Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation
来源期刊:中国有色金属学报(英文版)2012年第9期
论文作者:朱曙光 贺文智 李光明 周旭 张骁君 黄菊文
文章页码:2274 - 2281
关键词:废锂离子电池;回收;浸出;沉淀
Key words:spent lithium-ion batteries; recovery; leaching; precipitation
摘 要:基于废锂离子电池中的金属在不同价态时的化学行为差异,通过化学精制从废弃锂离子电池中获取高附加值的钴锂化学产品。将从正极中分离出来的活性物质溶解在硫酸和过氧化氢溶液中,加入(NH4)2C2O4生成CoC2O4·2H2O沉淀。在获得CoC2O4·2H2O后,加入Na2CO3获得Li2CO3沉淀。实验结果表明:96.3% 的Co和87.5% 的Li溶解在2 mol/L H2SO4和2.0% H2O2(体积分数)溶液中,并且有94.7 % 的Co和71.0%的Li 以CoC2O4·2H2O和Li2CO3的形式沉淀下来。
Abstract: Cathode material of spent lithium-ion batteries was refined to obtain high value-added cobalt and lithium products based on the chemical behaviors of metal in different oxidation states. The active substances separated from the cathode of spent lithium-ion batteries were dissolved in H2SO4 and H2O2 solution, and precipitated as CoC2O4·2H2O microparticles by addition of (NH4)2C2O4. After collection of the CoC2O4·2H2O product by filtration, the Li2CO3 precipitates were obtained by addition of Na2CO3 in the left filtrate. The experimental study shows that 96.3% of Co (mass fraction) and 87.5% of Li can be dissolved in the solution of 2 mol/L H2SO4 and 2.0% H2O2 (volume fraction), and 94.7% of Co and 71.0% of Li can be recovered respectively in the form of CoC2O4·2H2O and Li2CO3.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 2274-2281
ZHU Shu-guang1, 2, HE Wen-zhi1, LI Guang-ming1, ZHOU Xu1, ZHANG Xiao-jun1, HUANG Ju-wen1
1. State Key Laboratory of Pollution Control and Resource Reuse, School of Environmental Science and Engineering, Tongji University, Shanghai 200092, China;
2. School of Environment and Energy Engineering, Anhui University of Architecture, Hefei 230022, China
Received 23 September 2011; accepted 10 April 2012
Abstract: Cathode material of spent lithium-ion batteries was refined to obtain high value-added cobalt and lithium products based on the chemical behaviors of metal in different oxidation states. The active substances separated from the cathode of spent lithium-ion batteries were dissolved in H2SO4 and H2O2 solution, and precipitated as CoC2O4·2H2O microparticles by addition of (NH4)2C2O4. After collection of the CoC2O4·2H2O product by filtration, the Li2CO3 precipitates were obtained by addition of Na2CO3 in the left filtrate. The experimental study shows that 96.3% of Co (mass fraction) and 87.5% of Li can be dissolved in the solution of 2 mol/L H2SO4 and 2.0% H2O2 (volume fraction), and 94.7% of Co and 71.0% of Li can be recovered respectively in the form of CoC2O4?2H2O and Li2CO3.
Key words: spent lithium-ion batteries; recovery; leaching; precipitation
1 Introduction
Li-ion batteries (LIBs), first produced by SONY in 1991, have been preferred portable source energy for small electronic devices [1,2]. Along with the updated function and decreased cost, LIBs output rose rapidly. World’s LIBs production reached 2.05 billion units in 2005 and has reached 4.6 billion in 2010 [3,4]. The life-span of LIBs is 1-3 years. The tremendous growth in the use of LIBs has resulted in a great number of spent LIBs. Disposal of these spent LIBs will cause serious environmental problems due to the hazardous components such as heavy metal and electrolyte. On the other hand, materials contained in the spent LIBs are valuable resource and could be recycled by proper methods. Especially Co (5%-15% in mass fraction), a kind of rare and precious metal, is admitted strategic resource. In addition, Li is also a kind of scarce resource. Therefore, the recycling of major components from spent LIBs is considered to be a beneficial way to prevent environmental pollution and alleviate resource shortage.
