fluoride (PVDF), and additional reagents, such as LiPF6 [11]. Similarly, the cathode is an Al foil enclosed by an admixture of active cathode materials, PVDF adhesive, electric conductor, and additional reagents. So far, researchers have published data on the recovery of LiFePO4 cathode materials from spent LIBs, and they have mainly focused on existing hydrometallurgical methods [5, 12, 13] and direct regeneration methods [14-16]. Recycling of waste LiFePO4 via the above- mentioned methods has been widely reported.Table 1 shows the different methods of recycling LiFePO4 cathode materials. In the leaching processes of recycling and regeneration of LiFePO4 powders, inorganic acids (such as HCl, H3PO4, and H2SO4) usually combined with hydrogen peroxide are used as leaching agents to dissolve all elements into the solution. Chemical precipitation methods are further adopted to solve the problems incurred in the separation procedure by using NaOH or Na3PO4 [5, 12, 13]. However, the solution after acid leaching treatment is not purified, which will result in a higher content of manganese, calcium, etc., all of which will affect the quality of the final product. The direct regeneration methods refer to recycling waste LIBs via some simple steps and regenerating them directly in the battery manufacturing process. In recycling waste LiFePO4 powders, those materials comprising the cathodes could be separated effectively and later reused following high-temperature heating in organic solvents [14-16]. However, recovered cathode materials are vulnerable to impurities; in addition, a cathode’s structure will also become drastically changed after multiple charge-discharge cycles, leading to low electrochemical performances in the process.
In general, if a viable method of recycling waste cathode materials is industrialized, the chemical industry technique needs to be simpler, more efficient, more economical, and more environmentally friendly. Under these circumstances, it is undoubtedly necessary to explore a green, environmentally friendly process route for recycling spent LiFePO4. In this paper, we present an overall method of recovering LiFePO4 from spent LIBs, mainly including the recovery of Al foil, Fe salt, and Li salt. The route of selective leaching and recovery of Li, Fe, and P from spent LIBs was designed by using stoichiometric Na2S2O8 as an oxidant and adding low-concentration H2SO4 as a leaching agent. It is totally different from the conventional methods of dissolving all of the elements into solution by using excess inorganic acid. The optimal method presented in this study provides a scalable, environmentally friendly, and simple route for selective leaching and recycling of valuable metals from spent LIBs.
2 Experimental
2.1 Materials
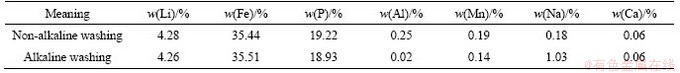
The chemical reagents used in this work include sodium persulfate, sulfuric acid solution, sodium hydroxide, and sodium carbonate (supplied by Shenghua Science Research Institution, Changsha, China), and all were of analytical grade. Before the leaching experiment, LiFePO4 powders were obtained from spent batteries (provided by Jinlu New Energy Co., Ltd., Yichun, Jiangxi,China) by adopting the ball-milling method, and its main elemental contents (wt.%) were 35.44% Fe, 4.28% Li, and 19.22% P.
Table 1 Various methods of recycling LiFePO4 cathode materials
2.2 Experimental procedures
Prior to beginning the recycling projects, the waste batteries were completely discharged and then disassembled, after which the cylindrical materials were removed from the waste LIBs. The experiments consisted of the following main procedures.
2.2.1 Separation of LiFePO4 from spent LIBs
The chosen separation method, ball-milling, was used to recover the LiFePO4 powders and Al foils, with the LiFePO4 powders effectively separated from the Al collectors by oscillating the sieve. During this experiment, the separation results obtained at different rotational speeds and different duration times were studied to determine the best conditions (300 r/min, 60 min); the best screen mesh (0.85 mm) was determined through comparison with results using different screen apertures.However, the residual aluminum slag (about 1.44%) after ball milling was mixed into LiFePO4 powder. Finally, the LiFePO4 powders were pretreated with NaOH solution (conditions: [OH-]=2.0 mol/L, solid/liquid (S/L) ratio=1 g:3 mL) to eliminate Al interference. Table 2 shows the effect of alkaline washing on the separation of LiFePO4 powder.
This procedure resulted in 97.63% of LiFePO4 and 98.56% of Al foils being separated effectively from the materials.
