
Surface pretreatment of Mg alloys prior to Al electroplating in TMPAC-AlCl3 ionic liquids
LIU Quan, LIU Kui-ren, HAN Qing, TU Gan-feng
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 30 October 2010; accepted 27 May 2011
Abstract: It is difficult to directly electroplate Al on Mg alloys. The effects of pretreatment parameters on the corrosion resistance of films obtained on AZ31 Mg alloy surface were studied by using potentiodynamic polarization curves, to produce a compact interfacial layer as zinc-immersion deposition. After the substrate was pretreated under optimized conditions, aluminum was electrodeposited on AZ31 from TMPAC-AlCl3 room temperature ionic liquids. The depositions were characterized by scanning electron microscope equipped with energy dispersion X-ray. The results show that the traditional pretreatment of Mg alloys was successfully used for the Al-electroplating process from TMPAC-AlCl3 ionic liquids. The entire procedure includes alkaline cleaning, chemical pickling, surface activation (400 mL/L HF acid, 10 min), zinc-immersion (20 min) and anhydrous treatment. A relatively compact zinc-immersion film was prepared on the substrate surface. A silvery-colored satin aluminum deposition was obtained on AZ31 from TMPAC-AlCl3 using direct current plating.
Key words: magnesium alloys; electroplating; pretreatment procedure; aluminum; ionic liquids
1 Introduction
Magnesium and its alloys as light metal (1.7 g/cm2) with so many merits have been applied in plane, automobile and electric industries [1]. However, the material is generally known to have a poor corrosion resistance [2]. The current surface treatment methods of Mg alloy include chemical conversion film, anodic oxidation, organic coating, surface modification and metal deposition and so on. The metal plating has a low production value, and its operation temperature is room temperature. Aluminum possesses a good anti-corrosion property and excellent mechanical property. The electroplating of Al on Mg alloy would not only prevent substrate from corrosion, but also not markedly increase its mass [3]. Magnesium and its alloys, wherever possible, are extremely easy to be oxidated under the existence of oxygen. To the authors’ experience, it is difficult to directly electroplate Al on Mg alloys. Therefore, the Al-electroplating process needs a compact intermediate layer deposited on Mg alloys. The surface activation and zinc immersion are two kinds of the traditional surface treatments used for Mg alloys. If they were used for the entire Al-electroplating pretreatment process, it will have important practice significance. Even though the similar pretreatments have been used for the process [4-5], their processing technology or technical formula is more or less different from the present work.
The electrodeposition of Al from anhydrous electrolytes has been studied widely on various kinds of metal substrates [6]. It is hard to electroplate Al on magnesium alloys owing to their high chemical reactivity. The first report on the subject was published by CHANG et al in 2007 [3]. CHANG et al [3, 7] and ZHANG et al [8] plated aluminum and its alloys on Mg alloys from anhydrous electrolytes, such as [EMIm]Cl-AlCl3 and AlCl3-NaCl-KCl-MnCl2. Those work exhibited an important significance to exploit a novel surface treatment technology used for Mg alloys. The TMPAC-AlCl3 ionic liquids (ILs) have a low melting point of -75 °C as x(AlCl3)=66.7%, good electro- chemical and thermal stability [9], and a relatively cheap price [10]. Its electric conductivity is low [11], but can be improved by the addition of benzene [12]. The electrodeposition of Al from TMPAC-AlCl3 was achieved on the W [10, 13, 14] and Al [13] substrate.
To our knowledge, less report was published on the electroplating of Al on the Mg alloy substrate from TMPAC-AlCl3 ionic liquids.
In the present work, both activation films and zinc-immersion depositions were prepared under different HF acid concentration, activation time and zinc-immersion time. The corrosion resistances were measured by potentiodynamic polarization curves. The AZ31 magnesium alloy was treated by the optimized surface pretreatment. And then aluminum was electroplated on AZ31 from trimethyl-phenyl- ammonium chloride and anhydrous aluminum chloride (TMPAC-AlCl3) quaternary ammonium room temperature ionic liquids mixed with benzene by direct current plating. The Al depositions were characterized.