In the practice of recycling spent LIBs, many technologies have been developed, including mechanical process [3,5,6], thermal treatment [7-12], mechano- chemical process [13], acid (or base) leaching [5,14-25], bioleaching [26,27], solvent extraction [28-31], chemical precipitation [32] and electrochemical process [33-35], etc. Because cobalt is the most valuable component in LIBs and lithium is also important in many industrial applications, most of the established technologies focused on the recycling of them. Hydrometallurgy is the main method to recycle LiCoO2 from spent LIBs. The leaching of LiCoO2 from cathodic material is usually carried out by using inorganic acids such as H2SO4 [5,6,19,21,22,29,30], HCl [3,14,17,24], and HNO3 [7,18,25,32] as leaching agents, and H2O2 is usually added in order to convert cobalt to the +2 state for subsequent recovery by electrochemical, precipitation or solvent extraction techniques. FREITAS and GARCIA [34] conducted electrochemical processes to recover cobalt from spent LIBs. Chemical precipitation was used by CONTESTABILE et al [14] and CASTILLO et al [32] to extract cobalt from the leaching solution. MANTUANO et al [29] and NAN et al [30] presented a solvent extraction method to recover cobalt.
Most of the proposed processes are based on hydrometallurgy chemistry and are developed at a laboratory scale. These processes provide a possibility for recycling LIBs in a large industrial scale. Although the spent LIBs will have to be recycled in the near future, the economical efficiency in the recycling process must be given particular consideration. Both cobalt oxalate and lithium carbonate are higher value-added chemical products. Cobalt oxalate can be used as catalyst, desiccant, additives, ceramic pigments, and foam stabilizer, etc; lithium carbonate can be used as catalyst, lithium salts, ceramics, and pharmaceuticals, etc. Based on the description above, the present work presented here aimed at a combined process to recycle cobalt and lithium, and obtain high value-added cobalt oxalate and lithium carbonate.
2 Experimental
2.1 Materials
The active substance used in this work was separated from spent mobile phone batteries (Li-ion batteries). Sulphuric acid was selected for leaching and hydrogen peroxide (H2O2) was employed as a reducing agent. Sodium hydroxide, ammonium oxalate and sodium carbonate were used for precipitation reaction. All solutions were prepared with distilled water and all reagents were of analytical grade.
2.2 Experimental procedure
The experimental procedures for recycling Co and Li from the active substance were as follows.
1) The LiCoO2 active substance sample was weighed accurately and then dissolved into H2SO4-H2O2 solution in a beaker and H2SO4 solution in an ultrasonic cleaner, respectively. Ultrasound-assisted leaching was performed in Kunshan KQ-50E ultrasonic cleaner (with a frequency of 40 kHz and a nominal power of 50 W; Kunshan Ultrasonic Instruments Co., Ltd., China).
2) The leaching solution was filtered to remove impurities, and then its pH was adjusted to 5 using NaOH solution.
3) An amount of (NH4)2C2O4 was added to the solution and was agitated at a speed of 300 r/min for about 1 h to precipitate CoC2O4.
4) The suspending liquid was filtered, the precipitate was dried, and then the CoC2O4 powder was obtained.
5) An amount of Na2CO3 solution was added to the left filtrate and was agitated at a speed of 300 r/min for 1 h to precipitate Li2CO3.
6) The lithium carbonate precipitates were separated from the suspension by filtering and dried for analysis.
2.3 Analysis methods
The contents of cobalt, lithium and the other metals were analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES, Optima 2100 DV, Perkin Elmer, U.S.). The products of CoC2O4·2H2O and Li2CO3 were characterized by X-ray diffraction (XRD, Bruker D8 Advance,GER). The diffractometer equipped with Cu Kα radiation (λ=1.5406 ?), employing a scanning rate of 0.02 (°)/s and 2θ ranging from 5° to 80°. The accelerating voltage was set at 40 kV with a 40 mA flux. Diffraction patterns were compared with reference data in the ICDD PDF-2 database.