2.2.2 Selective leaching of cathode materials (LiFePO4)
In the leaching process, the theory of oxidation reduction was applied to investigating selective separation of Li+ and FePO4 from the cathode materials. In the recovery of waste LIBs such as LiCoO2 and LiNixCoyMn1-x-yO2 [17-19], it has been proved that hydrogen peroxide has strong oxidizing property in an acidic environment instead of exhibiting reducing property, and hydrogen peroxide can effectively promote the dissolution of Li, Co, Ni, etc. LI et al [5] pointed out that hydrogen peroxide can have better selectivity for lithium iron phosphate leaching at lower H+ concentration, and that the Li+ was expected to separate from LiFePO4 powders and dissolve in solution in which H2O2 acted as an oxidation during the reaction (see Eq. (1)). However, to achieve the best reaction conditions, the H2O2/Li molar ratio was about 4 times its theoretical value, which excessively increases the amount of hydrogen peroxide used.
2LiFePO4+H2SO4+H2O2→2FePO4↓+Li2SO4+2H2O (1)
Therefore, the key to recycling LIBs is to choose a more effective oxidant. The persulfate anion (S2O82-) is a strong two-electron oxidizing agent with a redox potential of 2.08 V and higher oxidizing power than hydrogen peroxide (EΘ=1.7 V) [20]. The following experiment was therefore conducted using sodium persulfate instead of hydrogen peroxide. During the leaching experiment, Li+ was selectively dissolved into the leaching solution. Meanwhile, Fe2+ in LiFePO4 powders is easily oxidized to Fe3+, which will combine with PO43- and remain in the leaching residue as FePO4. The following reactions occurred in the selective leaching process [21-23]:
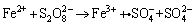 (2)
(2)
in which Fe2+ activates S2O82- decomposition and then promotes the formation of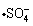 at a temperature of approximately 20 °C [24]. In the next reaction, excess Fe2+ reacts with
at a temperature of approximately 20 °C [24]. In the next reaction, excess Fe2+ reacts with 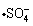 to transform Fe2+ into Fe3+, according to:
to transform Fe2+ into Fe3+, according to:
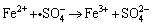 (3)
(3)
The total reaction in the leaching process could be concluded to be the one for the recovery of FePO4 with Na2S2O8; thus, the reaction equation is as follows:
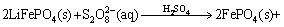
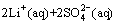 (4)
(4)
LiFePO4 materials are leached selectively by using a low concentration of H2SO4 and Na2S2O8/Li molar ratio. A low concentration of H2SO4 plays an important role in two aspects: improving the leaching rate of Li+ and reducing experimental costs. The agitation speed was set to 250 r/min during all of the leaching. To determine the technical feasibility of Li+ and FePO4 separation during leaching, the following operational variables were investigated: Na2S2O8/Li molar ratio (0–0.6), H2SO4 concentration (0–0.6 mol/L), temperature (30- 80 °C), duration (0.5–2.5 h), and H2SO4/Li molar ratio (0.39-0.72). The resulting solution was separated by filtration, and then the concentrations of Li, Fe, and P were determined with inductively coupled plasma–optical emission spectrometry (ICP-OES) (using a PerkinElmer Optima 5300DV ICP-OES system) after leaching.
Table 2 Main components of each element in separated LiFePO4 powder
2.2.3 Effect of pH values on Fe/P molar ratio of recovered FePO4
After selective leaching experiments, as shown in Figure 1, Li+ in solution would be expectedly separated with FePO4, but 1.39% of Fe3+ and 2.58% of PO43- were dissolved simultaneously into solution to avoid the interference of lithium phosphate on the final product, lithium carbonate. Therefore, the Fe3+/PO43- molar ratio in the solution was adjusted to 1.1:1 by the addition of Fe2(SO4)3 to precipitate and recover FePO4 [27]. This reaction can be expressed as:
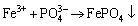 (5)
(5)
Finally, pH value are an important factor in controlling the precipitation of FePO4, and they were studied in the next process.
2.2.4 Recovery of Li2CO3
Recovery of Li2CO3 mainly includes the following processes: The filtrate was concentrated and boiled, then a saturated sodium carbonate solution was added and filtered, and the filter cake was washed [25]. Finally, the filter cake was dried to achieve a constant mass at 110 °C, and Li2CO3 was then obtained.
