DOI: 10.11817/j.ysxb.1004.0609.2021-40030
二价钛离子在NaCl-KCl熔盐中的活度分析
李经依1,李少龙2,车玉思2,宋建勋2,何季麟1, 2
(1. 郑州大学 化工学院,郑州 450001;
2. 郑州大学 材料科学与工程学院,河南省资源与材料工业技术研究院,郑州 450001)
摘 要:熔盐电化学工艺是提取与提纯金属钛最具前景的方法。钛离子活度的确定对研究钛的熔盐电解机制具有重要意义。因此,为得到熔盐中二价钛离子浓度与其活度的关系,本文采用开路电位法在三电极体系下测定了含定量钛离子及饱和金属钛的NaCl-KCl共晶盐中的离子平衡电位,并由Nernst方程计算得出二价钛离子的活度,进而获得了对应的活度因子。结果表明:在1023 K下,当熔盐中二价钛离子摩尔分数介于0.296%~2.378%时,其活度值单调地介于0.00205~0.01877,且其活度因子为0.693~0.998。本研究可为精确确定熔盐中钛离子活度及进一步研究钛离子歧化反应的平衡常数提供重要基础。
关键词:二价钛离子;熔盐;活度;开路电位法
文章编号:1004-0609(2021)-08-2210-08 中图分类号:TG146.2 文献标志码:A
引文格式:李经依, 李少龙, 车玉思, 等. 二价钛离子在NaCl-KCl熔盐中的活度分析[J]. 中国有色金属学报, 2021, 31(8): 2210-2217. DOI: 10.11817/j.ysxb.1004.0609.2021-40030
LI Jing-yi, LI Shao-long, CHE Yu-si, et al. Activity analysis of divalent titanium in NaCl-KCl molten salt[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(8): 2210-2217. DOI: 10.11817/j.ysxb.1004.0609.2021-40030
金属钛具有优异的物理化学性质,如高熔点、高强度、抗腐蚀、耐高温、形状记忆等,因而被广泛应用于化工、航空航天、生物医疗、国防科技及汽车等领域[1-6]。目前,工业上主要采用Kroll 法[7]生产金属钛。然而,该方法流程繁琐、设备成本高,间接导致了金属钛价格昂贵,一定程度上限制了钛的应用。熔盐电解工艺被认为是取代Kroll工艺制造金属钛的最具前景的方法[7-8]。基于此,钛离子在熔盐中的电化学性质得到了重视并被广泛研究。
钛离子在熔盐中的离子价态主要为二价、三价和四价。在氯化物熔盐体系中主要发生的钛离子歧化反应为[9]:
3Ti2+  2Ti3++Ti (1)
2Ti3++Ti (1)
研究表明,熔盐中钛离子的歧化反应会影响到钛离子的平均价态,从而影响到钛离子的阳极析出和阴极沉积过程,进而对生产过程中的电流效率及电解产物的品质造成影响[9-10]。另外,含低价钛离子电解质[11]与高纯钛粉[12-13]等的制取过程及金属钛的精炼[14]也都涉及到熔盐中钛离子的歧化反应。因此,研究钛离子在熔盐中的平衡行为具有重要的意义。
钛离子歧化反应(1)的平衡常数表达式如下:
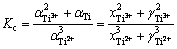 (2)
(2)
式中: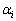 和
和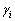 分别为i的活度和活度因子;xi为i的浓度(熔盐中的摩尔分数)。在NaCl-KCl熔盐中,xi的定义如下:
分别为i的活度和活度因子;xi为i的浓度(熔盐中的摩尔分数)。在NaCl-KCl熔盐中,xi的定义如下:
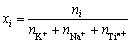 (3)
(3)
本研究团队前期在对平衡常数研究过程中[9-10, 15-18],考虑到熔盐中离子浓度较低的情况下,由Henry定律得出离子的摩尔分数近似等于其活度,因此Kc的表达式可化简为:
 (4)
(4)
但由式(4)计算得出的平衡常数Kc为近似值。如果采用活度计算Kc,得到的结果将更精准,有利于更好地从理论上揭示钛离子行为。基于此,有必要分析钛离子在熔盐中的活度。研究人员先后提出了不同的理论公式和经验公式来计算一定体系内分子或离子的活度,这使得原有活度计算公式的适用范围大大扩展[19-20]。本文采用基于Nernst方程的开路电位法(EMF):
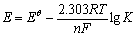 (5)
(5)
JINDAL[21]采用该方法研究了熔盐中二氯化镍的活度;朱吉庆等[22]采用该方法计算得到了氯化镁在三元熔体中的活度及其他热力学参数。然而很少有关于钛离子在熔盐中的活度分析的研究工作。
本文将采用开路电位法研究1023 K下含饱和金属钛的NaCl-KCl熔盐中二价钛离子的活度,并进一步获得相关的热力学参数。研究熔盐中歧化反应平衡时钛离子的活度可为歧化反应平衡常数的精确计算提供基础数据支撑,可进一步在理论上揭示钛离子在熔盐中的电化学行为,为开发和提升钛冶炼工艺提供重要参考。
1 实验
1.1 实验原料
本研究采用的含低价钛离子盐(以下简称“钛盐”)由实验室制取,所含成分为TiCln、NaCl和KCl。使用的高纯钛棒采购于清河县琪睿金属材料有限公司,钛棒的直径为5 mm,纯度>99.99%。实验中所采用的NaCl、KCl、浓盐酸、FeNH4(SO4)2、抗坏血酸、二安替比林甲烷、钛离子标准溶液均为分析纯试剂。
1.2 钛盐中钛离子质量分数测定
为确定加入定量钛盐的熔盐中各价态钛离子的浓度,首先需要对钛盐中各价态钛离子的质量分数进行测定。不同价态的钛离子含量可采用SONG等[15]提出的化学分析方法测定,即分别采用量氢法、滴定法及分光光度法对Ti2+、Ti3+和Ti4+含量进行测定。
取定量浓盐酸与除氧处理后的去离子水混合以配制1 mol/L稀盐酸溶液,倒入图1所示的量氢装置中。待两滴定管中液面平齐后加入定量钛盐,溶液中的Ti2+与H+发生如下反应:
2Ti2++2H+=H2(g)+2Ti3+ (6)
读取反应前后滴定管中液面差,换算后即为反应产生的氢气体积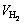 ,继而使用式(7)计算Ti2+质量分数:
,继而使用式(7)计算Ti2+质量分数:
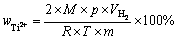 (7)
(7)
式中:M为钛的相对原子质量,M=47.86 g/mol;p为绝对标准大气压,p=1×105 Pa;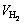 为反应产生的氢气体积,m3;R为摩尔气体常数,R=8.314 J/(mol·K);T为室温,取298 K;m为称取的钛盐的质量,g。
为反应产生的氢气体积,m3;R为摩尔气体常数,R=8.314 J/(mol·K);T为室温,取298 K;m为称取的钛盐的质量,g。
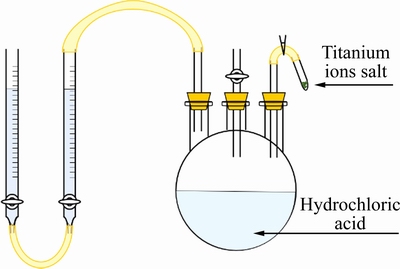
图1 量氢装置示意图
Fig. 1 Schematic diagram of hydrogen measuring device
将量氢反应后的溶液定容于500 mL容量瓶中,分成两份250 mL待测溶液,其中一份用于硫酸高铁铵滴定法测量Ti3+浓度,另一份用于分光光度法测量Ti4+浓度。滴定装置如图2所示。称取定量硫酸高铁铵以配制0.05 mol/L FeNH4(SO4)2标准溶液。向滴定装置中通入高纯Ar气,提供保护气氛。向三口烧瓶中加入2 g硫氰化钾,作为指示剂。待硫氰化钾完全溶解,用0.05 mol/L硫酸高铁铵溶液滴定。
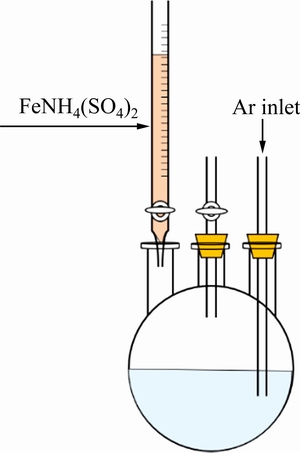
图2 滴定装置示意图
Fig. 2 Schematic diagram of titration device
溶液中的Ti3+与Fe3+发生如下反应:
Ti3++Fe3+=Ti4++Fe2+ (8)