3 Results and discussion
3.1 Leaching experiments
Dissolution of LiCoO2, the process of the reduction of Co3+ in the solid species to Co2+ in the aqueous phase, belongs to a surface chemical reaction [18]. The leaching process of LiCoO2 in H2SO4 solution could be represented as follows [22]:
2LiCoO2(s)+3H2SO4(aq)+H2O2(aq)→
2CoSO4(aq)+Li2SO4(aq)+4H2O(g)+O2(g) (1)
Generally, solution concentration, temperature, reaction time and the amount of added material could affect the reaction. To obtain the optimized leaching conditions for the mixed powder, the present work respectively examined the variation of leaching efficiency with sulphuric acid concentration, temperature, reaction time, ultrasound-assisted condition and the amount of hydrogen peroxide added. During the leaching process, the ratio of solid mass to liquid volume (represented by S:L) was maintained at 33 g/L.
Figure 1 shows the effect of H2SO4 concentration (represented by c(H2SO4)), H2O2 amount and ultrasound- assisted condition on the leaching of LiCoO2 at 60 ?C for 2 h. From Fig. 1, it can be noted that increasing the sulphuric acid concentration could enhance the leaching efficiencies of cobalt and lithium. When c(H2SO4) varied from 0.5 mol/L to 2.0 mol/L at reductant amount of 2.0% H2O2, the leaching efficiencies of cobalt and lithium increased from 82.87% to 96.28% and from 72.70% to 85.80%, respectively. However, with a further increase in the c(H2SO4) from 2.0 mol/L to 2.5 mol/L, the leaching efficiency of Co or Li did not change significantly. This phenomenon could be explained by the chemical reaction equation of dissolving LiCoO2 in the H2SO4 and H2O2 solution (Eq. (1)). Equation (1) indicates that the addition of the reacting substances can facilitate the forward reaction resulting in the increase in leaching efficiency. Meanwhile, the increase of CoSO4 or Li2SO4 in the solution reversely enhances the backward reaction, and with the continuous increase in c(H2SO4), the backward reaction gradually turns to be in equilibrium with the forward reaction. As a result, the leaching efficiencies of Co and Li did not change significantly.

Fig. 1 Effects of H2SO4 concentration, H2O2 amount and ultrasound-assisted condition on leaching of LiCoO2 at 60 ℃ for 2 h (S:L of 33 g/L, agitation speed of 300 r/min)
The effect of H2O2 amount on the leaching of LiCoO2 with 2 mol/L H2SO4, indicates that the leaching efficiency increased from 63.55% to 96.59% for cobalt and 69.33% to 85.91% for lithium as the H2O2 amount increased from 0.9% to 2.0%. But, the efficiency did not increase significantly when more than 2.0% H2O2 was used. The phenomenon is probably because of the instability of H2O2. When heated, hydrogen peroxide could decompose (see Eq. (2)) in solution and the increase of its concentration could accelerate the decomposing.
![]() (2)
(2)
Therefore, in the leaching process at the condition of high H2O2 concentration (above 2.0%), the effectual H2O2 increased slowly with the increase in its concentration, resulting in no significant increase in the leaching efficiency.
Of particular interest observed in Fig. 1 is a remarkable leaching efficiency variation of the LiCoO2 induced by ultrasonic irradiation. When the H2SO4 concentration is low (0.5 mol/L), the leaching efficiencies of cobalt and lithium using ultrasound- assisted leaching are obviously higher than those in H2SO4 using H2O2 as reductant. In the leaching process at the condition of high H2SO4 concentration (above 1.0 mol/L), the leaching efficiencies of cobalt and lithium using ultrasound-assisted leaching are lower than those in H2SO4 or H2O2. It is likely because H2O2 is easily generated by the action of ultrasonic waves on low H2SO4 concentration solution. When ultrasonic waves are irradiated into an aqueous solution, cavitation occurs. The cavitation provides a so-called “hot spot” which has an extremely high temperature (more than 5000 K) and pressure (more than 5.07×107 Pa) [36,37]. In the hot cavities, free-radical reactions (e.g., involving ?OH derived from the decomposition of H2O) are possible. The hot spot enables the generation of radical species such as ?OH and H2O2. Due to an associated decrease in water content with an increase in the H2SO4 concentration, it is not conducive to the formation of H2O2 by ultrasonic irradiation. Consequently, the equilibrium concentrations of hydrogen peroxide did not increase significantly, and the leaching efficiency of cobalt and lithium using ultrasound-assisted leaching is lower than that in H2SO4 or H2O2.