3 Results and discussion
3.1 Effect of different H2SO4 concentrations
Figure 1(a) shows the effect of different H2SO4 concentrations on leaching efficiency. Experiments were conducted at a Na2S2O8/Li molar ratio (no H2SO4 added, theoretical value 0.5) of 0.5, a liquor-to-solid (L/S) ratio of 10 mL/g, a temperature of 50 °C, and a duration of 2.0 h. When the H2SO4 concentration is 0, at this point the solution pH=7.5, the leaching efficiencies are 87.03% for Li+, 0.00% for Fe3+, and 0.02% for PO43-. These results clearly reveal that sodium persulfate has a strong oxidizability and a good selective leaching effect without sulfuric acid assistance. A reasonable explanation for this phenomenon is that lithium iron phosphate has a FeO6 octahedron and a PO4 tetrahedron as a skeleton structure [26], and it is difficult to decompose it under the action of lower H+ concentration, but Li+ easily escapes from the olivine-type LiFePO4 material through a two- dimensional channel. With H2SO4 concentrations increasing from 0.00 to 0.30 mol/L, the leaching ratio of Li+ significantly increased, from 87.03% to 96.62%, while Fe3+ and PO43- still remained at a low leaching rate (Fe3+ and PO43- less than 1.32% and 1.98%, respectively). However, when the concentration of H2SO4 was in the range 0.30-0.60 mol/L (pH values simultaneously changed from 1.8 to 0.4), the leaching ratio of Li+ hardly increases with the further increase of H2SO4 concentration, but it leads to the continuous increase of Fe3+ concentration (from 1.32% to 45.12%) and PO43- concentration (from 1.98% to 48.24%). To achieve the maximum efficiency of Li and lower Fe and P leaching efficiency, the optimal H2SO4 concentration was determined to be 0.30 mol/L by experiments.
3.2 Effect of Na2S2O8-to-Li molar ratio
When the operational variables were controlled at a H2SO4 concentration of 0.30 mol/L, a temperature of 50 °C, an L/S ratio of 10 mL/g, and a duration of 2.0 h, experiments with a different Na2S2O8/Li molar ratios were conducted. When the Na2S2O8/Li molar ratio changed from 0 (without addition of Na2S2O8) to 0.45, the Li leaching ratio increased by approximately 77% (from 18.32% to 95.83%), and the Fe and P leaching rates were reduced from 15.34% to 1.45% and from 17.87% to 1.34%, respectively. The results (Figure 1(b)) show that when combined with Na2S2O8, the leaching rate of Li is remarkably increased and that the leaching rates of Fe and P are thus suppressed. A reasonable explanation for this is that Na2S2O8 acts as an oxidant for transferring Fe from the 2+ state to the 3+ state, which is beneficial to the formation of FePO4 precipitate, thus making the structure of LiFePO4 easy to decompose in H2SO4 solution. Moreover, sulfuric acid also improves the leaching efficiency of Li and achieves the purpose of reducing the amount of sodium persulfate. When the Na2S2O8/Li molar ratio was further increased to above 0.6, a negligible effect on leaching efficiency was obvious. Therefore, the Na2S2O8-to-Li molar ratio should be set at 0.45, which also greatly reduces the leaching efficiency of Fe and P.
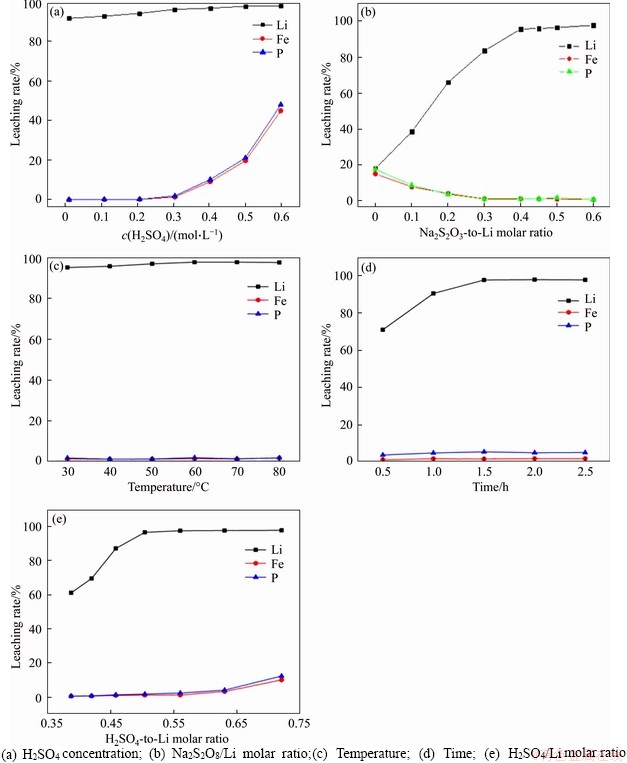
Figure 1 Effects of various conditions on leaching rates of Li, Fe, and P:
3.3 Effect of reaction temperature and time
Figure 1(c) shows the effect of reaction temperature on leaching efficiency. The operational variables are as follows: Na2S2O8/Li molar ratio 0.45, H2SO4 concentration 0.30 mol/L, L/S ratio 10 mL/g, and duration 2.0 h. With reaction temperature increasing from 30 °C to 60 °C, the leaching rates of all of the elements increase slightly. When the reaction temperature is above 60 °C, the leaching rate of Li+ hardly changes as temperature increases. Therefore, the optimal leaching temperature should be set at 60 °C to obtain the highest leaching efficiency of Li (up to 97.87%). However, temperatures below 60 °C are also feasible due to the small effect on leaching, especially when considering cost issues in industrial applications.