根据硫酸高铁铵溶液消耗量由式(9)计算Ti3+质量分数:
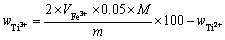 (9)
(9)
式中: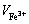 为硫酸高铁铵溶液消耗量,m3。
为硫酸高铁铵溶液消耗量,m3。
利用钛离子标准溶液、10%抗坏血酸溶液(隐蔽剂)和2%二安替比林甲烷溶液(显色剂)配制钛离子浓度分别为0.4、0.8、1.2、1.6和2.0 μg/mL的溶液,以去离子水代替钛离子标准液体配制参比溶液测得388 nm波长的紫外可见光下相应的吸光度(Abs);以钛离子溶液中的钛离子含量为横坐标,对应的吸光度为纵坐标,绘制钛离子浓度测定的标准曲线如图3所示。图中数据的直线拟合方程为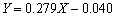 ,其拟合度为0.994,可用于钛离子浓度的测定。
,其拟合度为0.994,可用于钛离子浓度的测定。
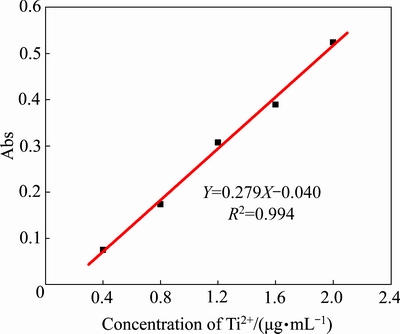
图3 标定中钛离子浓度与吸光度之间的关系
Fig. 3 Relationship between titanium ion concentration and absorbance
为测定待测溶液中钛离子的总浓度,用移液枪准确量取0.5 mL待测液于50 mL容量瓶中,加入1:1盐酸20 mL、10%抗坏血酸溶液1 mL、2%二安替比林甲烷溶液10 mL,用去离子水定容,摇匀静置10 min以配制样品溶液;向50 mL容量瓶中加入1:1盐酸20 mL、10%抗坏血酸溶液1 mL、2%二安替比林甲烷溶液10 mL,用纯水定容,摇匀后静置10 min以配制参比溶液。用分光光度计测量波长同为388 nm的紫外可见光下样品溶液的吸光度。由标准曲线拟合方程计算得到样品溶液中的钛离子浓度,再换算得到钛盐中钛离子的质量分数,计算式如下:
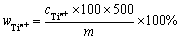 (10)
(10)
式中: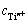 为样品溶液所含钛离子浓度。
为样品溶液所含钛离子浓度。
钛盐中Ti2+、Ti3+和Tin+的质量分数都已得到,则Ti4+的质量分数计算式为:
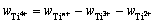 (11)
(11)
1.3 预熔熔盐电解质
将等摩尔比的NaCl-KCl装入刚玉坩埚中,混合均匀后放入熔盐电解炉中;抽真空后充入氩气,反复三次;在氩气气氛下先升温至573 K,保温1 h以除去混合熔盐中的水分,再升温至1023 K,保温4 h,使熔盐充分熔化形成共晶盐,得到NaCl-KCl共晶熔盐。
1.4 三电极体系及参比电极的标定
本研究工作的电化学测试采用三电极体系。为得到熔盐中钛离子(Ti2+)与金属钛的平衡电位,本文以高纯钛棒(d 5 mm)为工作电极,高纯铂棒作为参比电极,石墨为辅助电极,实验装置如图4所示。
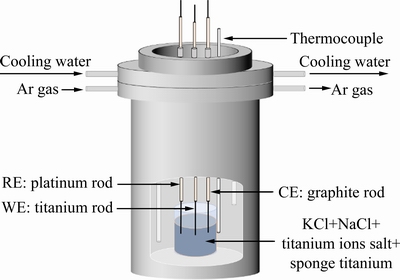
图4 电化学测试三电极体系及装置示意图
Fig. 4 Schematic diagram of three electrode system for electrochemical test
为标定参比电极以获得电极电势,利用金电极为工作电极、采用线性扫描伏安法测量体系中氯气的析出电位,即工作电极的极化曲线。在1023 K下的熔盐电解质中,将待标定的高纯铂棒作为参比电极,高纯金棒(d 2 mm)为工作电极,石墨为辅助电极。对电极体系施加一个从零开始线性增长的电压,记录工作电极上的电解电流值。在某个电压值附近,通过电极体系的电流迅速增大。低于该电压时,由于工作电极上很稳定,在较低电压下不会发生电解反应,故而电流值稳定在0 A附近;当高于该电压值时,熔盐中的氯离子在工作电极上发生氧化反应生成Cl2并在阳极析出,此时发生了电子交换,使得电极体系的电流迅速增大,如图5所示。