The effects of temperature and reaction time on the leaching efficiency were studied using 2.0 mol/L H2SO4 at a S:L ratio of 33 g/L and at a reductant concentration of 2.0%. The leaching results for cobalt and lithium are shown in Figs. 2(a) and (b), respectively. This figure illustrates that the metal leaching is significantly affected by temperature and reaction time. The increase in temperature or time significantly enhances the leaching efficiencies of Co and Li. The results indicate that only 33.67% of cobalt and 21.83% of lithium can be leached out at 20 ℃ within 0.5 h. The leaching efficiencies of the metals increase with an increase in the leaching temperature or the reaction time. At 60 ℃ for 2 h, 95.88% of the cobalt and 88.23% of the lithium are leached out. A further increase in the temperature and reaction time does not show any significant increase in the recovery of cobalt or lithium. As described by SAKUITUNG et al [21], the leaching process of both metals from the active substance is an endothermic reaction. LEE and RHEE [18] reported that the apparent activation energies of Co and Li calculated from an Arrhenius plot were 12.5 and 47.73 J/mol, respectively. Both the chemical reaction rate and the ion transfer rate are significantly affected by the temperature.

Fig. 2 Effects of leaching temperature and leaching time on leaching of LiCoO2 (c(H2SO4)=2 mol/L, φ(H2O2)=2%, S:L=33 g/L and agitation speed=300 r/min): (a) Leaching efficiency of Co; (b) Leaching efficiency of Li
3.2 Precipitation of CoC2O4
After filtration of the leaching solution to remove impurities, CoC2O4?2H2O was precipitated by adding ammonium oxalate in the filtrate. The precipitating process could be expressed by:
CoSO4(aq)+(NH4)2C2O4(aq)→
CoC2O4(s)↓+(NH4)2SO4(aq) (3)
The effects of temperature, pH and the ammonium oxalate concentration on the precipitation efficiency of cobalt were investigated. The experiment results are illustrated in Fig. 3.
Figure 3(a) indicates that the precipitation process is significantly affected by temperature. The recovery rate of Co increased with temperature (up to 50 ℃) in the beginning and then decreased. This phenomenon was caused by the relationship between the formation of CoC2O4 and its dissolution with temperature. Because the precipitation of CoC2O4 is an endothermic process, the increase in temperature facilitated the formation of CoC2O4, resulting in the increase in the recovery rate of Co. On the contrary, the increase of temperature also made for the dissolution of precipitated cobalt oxalate, causing the decrease of the Co-recovery rate. When the temperature was low (less than 50 ℃), the precipitation of CoC2O4 dominated the reaction depicted by Eq. (3). Therefore, with the increase in temperature from 20 ℃ to 50 ℃, the recovery rate of Co increased from 74.73% to a maximum of 98.85%. With further increasing the reaction temperature (higher than 50 ℃), the dissolution of precipitated cobalt oxalate eventually becomes the prevailing factor, causing the decrease in the recovery rate of Co at a higher reaction temperature.
From Fig. 3(b), the production of CoC2O4 also presented the trend of first increase and then decrease with the equilibrium pH. Two different mechanisms can be used to explain the variation of recovery rate of Co with the equilibrium pH. When the pH value was low (less than 2), the increase in equilibrium pH is beneficial to the precipitation of CoC2O4. However, with increasing the concentration of CoC2O4, ammonium and Co2+ can easily combine to form Co(NH3)62+, resulting in the decrease of CoC2O4. With the continuous increase of pH (higher than 2), the combination of ammonium and Co2+ gradually became the prevailing factor, causing the decrease in the net content of CoC2O4.