Figure 1(d) shows the effect of reaction time on leaching efficiency with Na2S2O8/Li molar ratio 0.38, H2SO4 concentration 0.30 mol/L, L/S ratio 10 mL/g, and temperature 60 °C. As above, a duration of 1.5 h was concluded to be optimal leaching time. Simultaneously, it was seen from the relationship diagram of reaction time that the leaching efficiency of P was always higher than Fe under different conditions. The main reason for this phenomenon was that the whole reaction was carried out in a weakly acidic environment, and Fe3+ in the solution was easily precipitated with Fe(OH)3 in the process.
3.4 Effect of H2SO4-to-Li molar ratio
To clearly demonstrate the influence of the amounts of H2SO4 and LiFePO4, different H2SO4/Li molar ratios instead of L/S ratios were studied under the following conditions: Na2S2O8/Li molar ratio 0.45, H2SO4 concentration 0.30 mol/L, temperature 60 °C, and duration 1.5 h. Since H2SO4 concentration and LiFePO4 quality used in each experiment were both fixed, the higher H2SO4/Li molar ratio means that the more H2SO4 is added, the larger the solution volume. The results (Figure 1(e)) clearly show the effect of H2SO4/Li molar ratio on the leaching efficiency of Li. As the molar ratio increases throughout the selected range, the Li leaching rate rises from 61.23% to 97.53%. However, when the H2SO4/Li molar ratio ranges from 0.39 to 0.56, there is no significant change in the leaching rate of Fe and P. As the molar ratio continues to increase, more Fe and P are dissolved in the solution. To reduce the additional amount of sulfuric acid to decrease the amount of alkali added and the volume of the leachate in the next impurity removal step to be as low as possible, a H2SO4/Li molar ratio of 0.56 (L/S ratio 11.1 mL/g) can obtain the best leaching results.
3.5 Removal of Fe3+ and PO43- from leaching solution
After leaching under the optimized conditions, 1.39% of Fe3+ and 2.58% of PO43- were dissolved simultaneously into solution. The Fe3+/PO43- molar ratio in the solution was adjusted to 1.1:1 by the addition of Fe2(SO4)3 at this point, with a system pH of 0.45. As shown in Figures 2 and 3, when the pH was greater than 1.0, precipitate began to appear in solution. Figure 2 shows that the Fe3+/PO43- molar ratio in the precipitation gradually increases with pH value. When pH values were adjusted to between 1.9 and 2.1, the Fe3+/PO43- molar ratio was analyzed as 0.97–1.01, so the precipitate was substantially in the form of FePO4, and when the pH value was 2.0, the removal efficiencies of PO43- and Fe3+ were 99.3% and 90.3%, respectively. With pH value increasing from 2.0 to 3.5, traces of PO43- and excess Fe3+ in solution can be further removed. When the final pH value was 3.5, the removal rate of PO43- reached 99.95% and the removal rate of Fe3+ was greater than 99.99%. Figure 4 shows the TG-DSC curve of the recovered FePO4/C during selective leaching. From the TG curve, it can be seen that there are continuous quality changes in the temperature range between 37 °C and 550 °C, which reveals a total weight loss of approximately 4.0%. In addition, the DSC curve shows that there are two upward exothermic peaks of different intensities at 430.0 °C and 550.0 °C. This result is due to the combustion of acetylene black and the decomposition of the binder residue (PVDF) in the FePO4/C materials. With temperature continuously increasing, a downward endothermic peak is shown at 658 °C and no loss of mass occurs, indicating that FePO4 began to undergo a phase transition at this temperature (i.e., from amorphous to an alpha quartz structure).