2 Experimental
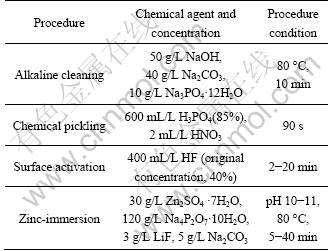
The trimethyl-phenyl-ammonium (TMPAC) of technical grade quality (>98%) was dried under vacuum at 100 °C for 24 h. All other reagents were of analytical grade. The acidic ionic liquid with the molar ratio of 2:1 was prepared by the slow addition of AlCl3 to TMPAC at room temperature under a continuous magnetic bar stirring (the details were showed in Ref. [10]). The cathodes with dimensions of 40 mm?15 mm?3 mm were made of AZ31 magnesium alloys. The AZ31 samples were pretreated by the wet paper polishing, alkaline cleaning, chemical pickling, HF acid surface activation and zinc-immersion. And then they were fully cleaned with ethanol and toluene. Table 1 shows the details of the pretreatment procedure of magnesium alloy. The pure Al sheets with a purity of 99.9% used as anodes (20 mm? 20 mm?1 mm) were cleaned with acetone, fully rinsed with water, and then pickled in the 1 mol/L NaOH solution at 80 °C. Eventually, both the cathode and anode were immediately rinsed with ethanol and toluene, respectively, before they were stored in a flask of toluene. In the ionic liquids with a benzene content of 50% (volume fraction), the Al plating was performed at 40-42 °C under an agitating speed of 200 r/min.
Table 1 Formulations and operation conditions of magnesium alloy pretreatment procedure
The morphology and components of cross-section or surface of depositions were examined on a scanning electron microscope equipped with an energy dispersion X-ray spectrometer (SEM-EDX, SSX-550, SHIMAZDU, Japan). The corrosion-resistances of samples were investigated using potentiodynamic polarization measurements in NaCl solution. A parallel team included three samples under the same conditions. All the measurements were performed in an ordinary three-electrode cell filled with 3.5% NaCl solution (mass fraction) at room temperature with an electrochemical workstation IM6e (ZAHNER, Germany). The working electrode was the films or depositions with an exposed surface area of 1 cm2. The remainder was sealed with ethoxyline resin. The counter electrode and reference electrode were a platinum sheet and a saturated calomel electrode (SCE), respectively. The polarization resistance (Rp) was calculated according to the polarization curves. The mean value and standard error of the parallel team were calculated.
3 Results and discussion
3.1 Surface pretreatment
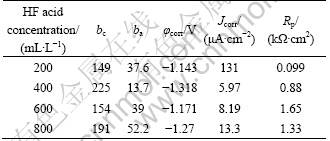
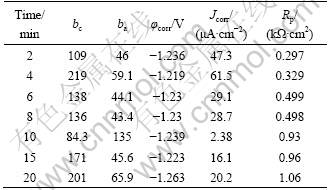
It is necessary to achieve a compact activation film in the previous step of zinc immersion. The samples were obtained after reaction time of 10 min in HF activation solution with different concentrations. Their corrosion-resistant properties were tested by polarization curves in a 3.5% (mass fraction) NaCl solution. Figure 1 shows the polarization curves and the relationship curves between HF acid concentration and polarization resistance of activation films. The Rp of activation films increases with the increase of HF concentration. Table 2 shows the relative data of polarization curves from Fig. 1. From Fig. 1 and Table 2, as c(HF)=200 mL/L, the obtained activation film has a low Rp and a high corrosion current density (Jcorr). As c(HF)=400 mL/L, Rp rises and Jcorr decreases. It indicates that the activation film prepared c(HF)=400 mL/L has a more compact surface than the one at c(HF)=200 mL/L. With the increase of c(HF) from 400 mL/L to 800 mL/L, the Jcorr slowly increases, and the mean Rp value has no significant increase considering the standard error (see Fig. 1(b)). In the present work, the HF volume concentration in activation solution was defined as 400 mL/L.