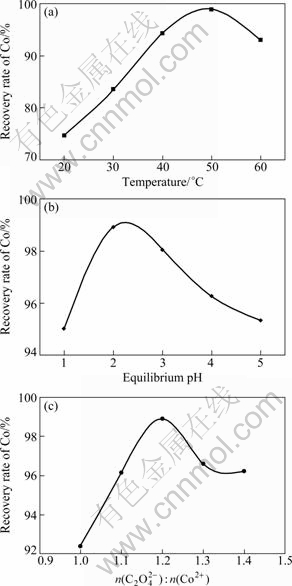
Fig. 3 Variation of recovery rate of Co with factors: (a) Temperature (pH=2, agitation speed 300 r/min and 1 h); (b) Equilibrium pH (50 ℃, agitation speed 300 r/min and 1 h); (c) Ammonium oxalate concentration (pH 2, agitation speed 300 r/min, 50 ℃ and 1 h)
From Fig. 3(c), the recovery rate of Co increased from 92.39% to 98.91% with the increase in molar ratio of ammonium oxalate to Co2+ from 1.0:10 to 1.2:1.0; while, the recovery rate of Co decreased from 98.91% to 96.24% with the increase in molar ratio of ammonium oxalate to Co2+ from 1.2:1.0 to 1.4:1.0. The above results could be due to two reasons: the common ion effect and the ion-interactions. Due to the common ion effect, the solubility product of oxalate ion and cobalt ion is constant. Therefore, with the increase of the ammonium oxalate concentration, the oxalate ions increased, leading to the decrease of cobalt ions in the aqueous phase. As a result, the cobalt ions in the solid species increased. Whereas too excessive use of ammonium oxalate (more than 1.2:1.0 molar ratio of ammonium oxalate to Co2+), could enhance ion-interactions, making the opportunity for formation of the CoC2O4 molecules reduce.
Based on the experiment results above, the optimum condition of recovering CoC2O4 is determined as follows: the optimum molar ratio of ammonium oxalate to Co2+ is 1.2:1.0, equilibrium pH is 2, temperature is 50 ℃, reaction time is 1 h, and agitation speed is 300 r/min. The XRD pattern of cobalt oxalate powder is shown in Fig. 4. Table 1 lists the composition of the product. It is shown that about 94.7% cobalt was deposited as oxalate with less than 0.32% impurities. Lithium was not detected in samples.

Fig. 4 Photo (a) and XRD pattern (b) of products of CoC2O4·2H2O obtained by sedimentation
Table 1 Composition analysis of CoC2O4 samples (mass fraction, %)
![]()
3.3 Precipitation of Li2CO3
After recovering CoC2O4, the precipitate of lithium carbonate was formed by adding an excessive amount of sodium carbonate in the left filtrate. The reaction in this system is shown as follows:
2Li+Na2CO3→Li2CO3↓+2Na+ (4)
Because the content of Li+ in leaching solution was much low, the left filtrate after recovering CoC2O4 was condensed in order to precipitate Li2CO3 as much as possible by adding sodium carbonate in the solution. Figure 5(a) shows the relationship between Li-recovery rate and the concentration of Li+ in the condensed solution. From Fig. 5, it can be noted that with the increase in Li+ concentration, Li-recovery rate increased markedly at the beginning. When Li+ concentration was raised to 20 g/L, the Li-recovery no longer increased significantly. So, in the experiment to investigate the influence of temperature, equilibrium pH and the amount of sodium carbonate on the precipitation efficiency of lithium, the Li+ concentration was maintained at 20 g/L.
Similar to the formation of CoC2O4, the precipitation of Li2CO3 is also an endothermic reaction that increasing the temperature is beneficial to obtain Li2CO3 and also in favor of the dissolution of precipitated Li2CO3 in the solution. Figure 5(b) shows that at the condition of temperature lower than 50 ℃, the precipitation of Li2CO3 dominated the reaction depicted by Eq. (4), resulting in a significant increase in the Li-recovery rate with the temperature. When the reaction temperature was raised to 50 ℃, the dissolution of precipitated Li2CO3 turned to be in equilibrium with the formation of Li2CO3 that the recovery rate of Li showed almost no change with the increase of temperature.