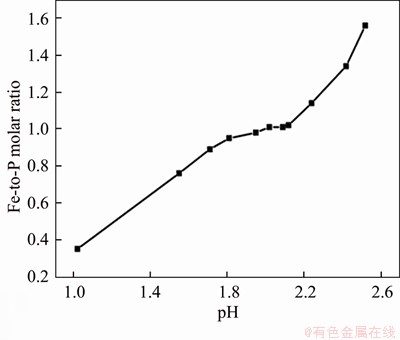
Figure 2 Effect of pH values on Fe/P molar ratio of FePO4
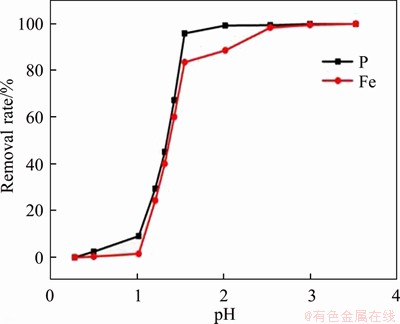
Figure 3 Effect of pH values on recovery of FePO4
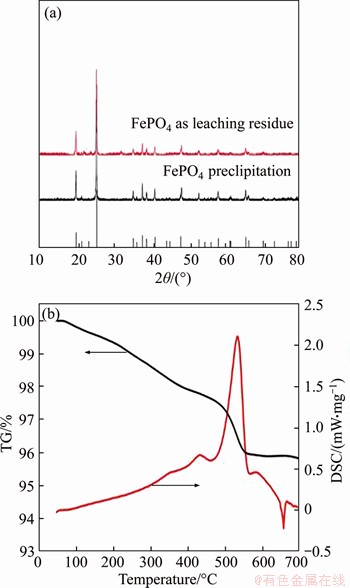
Figure 4 XRD patterns of recovered FePO4 (a) and TG-DSC curves (b) of recycled FePO4/C
To further analyze the crystal structure of the recovered FePO4 materials, X-ray-diffraction (XRD) patterns of FePO4 precipitation and FePO4 leaching residue by burning at 580 °C for 3 h were studied. As shown in Figure 4, two XRD patterns are well consistent with the standard pattern (PDF No. 00-29-0715). This result clearly demonstrates that Fe3+ in solution is precipitated in the form of FePO4 and that FePO4 remains in the leaching residue during the selective leaching experiment.
3.6 Recovery of Li from leaching solution
After the recovery of PO43- and Fe3+ by controlling pH values, the mother liquor concentrated and maintained at a temperature of approximately 100 °C, and then a saturated sodium carbonate solution was added to precipitate Li2CO3. Since lithium carbonate solubility is inversely proportional to temperature in an aqueous solution, e.g.,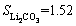 at 0 °C and
at 0 °C and  0.71 (g/100 g H2O) at 100 °C [25], Li2CO3 products can be effectively recovered after filtration and washing with hot water to eliminate residual mother liquor.
0.71 (g/100 g H2O) at 100 °C [25], Li2CO3 products can be effectively recovered after filtration and washing with hot water to eliminate residual mother liquor.
Finally, the result shows that approximately 80% of the Li is recovered as Li2CO3 precipitate. Figure 5 clearly reveals that the products of recovered Li2CO3 are pure and highly crystalline, and well matched compared to the pattern to Li2CO3 (PDF No. 22-1141). In addition, the diffraction peaks from the tests are highly coincident and sharp.