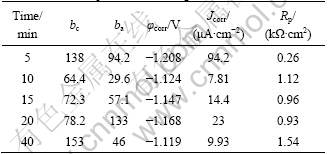
The samples were obtained after different reaction time (t) in 400 mL/L HF activation solution. Figure 2 shows the polarization curves and the relationship curves between reaction time and polarization resistance of activation films. Table 3 shows the relative data of polarization curves from Fig. 2. The Rp of activation films rapidly increases with the prolonged time t. After 10 min, Rp increases slowly. Considering the standard error of the mean Rp value, there is no significant difference for the samples’ resistance beyond 10 min (see Fig. 2(b)). In the present work, the activation time was defined as 10 min in the 400 mL/L HF acid solution.
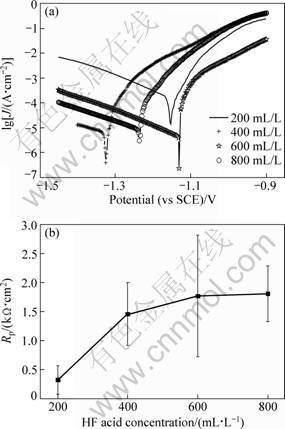
Fig. 1 Polarization curves (a) and relationship curve (b) between HF acid concentration and polarization impedance of acid activation films
Table 2 Relative parameters on polarization curves of activation films from Fig. 1
The samples were obtained after different reaction time (t) in the zinc-immersion solution. Figure 3 shows the polarization curves and the relationship curves between reaction time and polarization resistance of zinc-immersion depositions. Table 4 shows the relative data of polarization curves obtained from Fig. 3. The R of zinc depositions rapidly increases with the reaction time t. After 10 min, Rp increases slowly. Considering the standard error, Rp is of no significant difference when t is longer than 10 min (see Fig. 3). As t=20 min, the zinc-immersion process has a good repetition owing to a narrow distribution of the mean Rp value and its standard error. It is steadier than the samples prepared below 20 min and at 40 min. In short, it is sufficient to obtain a compact zinc deposition beyond 10 min. In the present work, the zinc-immersion time was defined as 20 min.
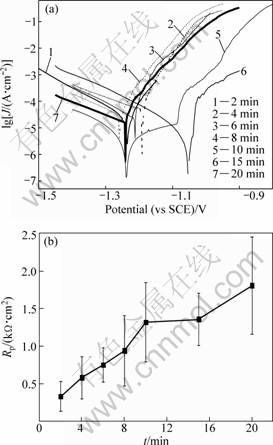
Fig. 2 Polarization curves (a) and relationship curve (b) between reaction time and polarization resistance of HF activation films
Table 3 Relative parameters on polarization curves of activation films from Fig. 2
The zinc deposition was prepared after 20 min on AZ31 substrate. The SEM image of its surface and the backscattered electron image of its cross-section are shown in Fig. 4. The zinc layer is porous. The layer is lighter than Mg substrate due to the higher atomic number of zinc than that of magnesium in the backscattered electron image (see Fig. 4(b)). The white-colored zinc-immersion film has a thickness of 1 μm and exists on the AZ31 substrate surface.