Because CO32- can easily combine with H+ to form HCO3-, reduction of H+ is beneficial to the precipitation of Li2CO3, i.e., the Li-recovery rate increased with the increase in equilibrium pH (see Fig. 5(c)). Meanwhile, increasing Li2CO3 precipitated in solution ever enhanced its dissolution. Therefore, when the equilibrium pH was increased to a certain value (about pH 10 in Fig. 5(c)), the precipitation of Li2CO3 turned to be equilibrious with dissolution of it. Consequently the Li- recovery rate did not increase significantly with the pH.
Taking the common ion effect into consideration, excess sodium carbonate sedimentation should be added to precipitate the lithium carbonate. Figure 5(d) shows that the Li-recovery rate increased from 72.76% to 80.61% with molar ratio of sodium carbonate to Li+ increased from 1.0:1.0 to 1.1:1.0. With a further increase in molar ratio of sodium carbonate to Li+ from 1.1:1.0 to 1.4:1.0, the Li recovery efficiency did not increase markedly.
From the description above, the optimum condition of recovering Li2CO3 is as follows: the molar ratio of sodium carbonate to Li+ is 1.1:1.0, equilibrium pH is 10, temperature is 50 ℃, lithium-ion concentration is 20 g/L, reaction time is 1 h, and agitation speed is 300 r/min. The crystalline Li2CO3 phase is clearly identified by XRD analysis shown in Fig. 6. The experimental results showed that around 71.0% of lithium was deposited as carbonate with less than 0.52% impurities. Cobalt was not detected in samples.

Fig. 5 Factors influencing recovery rate of Li: (a) lithium-ion concentration (pH 10, agitation speed 300 r/min, 50 ℃ and 1 h); (b) Temperature (pH 10, agitation speed 300 r/min and 1 h); (c) Equilibrium pH (agitation speed 300 r/min, 50 ℃ and 1 h); (d) Sodium carbonate concentration (pH 10, agitation speed 300 r/min, 50 ℃ and 1 h)
4 Conclusions
1) The experimental results show that solution concentration, temperature and leaching time have marked influence on the efficiency of dissolving the active substances, and the solution temperature, pH value and the amount of precipitant added significantly affect the precipitation of Co and Li products.
2) Within the range of experiment conditions performed, 96.3% of Co and 87.5% of Li can be leached using a 2 mol/L H2SO4 with 2.0% H2O2, 33 g/L S:L ratio, 2 h leaching time and temperature of 60 ℃.
3) The pearl colored CoC2O4?2H2O powder is precipitated using ammonium oxalate with a 1.2:1.0 molar ratio of ammonium oxalate to Co2+, an initial pH of 2, a temperature of 50 ℃ for 1 h. In the chemical precipitation process, more than 94.7% of Co element was recovered from the leach liquor.

Fig. 6 Photo (a) and XRD pattern (b) of products of Li2CO3 obtained by sedimentation
4) After collection of the CoC2O4?2H2O product by filtration, the Li2CO3 precipitates are obtained by addition of Na2CO3 in the left filtrate when the molar ratio of sodium carbonate to Li+ is 1.1:1.0, equilibrium pH is 10, temperature is 50 ℃, lithium-ion concentration is 20 g/L, reaction time is 1 h, and agitation speed is 300 r/min. By this process, 71.0% of Li can be recovered in the form of Li2CO3.
References
[1] BROUSSELY M, ARCHDALE G. Li-ion batteries and portable power source prospects for the next 5-10 years [J]. J Power Sources, 2004, 136(2): 386-394.
[2] HOWARD W F, SPOTNITZ R M. Theoretical evaluation of high-energy lithium metal phosphate cathode materials in Li-ion batteries [J]. J Power Sources, 2007, 165(2): 887-891.
[3] LI J H, SHI P X, WANG Z F, CHEN Y, CHANG C C. A combined recovery process of metals in spent lithium-ion batteries [J]. Chemosphere, 2009, 77(8): 1132-1136.
[4] DAI Yong-nian, YANG Bin, YAO Yao-chun, MA Wen-hui, LI Wei-hong. Development status of Li-ion batteries [J]. Battery Bimonthly, 2005, 35(3): 193-195. (in Chinese)
[5] SHIN S M, KIM N H, SOHN J S, YANG D H, KIM Y H. Development of a metal recovery process from Li-ion battery wastes [J]. Hydrometallurgy, 2005, 79(3-4): 172-181.