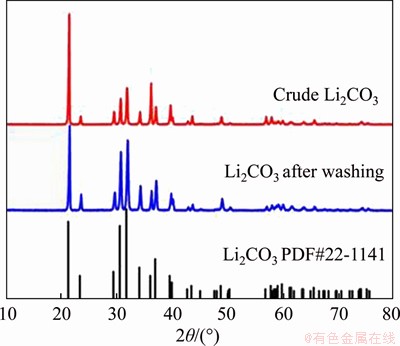
Figure 5 XRD patterns of synthesized Li2CO3
4 Conclusions
In this paper, we introduced a relatively simple recovery method of FePO4 and Li2CO3 from spent LIBs. Use of a selective leaching-chemical precipitation method was proposed for the orderly recovery of valuable metals in waste LiFePO4 batteries. As shown in Figure 6, an overall process flowchart mainly includes the following processes: the amount of acid and alkali required greatly reduced during leaching and precipitation compared with conventional hydrometallurgy method; the impurities in the leachate were low in content and easy to purify; the obtained products achieved effective recovery of elements, such as Li, Fe, P, and reduced environmental problems, such as phosphorus pollution. The conclusions are mainly as follows:
1) For the following operational variables, Na2S2O8/Li molar ratio 0.45, H2SO4 concentration 0.30 mol/L, L/S ratio 11.1 mL/g, temperature 60 °C, and duration 1.5 h, results indicate that 97.55% of Li+, 1.39% of Fe3+, and 2.58% of PO43- are leached at different levels of leaching efficiency.
2) The Fe3+/PO43- molar ratio in the solution is adjusted to 1.1:1 by the addition of Fe2(SO4)3 and the pH value increased to 2.0 to achieve precipitation of FePO4. Then, with the pH value increasing from 2.0 to 3.5, traces of PO43- and excess Fe3+ in the solution can be further removed.
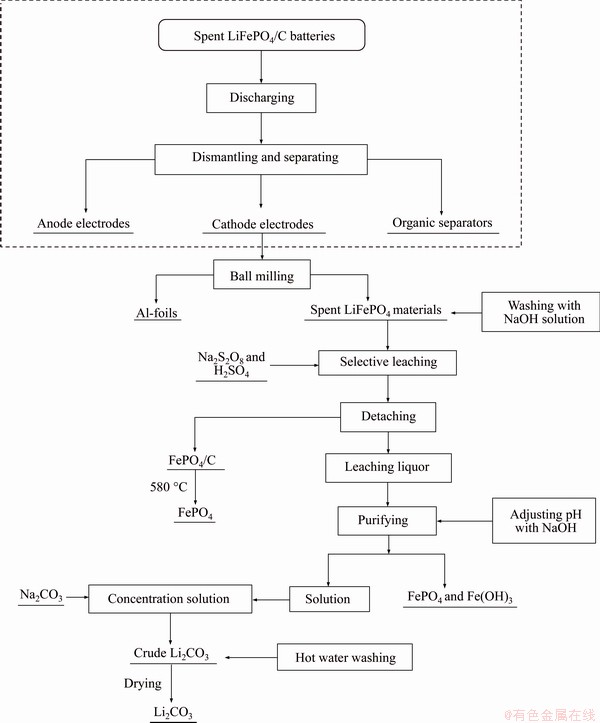
Figure 6 Flowchart for leaching and recovery process
3) The mother liquor is concentrated and maintained at a temperature of approximately 100 °C, and then a saturated sodium carbonate solution was added to precipitate the Li2CO3.
Contributors
YE Hua provided the idea of the study and developed the research goal. LI Hao-yu led the research activity planning and execution. SUN Ming-cang made great contribution to the improvement of manuscript after the initial edition. CHEN Wu-jie offered some valuable suggestions for analyzing the test data. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
LI Hao-yu, YE Hua, SUN Ming-cang and CHEN Wu-jie declare that they have no conflict of interest.
References
[1] KERMAN K, LUNTZ A, VISWANATHAN V, CHIANG Y M, CHEN Zhe-bo. Review: Practical challenges hindering the development of solid state Li ion batteries [J]. Journal of the Electrochemical Society, 2017, 164(7): A1731-A1744. DOI: 10.1149/2.1571707jes.
[2] EL KHARBACHI A, ZAVOROTYNSKA O, LATROCHE M, CUEVAS F, YARTYS V, FICHTNER M. Exploits, advances and challenges benefiting beyond Li-ion battery technologies [J]. Journal of Alloys and Compounds, 2020, 817: 153261. DOI: 10.1016/j.jallcom.2019.153261.
[3] STEWARD D, MAYYAS A, MANN M. Economics and challenges of Li-ion battery recycling from end-of-life vehicles [J]. Procedia Manufacturing, 2019, 33: 272-279. DOI: 10.1016/j.promfg. 2019.04.033.
