文章编号:1004-0609(2009)06-1142-06
KOH亚熔盐法分解钛铁矿的动力学分析
刘玉民1,齐 涛2,张 懿2
(1. 河南师范大学 化学与环境科学学院,新乡 453007;
2. 中国科学院 过程工程研究所,北京 100080)
摘 要:对钛铁矿在氢氧化钾亚熔盐体系中的分解动力学进行研究,考察反应温度及矿粒粒径对钛铁矿分解率的影响。结果表明:钛铁矿在氢氧化钾亚熔盐体系中的分解符合未反应收缩核模型,受界面化学反应控制;提高反应温度或减小矿粒粒径均能提高钛铁矿的分解率。在一定的操作条件下,根据Arrherius方程得到钛铁矿在氢氧化钾亚熔盐体系中的分解活化能,且活化能随矿粒粒径的减小而减小;当矿粒粒径为58~180 ?m时,钛铁矿的分解活化能为54.78~46.82 kJ/mol。
关键词:钛铁矿;亚熔盐法;KOH;分解
中图分类号:TF 823 文献标识码:A
Kinetics analysis of decomposition of ilmenite by
KOH sub-molten salt method
LIU Yu-min1, QI Tao2, ZHANG Yi2
(1. College of Chemistry and Environmental Science, Henan Normal University, Xinxiang 453007, China;
2. Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100080, China)
Abstract:The decomposing kinetics of ilmenite in KOH sub-molten salt was investigated. The effects of factors, such as reaction temperature and particle size of the ore, on the decomposition rate of ilmenite were also studied. The results show that the decomposing process of ilmenite in KOH sub-molten salt is well interpreted with the unreacted shrinking core model under chemical reaction control. The decomposition rate of ilmenite increases with increasing the reaction temperature and decreasing the particle size. Under the specific operating conditions, the activation energy for the decomposition of ilmenite is calculated according to Arrhenius expression, and its values decrease with decreasing the particle size of the ore. When the particle size of the ore varies from 58 ?m to 180 ?m, the activation energy varies from 46.82 kJ/mol to 54.78 kJ/mol.
Key words: ilmenite; sub-molten salt method; KOH; decomposition
我国的钛资源极其丰富,全国有20多个省市自治区有钛矿,其中98.9%是钒钛磁铁矿,1%左右是金红石[1?3]。目前,世界钛资源的90%以上用于生产钛白粉,而硫酸法和氯化法是目前工业生产钛白粉的两种工艺,但传统硫酸法钛白粉的生产工艺流程长、工艺复杂,对环境污染极其严重,已成为我国钛白粉行业发展的瓶颈;氯化法钛白粉的生产工艺流程短、三废量少、产品档次高,但对原料要求苛刻,钛精矿中钙镁总含量必须控制在0.5%以内,原料来源困难[4?9]。虽然我国钛矿资源丰富,但矿石的品位低,MgO等杂质含量高,不适宜采用氯化法,因此,探索适合我国钛资源特点的冶金新工艺意义重大。