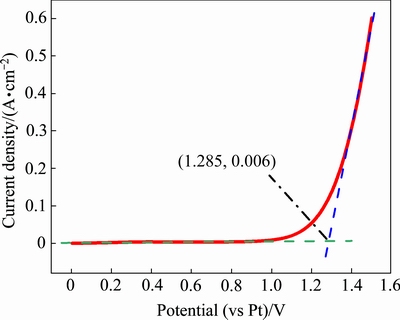
图5 金电极上的极化曲线(参比电极为铂)
Fig. 5 Polarization curve on gold electrode(RE: Pt)
极化曲线的切线交汇于(1.285,0.006)点,横坐标值即为氯气析出电位,从而得到了Cl2/Cl-标准电极相对于参比电极的电势。因此,在1023 K的NaCl-KCl共晶熔盐中,高纯铂棒参比电极相对于氯气析出的电位为1.285 V,三组平行实验的结果表明其误差范围在±0.39%。此后的分析中,所得的电位均相对于Cl2/Cl-电位。
1.5 开路电位测试
开路电位测试是计时电位法的一种特殊应用,用于采集开路电位随时间的变化。将三电极分别连接电化学工作站(AutoLab 302N),设置参数后即可开始开路电位测试。由于插入电极后破坏了体系中原本已建立的平衡,故需要进行长时间测试,待电位值在30 min内波动≤0.001 V时可结束测试,并认定该值为相应条件下的开路电位。
考虑到钛离子在熔盐中存在多种价态,本实验在NaCl-KCl共晶熔盐中加入过量的海绵钛,令钛盐中的高价态钛离子与海绵钛发生反应:2Ti3++Ti=3Ti2+和Ti4++Ti=2Ti2+。此时,熔盐中的钛离子存在价态为二价。体系中Ti2+的物质的量及摩尔分数分别可用式(12)和式(13)表示:
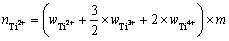 (12)
(12)
 (13)
(13)
熔盐中发生的平衡反应主要为TiCl2(aq) Ti(s)+Cl2(g),由该反应导出的Nernst方程为:
Ti(s)+Cl2(g),由该反应导出的Nernst方程为:
 (14)
(14)
式中: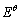 为Ti2+/Ti电极对的标准电极电势,V;
为Ti2+/Ti电极对的标准电极电势,V;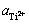 为体系中Ti2+的活度;
为体系中Ti2+的活度;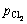 为体系中氯气的压强与标准大气压之比;R为摩尔气体常数;F为法拉第常数;n为反应的电子转移数;T为体系温度,K。在含微量TiCl2的NaCl-KCl熔盐中,氯离子相对于Ti3+是极其过量的,并且在增加熔盐中钛离子浓度时添加的钛盐的主要成分为NaCl-KCl,引入钛离子时也成比例地引入了氯离子,故实验过程中氯离子的活度可视为1,且氯离子的浓度几乎不变,即氯离子活度的变化可忽略。
为体系中氯气的压强与标准大气压之比;R为摩尔气体常数;F为法拉第常数;n为反应的电子转移数;T为体系温度,K。在含微量TiCl2的NaCl-KCl熔盐中,氯离子相对于Ti3+是极其过量的,并且在增加熔盐中钛离子浓度时添加的钛盐的主要成分为NaCl-KCl,引入钛离子时也成比例地引入了氯离子,故实验过程中氯离子的活度可视为1,且氯离子的浓度几乎不变,即氯离子活度的变化可忽略。
由于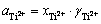 ,因此可由式(14)得到体系平衡电位和钛离子浓度的关系式(15):
,因此可由式(14)得到体系平衡电位和钛离子浓度的关系式(15):
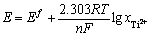 (15)
(15)
式中: 的含义如式(16):
的含义如式(16):
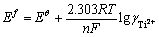 (16)
(16)
根据方程(14)可计算得到如下Ti2+活度计算表达式:
 (17)
(17)
在反应体系及实验温度确定后,式(17)右侧只有E一个变量。因此通过实验得到体系的开路电位后即可计算出体系中Ti2+的活度,再根据体系中钛离子的摩尔分数可计算得到其活度因子。
2 结果与讨论
2.1 熔盐中钛离子浓度分析