[4] YAN Kang, GUO Xue-yi, TIAN Qing-hua, LI Dong. Cobalt flow analysis of lithium-ion battery system in China [J]. Journal of Central South University: Science and Technology, 2017, 48(1): 25-30. DOI: 10.11817/j.issn.1672- 7207.2017. 01.004. (in Chinese)
[5] LI Huan, XING Sheng-zhou, LIU Yu, LI Fu-jie, GUO Hui, GE Kuang. Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 8017-8024. DOI: 10.1021/ acssuschemeng.7b01594.
[6] LI Huan, XING Sheng-zhou, LIU Yu, LI Fu-jie, GUO Hui, KUANG Ge. Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 8017-8024.
[7] LIU Chun-wei, LIN Jiao, CAO Hong-bin, ZHANG Yi, SUN Zhi. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review [J]. Journal of Cleaner Production, 2019, 228: 801-813. DOI: 10.1016/j.jclepro. 2019.04.304.
[8] BERTUOL D A, MACHADO C M, SILVA M L, CALGARO C O, DOTTO G L, TANABE E H. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction [J]. Waste Management, 2016, 51: 245-251. DOI: 10.1016/j.wasman.2016.03.009.
[9] ZHU Shu-guang, HE Wen-zhi, LI Guang-ming, ZHOU Xu, ZHANG Xiao-jun, HUANG Ju-wen. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(9): 2274- 2281. DOI:10.1016/s1003-6326(11)61460-x.
[10] JHA A K, JHA M K, KUMARI A, SAHU S K, KUMAR V, PANDEY B D. Selective separation and recovery of cobalt from leach liquor of discarded Li-ion batteries using thiophosphinic extractant [J]. Separation and Purification Technology, 2013, 104: 160-166. DOI: 10.1016/j.seppur. 2012.11.024.
[11] BERTUOL D A, TONIASSO C, JIMENEZ B M, MEILI L, DOTTO G L, TANABE E H, AGUIAR M L. Application of spouted bed elutriation in the recycling of lithium ion batteries [J]. Journal of Power Sources, 2015, 275: 627-632. DOI: 10.1016/j.jpowsour.2014.11.036.
[12] GOLMOHAMMADZADEH R, FARAJI F, RASHCHI F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review [J]. Resources, Conservation and Recycling, 2018, 136: 418-435. DOI: 10.1016/j.resconrec.2018.04.024.
[13] GAO Rui-chuan, SUN Cong-hao, XU Li-jun, ZHOU Tao, ZHUANG Lu-qi, XIE Hua-sheng. Recycling LiNi0.5Co0.2Mn0.3O2 material from spent lithium-ion batteries by oxalate co-precipitation [J]. Vacuum, 2020, 173: 109181. DOI: 10.1016/j.vacuum.2020.109181.
[14] BIAN Dou-cheng, SUN Yong-hui, LI Sheng, TIAN Yuan, YANG Ze-heng, FAN Xiao-ming, ZHANG Wei-xin. A novel process to recycle spent LiFePO4 for synthesizing LiFePO4/C hierarchical microflowers [J]. Electrochimica Acta, 2016, 190: 134-140. DOI: 10.1016/j.electacta.2015.12.114.
[15] KIM H S, SHIN E J. Re-synthesis and electrochemical characteristics of LiFePO4 cathode materials recycled from scrap electrodes [J]. Bulletin of the Korean Chemical Society, 2013, 34(3): 851-855. DOI: 10.5012/bkcs.2013.34.3.851.
[16] CHEN Jiang-ping, LI Qing-wen, SONG Ji-shun, SONG Da-wei, ZHANG Lian-qi, SHI Xian-xing. Environmentally friendly recycling and effective repairing of cathode powders from spent LiFePO4 batteries [J]. Green Chemistry, 2016, 18(8): 2500-2506. DOI: 10.1039/c5gc02650d.
[17] SONG X, HU T, LIANG C, LONG H L, ZHOU L, SONG W, YOU L, WU Z S, LIU J W. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method [J]. RSC Advances, 2017, 7(8): 4783-4790. DOI: 10.1039/c6ra27210j.
[18] DORELLA G, MANSUR M B. A study of the separation of cobalt from spent Li-ion battery residues [J]. Journal of Power Sources, 2007, 170(1): 210-215. DOI: 10.1016/ j.jpowsour.2007.04.025.