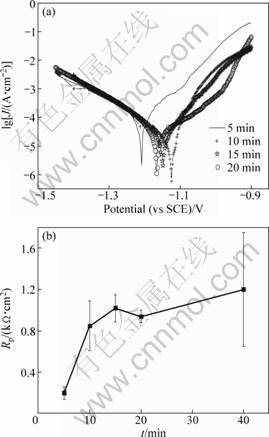
Fig. 3 Polarization curves (a) and relationship curves (b) between reaction time and polarization impedance of zinc-immersion
Table 4 Relative parameters on polarization curves of zinc-immersion depositions from Fig. 3
3.2 Electrodeposition of Al
The silvery-colored satin Al layer was constant- current electrodeposited on AZ31 with a zinc deposition from the TMPAC-AlCl3 ionic liquids mixed with benzene with a volume content of 50%. The depositions were prepared at 5.8 mA/cm2 for 60 min, at 12.3 mA/cm2 for 60 min, and at 12.3 mA/cm2 for 120 min, respectively. Their SEM images and EDX analysis are shown in Fig. 5. The Al layer has a bulk grain microstructure prepared at a low current density (J=5.8 mA/cm2) (Fig. 5(a)). Nevertheless, it has a spherical grain structure at relatively high current density (J=12.3 mA/cm2), as shown in Fig. 5(b). In Fig. 5(a), there are many pinholes in Al layer, which results in the less compact surface. The Al deposition with spherical equiaxed grains is more compact than that with bulk grains. It is quite different for the microstructures obtained at high and low current. The nucleation rate of a new grain increases more rapidly than its growing rate as the current density rises. At a relatively high current density, the nucleation rate controls the growth of Al deposition. It promotes the formation of the equiaxed crystal grains with spherical shape [15]. Thanks to no pore and crack, the deposition (Fig. 5(b)) is more smooth and compact than that shown in Fig. 5(a). The grains grow fully and their size increases with the rising of deposition time (see Figs. 5(b) and (c)). The EDX analysis of this layer (Fig. 5(b)) shows that the as-deposited layer consists of Al (Fig. 5(d)).

Fig. 4 SEM images of zinc-immersion film (a) obtained after 20 min and its cross-section (b)
The deposition was prepared at 12.3 mA/cm2 for 120 min on AZ31 substrate. The SEM image and linear composition analysis of its cross-section are shown in Fig. 6. The Al layer is uniformly deposited on the substrate (Fig. 6(a)). A close bonding e bonding among Al, zinc and the substrate is beneficial to protect the AZ31 Mg alloy substrate. According to the black arrow direction in Fig. 6(a), the linear analysis was obtained (Fig. 6(b)). It indicates that the Al layer has a thickness of about 13 μm. A white-colored zinc-immersion layer exists between the Al deposition and Mg alloy substrate.
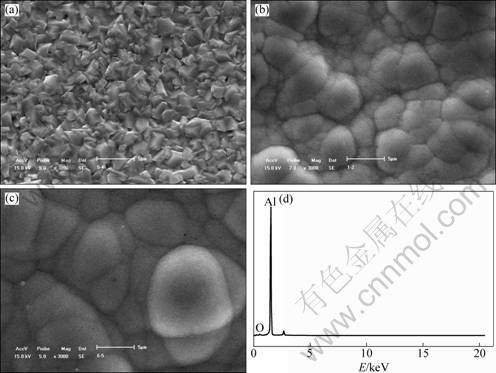
Fig. 5 SEM images of as-deposited Al depositions on AZ31 magnesium alloy surface obtained at 5.8 mA/cm2 for 60 min (a), 12.3 mA/cm2 for 60 min (b) and 12.3 mA/cm2 for 120 min (c) and EDX spectrum (d)
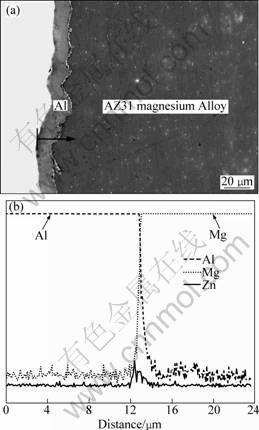
Fig. 6 SEM image (a) and linear composition distribution (b) of as-deposited Al deposition cross-section on AZ31 obtained at 12.3 mA/cm2 for 120 min
4 Conclusions
1) A compact activation film on AZ31 Mg alloy surface was obtained within 10 min in 400 mL/L HF acid solution. Then, a relatively compact zinc film is prepared after immersion for 20 min. Those optimized pretreatments were successfully used for Al-electroplating process from TMPAC-AlCl3 ionic liquid.
2) A compact silvery-colored satin Al layer with spherical-shape equiaxed grains was electroplated at constant current 12.3 mA/cm2 from TMPAC-AlCl3 ionic liquid with 50% content benzene.
References
[1] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—A critical review [J]. J Alloys Compd, 2002, 336: 88-113.
[2] LIU L J, SCHLESINGER M. Corrosion of magnesium and its alloys [J]. Corros Sci, 2009, 51: 1733-1737.