通过3组平行的化学分析和分光光度法测定,本文得到了钛盐中各价态钛离子的质量分数。本研究钛盐中钛离子浓度( 、
、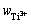 和
和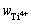 )分别为1.20%、2.43%和1.36%,误差范围为0.8%~5.7%。
)分别为1.20%、2.43%和1.36%,误差范围为0.8%~5.7%。
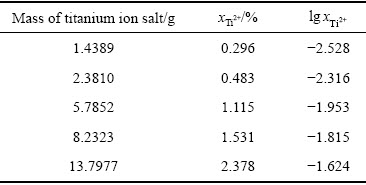
在钛离子活度测定中,为获得不同浓度的Ti2+,研究中将不同量的钛盐加入到NaCl-KCl熔盐中。由式(12)和式(13)计算得到加入不同量钛盐与过量海绵钛后的熔盐中Ti2+的摩尔分数,结果如表1所示。
表1 NaCl-KCl熔盐中Ti2+摩尔分数计算结果
Table 1 Mole fraction of divalent titanium ion in NaCl-KCl molten salt
2.2 二价钛离子活度分析
5组不同钛离子浓度下的开路电位测试所得数据如图6所示。当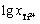 =-1.624时,在0~2400 s内开路电位数值有明显的波动。这是由于工作电极(钛棒)插入后,体系中的离子平衡被破坏,体系需重新建立平衡。从图中可以看出,开路电位逐渐趋于稳定,且从2400 s开始电位稳定在-1.282 V。同理,从图中可得知体系中:
=-1.624时,在0~2400 s内开路电位数值有明显的波动。这是由于工作电极(钛棒)插入后,体系中的离子平衡被破坏,体系需重新建立平衡。从图中可以看出,开路电位逐渐趋于稳定,且从2400 s开始电位稳定在-1.282 V。同理,从图中可得知体系中: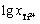 =-1.815时电位稳定在-1.287 V;
=-1.815时电位稳定在-1.287 V;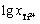 =-1.953时电位稳定在-1.297 V;
=-1.953时电位稳定在-1.297 V;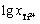 =-2.316时电位稳定在-1.308 V;
=-2.316时电位稳定在-1.308 V;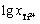 =-2.528时电位稳定在-1.325 V。此外,各体系中稳定的电位值随着
=-2.528时电位稳定在-1.325 V。此外,各体系中稳定的电位值随着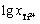 的增大而增大。由Nernst方程导出的E与
的增大而增大。由Nernst方程导出的E与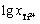 的关联式(15)可知,这种变化关系符合实验预期。
的关联式(15)可知,这种变化关系符合实验预期。
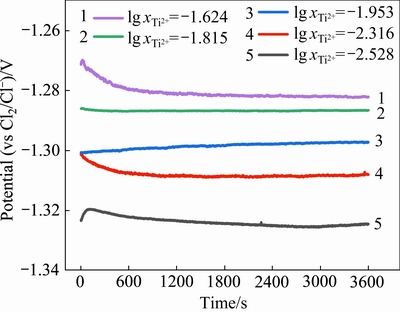
图6 不同钛离子浓度下的开路电位图
Fig. 6 Apparent potential data at various titanium ion concentrations
以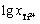 为横坐标、相应的开路电位为纵坐标绘制散点图,并对其进行线性拟合,结果如图7所示。拟合得到的直线方程为
为横坐标、相应的开路电位为纵坐标绘制散点图,并对其进行线性拟合,结果如图7所示。拟合得到的直线方程为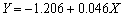 ,拟合度为0.969,表明实验测得的开路电位E与
,拟合度为0.969,表明实验测得的开路电位E与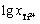 基本符合式(15)所示的线性关系。