[6] LI J G, ZHAO R S, HE X M, LIU H C. Preparation of LiCoO2 cathode materials from spent lithium-ion batteries [J]. Ionics, 2009, 15: 111-113.
[7] LEE C K, RHEE K I. Preparation of LiCoO2 from spent lithium-ion batteries [J]. J Power Sources, 2002, 109(1): 17-21.
[8] KIM D S, SOHN J S, LEE C K, LEE J H, HAN K S, LEE Y I. Simultaneous separation and renovation of lithium cobalt oxide from the cathode of spent lithium ion rechargeable batteries [J]. J Power Sources, 2004, 132(1-2): 145-149.
[9] LIU Yun-jian, HU Qi-yang, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun. Recycle and synthesis of LiCoO2 from incisors bound of Li-ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 956-959.
[10] BAHGAT M, FARGHALY F E, ABDEL BASIR S M, FOUAD O A. Synthesis, characterization and magnetic properties of microcrystalline lithium cobalt ferrite from spent lithium-ion batteries [J]. J Mater Process Technol, 2007, 183(1): 117-121.
[11] FOUADA O A, FARGHALYA F I, BAHGAT M. A novel approach for synthesis of nanocrystalline γ-LiAlO2 from spent lithium-ion batteries [J]. J Anal Appl Pyrolysis, 2007, 78(1): 65-69.
[12] PAULINO J F, BUSNARDO N G, AFONSO J C. Recovery of valuable elements from spent Li-batteries [J]. J Hazard Mater, 2008, 150(3): 843-849.
[13] ZHANG Q W, LU J F, SAITO F, NAGATA C, ITO Y. Room temperature acid extraction of Co from LiCo0.2Ni0.8O2 scrap by a mechanochemical treatment [J]. Advanced Powder Technol, 2000, 11(3): 353-359.
[14] CONTESTABILE M, PANERO S, SCROSATI B. A laboratory-scale lithium-ion battery recycling process [J]. J Power Sources, 2001, 92(1-2): 65-69.
[15] FR?HLICH S, SEWING D. The BATENUS process for recycling mixed battery waste [J]. J Power Sources, 1995, 57(1-2): 27-30.
[16] ZHANG P W, YOKOYAMA T, ITABASHI O, WAKUI Y, SUZUKI T M, INOUE K. Hydrometallurgical process for recovery of metal values from spent nickel metal hydride secondary batteries [J]. Hydrometallurgy, 1998, 50(1): 61-75.
[17] ZHANG P W, YOKOYAMA T, ITABASHI O, SUZUKI T M, INOUE K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries [J]. Hydrometallurgy, 1998, 47(2-3): 259-271.
[18] LEE C K, RHEE K I. Reductive leaching of cathodic active materials from lithium ion battery wastes [J]. Hydrometallurgy, 2003, 68(1-3): 5-10.
[19] NAN J M, HAN D M, ZUO X X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction [J]. J Power Sources, 2005, 152(1): 278-284.
[20] RA D I, HAN K S. Used lithium ion rechargeable battery recycling using Etoile-Rebatt technology [J]. J Power Sources, 2006, 163(1): 284-288.
[21] SAKUITUNG S, PRUKSATHORN K, HUNSOM M. Simultaneous recovery of valuable metals from spent mobile phone battery by an acid leaching process [J]. Korean J Chem Eng, 2007, 24(2): 272-277.
[22] SWAIN B, JEONG J, LEE J C, LEE G H, SOHN J S. Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries [J]. J Power Sources, 2007, 167(2): 536-544.
[23] SWAIN B, JEONG J, LEE J C, LEE G H. Development of process flow sheet for recovery of high pure cobalt from sulfate leach liquor of LIB industry waste: A mathematical model correlation to predict optimum operational conditions [J]. Sep Purif Technol, 2008, 63(2): 360-369.
[24] WANG R C, LIN Y C, WU S H. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries [J]. Hydrometallurgy, 2009, 99(3-4): 194-201.