中国科学院过程工程研究所提出了亚熔盐法二氧化钛清洁生产新工艺,该工艺是在适合于处理两性矿产资源的亚熔盐清洁生产技术平台上对钛冶金的具体应用,完全不同于硫酸法和氯化法。该工艺可以在低温下将钛铁矿中的钛高效高选择性地转化为钛酸盐,钛酸盐经水解、煅烧后可得二氧化钛;而钛铁矿中的铁、镁、钙等有价元素不与亚熔盐体系反应而形成渣相,实现钛酸盐与弱磁性铁渣的高选择性分离,而铁渣可进一步资源化,从而实现二氧化钛的清洁生产,可望为解决我国高钙镁钛资源综合利用的重大科技难题开辟一条新途径。亚熔盐法分解精矿是利用在常压下流动的高浓介质中的拟均相反应分解矿石,可极大地强化反应和质量、热量传递,在相对较低的温度下获得较高的矿石分解率
[10?13]。但亚熔盐法处理钛铁矿作为一个尚未开拓的领域,急需开展大量的基础研究工作,而有关钛铁矿在KOH亚熔盐体系中的分解动力学研究尚未见报道。针对这一问题,本文作者研究了反应温度及矿粒粒径对钛铁矿在KOH亚熔盐体系中分解率的影响,得到钛铁矿的分解动力学模型及不同矿粒径时钛铁矿的分解活化能,这对钛铁矿的亚熔盐冶金清洁生产新工艺的设计具有重要的理论意义和实际意义。
1 实验
1.1 实验原理
钛铁矿在KOH亚熔盐介质中反应生成钛酸钾的过程是一个典型的液?固反应过程,生成的钛酸钾产物经分离后的XRD谱(见图1)及组分分析表明,所得
的钛酸钾为K4Ti3O8,则钛铁矿在KOH亚熔盐中的反应可表示为:


图1 产物的XRD谱
Fig.1 XRD pattern of product
1.2 原料与仪器
实验中所用KOH为分析纯试剂,钛铁矿为济南裕兴化工总公司提供,其主要化学组分如表1所列。
表1 钛铁矿的化学组分
Table 1 Chemical compositions of ilmenite

反应器为自制设备,由不锈钢制成,通过CKW?2100型可控硅温度控制器控温,用镍铬?镍硅热电耦测温,温控精度为±2 ℃,反应器上装有回流冷凝管;搅拌转速由D?8401?WZ型数显控速搅拌机控制,实验装置如图2所示。
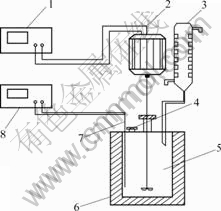
图2 实验装置示意图
Fig.2 Schematic diagram of experimental apparatus: 1—Adjustable voltage speed controller; 2—Agitator; 3—Reflux condenser; 4—Sample point; 5—Reactor; 6—Heating furnace; 7—Thermocouple; 8—Temperature controller
1.3 实验步骤
称取一定质量的分析纯固体KOH于反应釜中,加入去离子水至设定浓度。然后将反应釜缓慢升温至设定温度,开通搅拌,使体系恒温5 min,按碱矿比R(KOH与钛铁矿的质量比)加入钛铁矿,在设定的时间取样约2 g,样品经骤冷、称量、溶解、过滤、洗涤,滤液定容至100 mL,从中取2 mL稀释至50 mL,用ICP-AES分析其中的Ti含量,从而计算钛铁矿的分 解率。
2 结果与讨论
2.1 矿粒粒径的影响
确定适当的矿粒粒径具有重有意义,因为矿粒粒径的大小与反应时间、反应器的大小及矿粒的制备方法密切相关,同时考察矿粒粒径对钛铁矿分解速率的影响和对动力学控制步骤的判定也具有非常重要的作用。为此,在搅拌速度为1 000 r/min、反应温度为200 ℃、碱矿质量比为5?1、初始碱浓度为80%的实验条件下,考察了矿粒粒径对钛铁矿分解率的影响,结果如图3所示。
由图3可知,矿粒粒径的大小对钛铁矿的分解率有明显的影响,随着矿粒径的减小钛铁,矿的分解率显著增大,在反应时间为240 min、矿粒粒径为58~75 μm的分解率比矿粒粒径为160~180 μm的分解率提高了约24%。这是因为随矿粒粒径的减小,比表面积增大,钛铁矿与KOH接触面增大,内扩散阻力变小,加快了钛铁矿的分解率,从而显著提高反应速率和分解率。
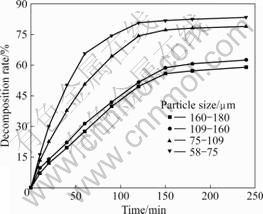
图3 矿粒粒径对钛铁矿分解率的影响
Fig.3 Effect of particle size of ilmenite ore on decomposition rate
2.2 反应温度的影响
在搅拌速度为1 000 r/min,碱矿质量比为5?1、初始碱浓度为80%的条件下考察了反应温度对不同粒度钛铁矿分解率的影响,结果如图4所示。由图4可以看出,反应温度是决定钛铁矿分解率的重要操作参数,提高反应温度,不同粒径钛铁矿的分解率均大幅度提高。当钛铁矿的粒径为109~160 ?m、反应时间为240 min时,钛铁矿在反应温度为180 ℃的分解率达到40%;而在反应温度为220 ℃时,钛铁矿的分解率接近74%,此时的分解率提高了近34%。这是因为温度升高时,化学反应速率及反应物、反应产物的扩散速率也随之加快,从而加速了钛铁矿的分解,使钛铁矿的分解率提高。但若温度过高,会造成体系内水分大量蒸发,KOH浓度变大,反应物料的粘度增加,从而影响反应效果,降低反应速度,钛铁矿的分解率也不再增加。