基本符合式(15)所示的线性关系。
在测得不同钛离子浓度条件下含饱和金属钛的NaCl-KCl熔盐的开路电位后,根据式(17)可得到体系中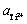 ,计算结果如图8所示。可以看出,Ti3+的活度与其浓度具有单调的变化趋势,这是由于Ti3+在熔盐中的浓度增大后,其在对应的单位体积内与氯离子形成的二氯化钛活化分子的总量增加,进而单位时间单位体积内活化分子碰撞的几率增大,其反应活度值也相应增大。
,计算结果如图8所示。可以看出,Ti3+的活度与其浓度具有单调的变化趋势,这是由于Ti3+在熔盐中的浓度增大后,其在对应的单位体积内与氯离子形成的二氯化钛活化分子的总量增加,进而单位时间单位体积内活化分子碰撞的几率增大,其反应活度值也相应增大。
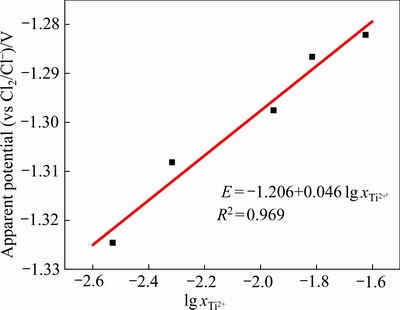
图7 开路电位E与 的关系及其线性拟合图
的关系及其线性拟合图
Fig. 7 Relationship and linear fitting diagram between apparent potentials(E) and 

图8 Ti2+在不同浓度下对应的活度
Fig. 8 Activities of Ti2+ at various concentrations
在无限稀的理想溶液中,离子的活度在数值上等于其浓度(摩尔分数);而在实际NaCl-KCl熔盐中,当Ti2+浓度介于0.296%至2.378%时,实验所得其活度相对于理想值存在负偏差。这可能是由于氯离子与高价态阳离子产生配合物,如Ti3+与氯离子形成诸如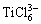 等氯离子配合物;这类结构的数量上升或稳定性增强都会造成系统中氯离子数量减少,使得Ti2+更难于与氯离子结合,从而造成Ti2+活度的负偏差。因此,熔盐中钛离子的活度相对于其浓度需要通过活度因子来校准。
等氯离子配合物;这类结构的数量上升或稳定性增强都会造成系统中氯离子数量减少,使得Ti2+更难于与氯离子结合,从而造成Ti2+活度的负偏差。因此,熔盐中钛离子的活度相对于其浓度需要通过活度因子来校准。
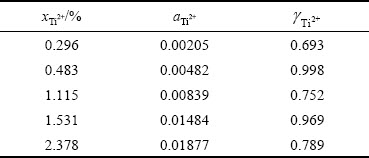
根据钛离子浓度及计算出的对应活度,可以得到各浓度下对应的活度因子,如表2所示。可以看出,在1023 K下,当体系中Ti2+摩尔分数介于0.296%~2.378%时,其活度因子在0.693~0.998之间。Ti2+活度因子与其浓度不具有单调的变化关系可能是由于实验过程中多因素误差造成的。根据亨利定律,低浓度下离子的浓度与活度符合线性关系,图8中的钛离子浓度与实际活度的拟合直线可视为在这一浓度范围内符合亨利定律的浓度与活度的线性关系,这一结果可为相关研究工作提供参考意见。
表2 1023 K下NaCl-KCl熔盐中Ti2+活度及活度因子
Table 2 Activities and activity coefficient of Ti2+ in NaCl-KCl molten salt at 1023 K
3 结论
1) 为获得精确的钛离子浓度,本文利用化学分析法、分光光度分析法等分别测定了Ti2+、Ti3+和Ti4+的浓度。为获取钛离子的活度,本文在1023 K下测定了不同钛离子浓度条件下含饱和金属钛的NaCl-KCl熔盐的开路电位。
2) 熔盐中钛离子浓度的对数与其开路电位呈直线关系,通过拟合及Nernst方程计算,获取了不同浓度的钛离子对应的活度及活度因子。
3) 钛离子的活度与浓度呈负偏差,当体系中Ti2+摩尔分数介于0.296%~2.378%时,其活度值单调地介于0.00205~0.01877,其活度因子位于0.693~0.998之间。本研究为精确测定熔盐中离子活度提供了理论及实验依据。
REFERENCES
[1] 贾 翃, 逯福生, 郝斌. 2019年中国钛工业发展报告[J]. 钛工业进展, 2019, 36(3): 42-48.