[3] CHANG J K, CHEN S Y, TSAI W T, DENG M J, SUN I W. Electrodeposition of aluminum on magnesium alloy in aluminum chloride (AlCl3)-1-ethyl-3-methylimidazolium chloride (EMIC) ionic liquid and its corrosion behavior [J]. Electrochem Commun, 2007, 9: 1602-1606.
[4] QIAN Hong-mei, LI Yan, LING Guo-ping. Influence of acid pickling on electrodeposition of aluminum on magnesium alloy in room temperature molten salts [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(5): 854-860. (in Chinese)
[5] LIU F Q, YANG S, SHAN P. Pretreatment process and electroplating on surface of magnesium alloy [J]. Special Cast and Nonferrous Alloys, 2009 , 29(9): 851-853. (in Chinese)
[6] SIMKA W, PUSZCZYK D, NAWRAT G. Electrodeposition of metals from non-aqueous solutions [J]. Electrochim Acta, 2009, 54: 5307-5319.
[7] CHANG J K, CHEN S Y, TSAI W T, DENG M J, SUN I W. Improved corrosion resistance of magnesium alloy with a surface aluminum coating electrodeposited in ionic liquid [J]. J Electrochem Soc, 2008, 155: C112-C116.
[8] ZHANG J F, YAN C W, WANG F H. Electrodeposition of Al-Mn alloy on AZ31B magnesium alloy in molten salts [J]. Appl Surf Sci, 2009, 255: 4926-4932.
[9] ZHAO Y, VANDERNOOT T J. Review: Electrodeposition of aluminium from nonaqueous organic electrolytic systems and room temperature molten salts [J]. Electrochim Acta, 1997, 42: 3-13.
[10] ZHAO Y, VANDERNOOT T J. Electrodeposition of aluminium from room temperature AlCl3-TMPAC molten salts [J]. Electrochim Acta, 1997, 42: 1639-1643.
[11] PAPAGEORGIOU N, EMMENEGGER F P. The effect of cosolvents and additives on the electrochemical properties of [(Me)3PhN][Al2Cl7] melts [J]. Electrochim Acta, 1993, 38: 245-252.
[12] MOY R, EMMENEGGER F P. Co-cosolvents for chloroaluminate electrolytes [J]. Electrochim Acta, 1992, 37: 1061-1068.
[13] JIANG T, CHOLLIER BRYM M J, DUBE G, LASIA A, BRISARD G M. Electrodeposition of aluminium from ionic liquids: Part Ⅱ— Studies on the electrodeposition of aluminum from aluminum chloride (AlCl3)-trimethylphenylammonium chloride (TMPAC) ionic liquids [J]. Surf Coat Technol, 2006, 201: 10-18.
[14] MOFFAT T P. Electrodeposition of Al-Cr metallic glass [J]. J Electrochem Soc, 1994, 141: L115-117.
[15] PAUNOVIC M, SCHLESINGER M. Fundamentals of electrochemical deposition [M]. 2nd ed. New York: John Wiley & Sons, Inc., 2006: 276.
AZ31镁合金离子液体镀铝前处理
柳 泉,刘奎仁,韩 庆,涂赣峰
东北大学 材料与冶金学院,沈阳 110004
摘 要:通过优化镁合金活化及浸锌前处理工艺,在前处理后的镁合金AZ31表面上实施电镀铝。采用SEM/EDX以及极化曲线等手段对镁合金表面上的膜层或镀层进行表征。结果表明,可用于离子液体电镀铝的镁合金前处理工序为碱洗、酸洗、HF活化(400 mL/L 40% HF,10 min)、浸锌(20 min)、除水工序。通过该工序,可在镁合金表面上获得致密的浸锌层。采用TMPAC-AlCl3离子液体直流电镀铝,可获得致密的银白色电镀铝层。
关键词:镁合金;电镀铝;前处理;浸锌;离子液体
(Edited by YUAN Sai-qian)
Corresponding author: LIU Kui-ren; Tel/Fax: +86-24-83686997; E-mail: liukr@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)60981-3