图4 反应温度对不同粒径钛铁矿分解率的影响
Fig.4 Effect of reaction temperature on decomposition rate of ilmenite with different particle sizes: (a) 160?180 ?m; (b) 109?160 ?m; (c) 75?109 ?m; (d) 58?75 ?m
2.3 反应过程控制步骤的确定及反应活化能的确定
由反应式(1)可知,钛铁矿在KOH亚熔盐中反应时有固体产物生成,因此可以认为反应过程适用于未反应收缩核模型,传质反应过程由以下步骤组 成[14?17]:
1) 溶液中的KOH由流体本体通过液相边界层向固体颗粒外表面的扩散(外扩散);
2) 未反应的反应物(钙、镁、锰的氧化物)通过固体产物层向未反应固体表面的扩散(内扩散);
3) KOH与固体颗粒在界面上发生化学反应(表面反应过程);
4) 反应产物(钛酸钾)离开反应界面从固体内部向固体颗粒外表面的扩散(内扩散);
5) 反应产物(钛酸钾)离开固体颗粒表面向溶液主体相扩散(外扩散)。
以上各步骤中,外扩散过程除了受溶液的物性影响之外,还取决于实际反应器中的速度场、温度场和浓度场,因此外扩散过程与反应器的结构、是否有搅拌以及搅拌强度等因素有关。由于实验中所用的搅拌转速为1 000 r/min,同时反应器较小,因此可认为反应过程中已基本消除外扩散的影响。内扩散过程除了受溶液物性影响之外,更主要的是受固体颗粒空隙结构的影响。表面反应过程则主要取决于反应物、反应产物的性质以及固体颗粒的性质,包括固体颗粒中参加反应的物质的结晶度、晶粒大小以及其它惰性物质对反应的影响等。
为确定反应过程的控制步骤,根据界面化学反应控制动力学方程(式(2))和产物层内扩散控制动力学方程(式(3))分别对反应时间t作图,根据各自的线性相关系数的大小确定钛铁矿与KOH亚熔盐反应的控制步骤。结果表明,在钛铁矿分解反应最快的阶段,[1?(1?X)1/3]与反应时间t的线性关系非常好,而[1+2(1?X)?3(1?X)2/3]与反应时间t的线性关系则较差,说明钛铁矿在KOH亚熔盐介质中反应过程的初始阶段更符合界面化学反应控制。
1?(1?X)1/3=k1t (2)
1+2(1?X) ?3(1?X)2/3=k2t (3)
式中 t为反应时间;X为反应时间为t时钛铁矿的分解率;k1和k2分别为反应速率常数。
利用图4中钛铁矿粒径为109~160 ?m时其分解率与反应温度的关系,作不同反应温度下[1?(1?X)1/3]与反应时间t的关系图,结果如图5所示。

图5 [1?(1?X)1/3]与反应时间的关系
Fig.5 Relationship between [1?(1?X)1/3] and reaction time
由图5可知,在不同反应温度下,[1?(1?X)1/3]与反应时间t之间呈现良好的线性关系,这进一步说明钛铁矿KOH亚熔盐反应过程受界面化学反应控制。由图5中各直线可以求出其斜率k,各斜率分别代表不同温度下的反应速率常数,根据Arrheriys方程k=A·exp[?Ea/(RT)]有lnk=lnA?Ea/(RT)。将lnk对1/T作图,结果如图6所示。
由图6可知,lnk与1/T呈线性关系,从该直线斜率可以求出实验条件下反应的表观活化能Ea为51.54 kJ/mol。用同样的方法可以计算出当钛铁矿的粒径为160~180、75~109和58~75 ?m时,KOH亚熔盐分解钛铁矿的表观活化能Ea分别为54.78、48.75和46.82 kJ/mol。通常,当活化能大于41.8 kJ/mol时,过程为化学反应控制[18]。因此,可以证明钛铁矿在KOH亚熔盐介质中的分解过程为界面化学反应控制。

图6 ln k与1/T的关系
Fig.6 Relationship between ln k and 1/T
3 结论
1) 反应温度及矿粒粒径均对钛铁矿在KOH亚熔盐中的分解率有显著的影响,提高反应温度或者减小矿粒粒度均能提高钛铁矿的分解率。
2) 钛铁矿在KOH亚熔盐中的分解过程符合未反应收缩核模型,为界面化学反应控制,且钛铁矿的分解活化能与矿粒粒径有关。在搅拌速度为1 000 r/min、碱矿质量比为5?1、初始碱浓度为80%、矿粒粒径分别为160~180、109~160、75~109和58~75 ?m时,钛铁矿在KOH亚熔盐中分解的活化能分别为54.78、51.54、48.75和46.82 kJ/mol。
REFERENCES
[1] 王 志, 袁章福. 中国钛资源综合利用技术现状与新进展[J]. 化工进展, 2004, 23(4): 349?352.