[19] DORELLA G, MANSUR M B. A study of the separation of cobalt from spent Li-ion battery residues [J]. Journal of Power Sources, 2007, 170(1): 210-215.
[20] ZOU Hai-yang, GRATZ E, APELIAN D, WANG Yan. A novel method to recycle mixed cathode materials for lithium ion batteries [J]. Green Chemistry, 2013, 15(5): 1183-1191. DOI: 10.1039/ c3gc40182k.
[21] WANG Hong-gang, FRIEDRICH B. Development of a highly efficient hydrometallurgical recycling process for automotive Li–ion batteries [J]. Journal of Sustainable Metallurgy, 2015, 1(2): 168-178. DOI: 10.1007/s40831-015- 0016-6.
[22] BANERJEE M, KONAR R S. Comment on the paper “polymerization of acrylonitrile initiated by K2S2O8-Fe(II) redox system” [J]. Journal of Polymer Science: Polymer Chemistry Edition, 1984, 22(5): 1193-1195. DOI: 10.1002/ pol.1984.170220519.
[23] TSAO M S, WILMARTH W K. The aqueous chemistry of inorganic free radicals. I. The mechanism of the photolytic decomposition of aqueous persulfate ion and evidence regarding the sulfate–hydroxyl radical interconversion equilibrium [J]. The Journal of Physical Chemistry, 1959, 63(3): 346-353. DOI: 10.1021/j150573a006.
[24] LIANG Chen-ju, BRUELL C J, MARLEY M C, SPERRY K L. Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate– thiosulfate redox couple [J]. Chemosphere, 2004, 55(9): 1213-1223. DOI: 10.1016/ j.chemosphere.2004.01.029.
[25] LIANG Chen-ju, BRUELL C J, MARLEY M C, SPERRY K L Persulfate oxidation for in situ remediation of TCE. II. Activated by chelated ferrous ion [J]. Chemosphere, 2004, 55(9): 1225-1233. DOI: 10.1016/j.chemosphere.2004.01. 030.
[26] FORDHAM J W L, WILLIAMS H L. The persulfate-iron (II) initiator system for free radical polymerizations [J]. Journal of the American Chemical Society, 1951, 73(10): 4855-4859. DOI: 10.1021/ja01154a114.
[27] ZHANG Ping-wei, YOKOYAMA T, ITABASHI O, SUZUKI T M, INOUE K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries [J]. Hydrometallurgy, 1998, 47(2,3): 259-271. DOI: 10.1016/ S0304-386X(97)00050-9.
[28] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries [J]. Nature, 2001, 414(6861): 359-367. DOI: 10.1038/35104644.
[29] ZHENG Ru-juan, ZHAO Li, WANG Wen-hui, LIU Yuan-long, MA Quan-xin, MU De-ying, LI Ru-hong, DAI Chang-song. Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method [J]. RSC Advances, 2016, 6(49): 43613-43625. DOI: 10.1039/ c6ra05477c.
(Edited by YANG Hua)
中文导读
选择性浸出-沉淀法回收废磷酸铁锂电池
摘要:以废磷酸铁锂电池为原料,通过活性物质分离、选择性浸出、逐步化学沉淀等工序回收废旧锂离子电池中的有价金属。提出了使用化学计量比的Na2S2O8作为氧化剂并添加低浓度H2SO4作为浸出剂进行实验。该方法与传统上使用过量无机酸将所有元素溶解到溶液中的常规方法完全不同。在最佳条件下进行实验下(Na2S2O8和Li的摩尔比0.45;0.30 mol /L H2SO4;60 °C;1.5 h),记录Li+的浸出效率为97.53%,Fe3+的浸出效率为1.39%,PO43-的浸出效率为2.58%。然后通过控制pH为2.0使用沉淀法从浸出液中回收FePO4。将母液浓缩并保持在约100 °C的温度,然后加入饱和碳酸钠溶液以沉淀Li2CO3,此时锂回收率接近80%。
关键词:磷酸铁锂电池;选择性浸出;回收;过硫酸钠;碳酸锂
Foundation item: Project(Z20160605230001) supported by Hunan Province Non-ferrous Fund Project, China
Received date: 2019-01-22; Accepted date: 2020-08-10
Corresponding author: YE Hua, PhD, Professor; Tel: +86-18974801607; E-mail: 451250423@qq.com; ORCID: https://orcid.org/0000- 0002-8345-6913