JIA Hong, LU Fu-sheng, HAO Bin. Report on China titanium industry progress in 2019[J]. Titanium Industry Progress, 2019, 36(3): 42-48.
[2] 金和喜, 魏克湘, 李建明, 等. 航空用钛合金研究进展[J]. 中国有色金属学报, 2015, 25(2): 280-292.
JIN He-xi, WEI Ke-xiang, LI Jian-ming, et al. Research development of titanium alloy in aerospace industry[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(2): 280-292.
[3] MANMEET K, SINGH K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications[J]. Materials Science & Engineering C, 2019, 102: 844-862.
[4] 郝 芳, 辛社伟, 毛友川, 等. 钛合金在装甲领域的应用综述[J]. 材料导报, 2020, 34(S1): 293-296, 327.
HAO Fang, XIN She-wei, MAO You-chuan, et al. Review on application of titanium alloy in armor[J]. Materials Reports, 2020, 34(S1): 293-296, 327.
[5] IMAM M A, SAM F H F. Low cost titanium and developing applications[J]. Journal of Metals, 2010, 62: 17-20.
[6] 李 中. 钛及钛合金在汽车上的应用[J]. 中国有色金属学报, 2010, 20(S1): 1034-1038.
LI Zhong. Applications of titanium and titanium alloys in automotive field[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(S1): 1034-1038.
[7] KROLL W J. The production of ductile titanium[J]. Transactions American Electrochemical Society, 1940, 78: 35-47.
[8] 王 天, 王耀武, 王 宇, 等. 金属钛冶炼研究进展[J]. 中国有色冶金, 2020, 49(3): 1-6, 27.
WANG Tian, WANG Yao-wu, WANG Yu, et al. Research progress of titanium metal smelting[J]. China Nonferrous Metallurgy, 2020, 49(3): 1-6, 27.
[9] WANG Qiu-yu, SONG Jian-xun, HU Guo-jing, et al. The equilibrium between titanium ions and titanium metal in NaCl-KCl equimolar molten salt[J]. Metallurgical and Materials Transactions B, 2013, 114B: 906-913.
[10] SONG Jian-xun, XIAO Jiu-san, ZHU Hong-min. Electrochemical behavior of titanium ions in various molten alkali chlorides[J]. Journal of The Electrochemical Society, 2017, 164(12): E321-E325.
[11] 王芳平, 汪艳芳, 张劲斌, 等. 低价钛离子电解质的制备与电化学行为研究[J]. 中国有色冶金, 2018, 47(6): 74-77.
WANG Fang-ping, WANG Yan-fang, ZHANG Jin-bin, et al. Preparation of titanium subchlorides molten salt and study on it’s electrochemical behavior[J]. China Nonferrous Metallurgy, 2018, 47(6): 74-77.
[12] JIAO Han-dong, SONG Wei-Li, CHEN Hao-sen, et al. Sustainable recycling of titanium scraps and purity titanium production via molten salt electrolysis[J]. Journal of Cleaner Production, 2020, 261: 121314.
[13] WENG Qi-gang, LI Rui-di, YUAN Tie-chui, et al. Valence states, impurities and electrocrystallization behaviors during molten salt electrorefining for preparation of high-purity titanium powder from sponge titanium[J].Transactions of Nonferrous Metals Society of China, 2014, 24(2): 553-560.
[14] ZHAO Kun, WANG Yao-wu, PENG Jian-ping, et al. Formation of Ti or TiC nanopowder from TiO2 and carbon powders by electrolysis in molten NaCl-KCl[J]. RSC Advances, 2016, 6: 8644-8650.
[15] SONG Jian-xun, WANG Qiu-yu, KANG Min-ho, et al. The equilibrium between titanium ions and metallic titanium in the molten binary mixtures of LiCl[J]. Electrochemistry, 2014, 82(12): 1047-1051.