[25] FERREIRA D A, PRADOS L M Z, MAJUSTE D, MANSUR M B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries [J]. J Power Sources, 2009, 187(1): 238-246.
[26] MISHRA D, KIM D, RALPH D E, AHN J G, RHEE Y H. Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans [J]. Waste Manage, 2008, 28(2): 333-338.
[27] XIN B P, ZHANG D, ZHANG X, XIA Y T, WU F, CHEN S, LI L. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria [J]. Bioresour Technol, 2009, 100(24): 6163-6169.
[28] LAIN M J. Recycling of lithium ion cells and batteries [J]. J Power Sources, 2001, 97-98: 736-738.
[29] MANTTUANO D P, DORELLA G, ELIAS R C A, MANSUR M B. Analysis of a hydrometallurgical route to recover base metals from spent rechargeable batteries by liquid-liquid extraction with Cyanex 272 [J]. J Power Sources, 2006, 159(2): 1510-1518.
[30] NAN J M, HAN D M, YANG M J, CUI M, HOU X L. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries [J]. Hydrometallurgy, 2006, 84(1-2): 75-80.
[31] SWAIN B, JEONG J, LEE J C, LEE G H. Separation of cobalt and lithium from mixed sulphate solution using Na-Cyanex 272 [J]. Hydrometallurgy, 2006, 84(3-4): 130-138.
[32] CASTILLO S, ANSART F, LABERTY-ROBERT C, PORTAL J. Advances in the recovering of spent lithium battery compounds [J]. J Power Sources, 2002, 112(1): 247-254.
[33] ESPINOSA D C R, BERNARDES A M, TENORIO J A S. An overview on the current processes for the recycling of batteries [J]. J Power Sources, 2004, 135(1-2): 311-319.
[34] FREITAS M B J G, GARCIA E M. Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries [J]. J Power Sources, 2007, 171(2): 953-959.
[35] GARCIA E M, SANTOS J S, PEREIRA E C, FREITAS M B J G. Electrodeposition of cobalt from spent Li-ion battery cathodes by the electrochemistry quartz crystal microbalance technique [J]. J Power Sources, 2008, 185(1): 549-553.
[36] SUSLICK K S. Sonochemistry [J]. Science, 1990, 247: 1439-1445.
[37] LUO X F, WANG X Y, LIAO L, GAMBOA S, SEBASTIAN P J. Synthesis and characterization of high tap-density layered Li[Ni1/3Co1/3Mn1/3]O2 cathode material via hydroxide co-precipitation [J]. Journal of Power Sources, 2006, 158(1): 654-658.
朱曙光1, 2,贺文智1,李光明1,周 旭1,张骁君1,黄菊文1
1. 同济大学 环境科学与工程学院,污染控制与资源化研究国家重点实验室,上海 200092;
2. 安徽建筑工业学院 环境与能源工程学院,合肥 230022
摘 要:基于废锂离子电池中的金属在不同价态时的化学行为差异,通过化学精制从废弃锂离子电池中获取高附加值的钴锂化学产品。将从正极中分离出来的活性物质溶解在硫酸和过氧化氢溶液中,加入(NH4)2C2O4生成CoC2O4?2H2O沉淀。在获得CoC2O4?2H2O后,加入Na2CO3获得Li2CO3沉淀。实验结果表明:96.3% 的Co和87.5% 的Li溶解在2 mol/L H2SO4和2.0% H2O2(体积分数)溶液中,并且有94.7 % 的Co和71.0%的Li 以CoC2O4?2H2O和Li2CO3的形式沉淀下来。
关键词:废锂离子电池;回收;浸出;沉淀
(Edited by YANG Hua)
Foundation item: Project (51078286) supported by the National Natural Science Foundation of China; Project (2008BAC46B02) supported by the National Key Technologies R&D Program of China; Project (2011SQRL110) supported by the Excellent Youth Foundation of Anhui Education Department, China; Project (KJ2011z053) supported by the Natural Science Foundation of Anhui Education Department, China
Corresponding author: HE Wen-zhi; Tel: +86-21-65989215; E-mail: hithwz@163.com
DOI: 10.1016/S1003-6326(11)61460-X