[16] SONG Jian-xun, WANG Qiu-yu, HU Guo-jing, et al. Equilibrium between titanium ions and high-purity titanium electrorefining in a NaCl-KCl melt[J]. International Journal of Minerals, Metallurgy and Materials, 2014, 21(7): 660-665.
[17] SONG Jian-xun, WANG Qiu-yu, WU Jin-yu, et al. The influence of fluoride ions on the equilibrium between titanium ions and titanium metal in fused alkali chloride melts[J]. Faraday Discuss, 2016, 190: 421-433.
[18] WU Jin-yu, SONG Jian-xun, ZHU Hong-min, et al. Equilibrium between metallic titanium and titanium ions in MgCl2 LiCl molten salt[J]. Materials Transactions, 2019, 60(3): 374-378.
[19] 彭小奇, 宋国辉, 宋彦坡, 等. NaOH NaAl(OH)4 Na2CO3 H2O体系活度因子的计算模型[J]. 中国有色金属学报, 2009, 19(7): 1332-1337.
PENG Xiao qi, SONG Guo hui, SONG Yan po, et al. Calculation model of activity coefficient for NaOH NaAl(OH)4 Na2CO3 H2O system[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(7): 1332-1337.
[20] 陈辉煌, 陈启元, 刘常青, 等. 吉布斯吸附等温式的应 用—— 电解质溶液活度测定新方法[J]. 中国有色金属学报, 2014, 24(7): 1878-1882.
CHEN Hui-huang, CHEN Qi-yuan, LIU Chang-qing, et al. Application of Gibbs adsorption isotherm—A new method to measure activity of electrolyte solutions[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(7): 1878-1882.
[21] JINDAL H L. Activities of nickel chloride in molten chlorides[J]. Electrochimica Acta, 1974, 19: 441-443.
[22] 朱吉庆, THOMPSON W T. 电动势法测定MgCl2在MgCl2-KCl-CaCl2熔体中的活度[J]. 中南矿冶学院学报, 1988, 19(1): 94-101.
ZHU Ji-qing, THOMPSON W T. Determination of the activities of MgCl2 in the molten MgCl2-KCl-CaCl2 system by EMF measurements[J]. Journal of Central-South Institute of Mining and Metallurgy, 1988, 19(1): 94-101.
Activity analysis of divalent titanium in NaCl-KCl molten salt
LI Jing-yi1, LI Shao-long2, CHE Yu-si2, SONG Jian-xun2, HE Ji-lin1, 2
(1. School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, China;
(2. School of materials science and Engineering, Henan Province Industrial Technology Research Institute of Resources and Materials, Zhengzhou University, Zhengzhou 450001, China)
Abstract: Molten salt electrochemical process is the most promising method to extract and purify titanium. The determination of titanium ions activities is of great significance to the study of molten salt electrolysis mechanism of titanium. Therefore, in order to obtain the relationship between the concentration of divalent titanium ion and its activity in molten salt, the ions equilibrium potentials of NaCl-KCl eutectic salt system containing quantitative titanium ions and saturated metal titanium were measured by open circuit potential method in three-electrodes system. The activity of divalent titanium ion was calculated by Nernst equation, and the corresponding activity coefficient was obtained. The results show that the activity values of divalent titanium ions are monotonically ranged from 0.205 and 1.877, the activity coefficients range from 0.693 to 0.998, when the molar fraction of titanium ions ranges from 0.296% to 2.378% at 1023 K. This study can provide an important basis for accurately determining the activity of titanium ion in molten salt and further studying the equilibrium constant of titanium disproportionation reaction.
Key words: divalent titanium; molten salt; activity; open circuit potential method
Foundation item: Project(51804277) supported by the National Natural Science Foundation of China; Project (CNMRCUKF2008) supported by the State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, China
Received date: 2020-08-23; Accepted date: 2020-11-27
Corresponding author: SONG Jian-xun; Tel: +86-15039097174; E-mail: jianxun.song@zzu.edu.cn
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51804277);复杂有色金属资源清洁利用重点实验室开放课题(CNMRCUKF2008)
收稿日期:2020-08-23;修订日期:2020-11-27
通信作者:宋建勋,副教授,博士;电话:15039097174;E-mail:jianxun.song@zzu.edu.cn