WANG Zhi, YUAN Zhang-fu. Progress and status reporting on the comprehensive utilization technology of titanium resources in China[J]. Chemical Industry and Engineering Progress, 2004, 23(4): 349?352.
[2] 邓国珠, 王向东, 车小奎. 钛工业的现状和未来[J]. 钢铁钒钛, 2003, 24(1): 1?7.
DENG Guo-zhu, WANG Xiang-dong, CHE Xiao-kui. Today and tomorrow of titanium industry[J]. Iron Steel Vanadium Titanium, 2003, 24(1): 1?7.
[3] 王铁明, 邓国珠. 中国钛工业发展现状及原料问题[J]. 稀有金属快报, 2008, 27(6): 1?5.
WANG Tie-ming, DENG Guo-zhu. Situation of titanium industrial development and raw material in China[J]. Rare Metals Letters, 2008, 27(6): 1?5.
[4] SASIKUMAR C, RAL D S, SRIKANTH S, MUKHOPADHYAY N K, MEHROTRA S P. Dissolution studies of mechanically activated Manavalakurichi ilmenite with HCl and H2SO4[J]. Hydrometallurgy, 2008, 88: 154?169.
[5] 唐振宁. 钛白粉的生产与环境治理[M]. 北京: 化学工业出版社, 2000: 211?216.
TANG Zheng-ning. The production and environmental treatment of titanium dioxide[M]. Beijing: Chemical Industry Press, 2000: 211?216.
[6] LASHEEN T A. Soda ash roasting of titania slag product from Rosetta ilmenite[J]. Hydrometallurgy, 2008, 93: 124?128.
[7] LI Chun, LIANG Bin, WANG Hai-yu. Preparation of synthetic rutile by hydrochloric acid leaching of mechanically activated Panzhihua ilmenite[J]. Hydrometallurgy, 2008, 91: 121?129.
[8] LIANG Bin, LI Chun, ZHANG Cheng-gang, ZHANG Yong-kui. Leaching kinetics of Panzhihua ilmenite in sulfuric acid[J]. Hydrometallurgy, 2005, 76 : 173?179.
[9] FRANCIS A A, EL-MIDANY A A. An assessment of the carbothermic reduction of ilmenite ore by statistical design[J]. Journal of Materials Processing Technology, 2008, 199: 279?286.
[10] 张 懿, 范秀英, 张 微. 环保产业与高新技术[M]. 北京: 中国科学技术出版社, 2001: 134?135.
ZHANG Yi, FAN Xiu-ying, ZHANG Wei. Environmental protection industry and high technology[M]. Beijing: Chinese Science and Technique Press, 2001: 134?135.
[11] ZHANG Yi, LI Zuo-hu, QI Tao, WANG Zhi-kuan, ZHENG Shi-li. Green chemistry of chromate cleaner production [J]. Chinese Journal of Chemistry, 1999, 17(3): 258?266.
[12] XU H B, ZHENG S L, ZHANG Y, LI Z H, WANG Z K. Oxidative leaching of a Vietnamese chromite ore in highly concentrated potassium hydroxide aqueous solution at 300 ℃ and atmospheric pressure[J]. Minerals Engineering, 2005, 18: 527?535.
[13] ZHOU Hong-ming, ZHENG Shi-li, ZHANG Yi. Kinetics investigations on the leaching of niobium from a low-grade niobium-tantalum ore by concentrated KOH solution[J]. Chinese Journal of Chemical Engineering, 2004, 12(2): 202?207.
[14] 杨显万, 邱定藩. 湿法冶金[M]. 北京: 冶金工业出版社, 1998: 154?188.
YANG Xian-wan, QIU Ding-fan. Hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 1998: 154?188.
[15] LEVENSPIEL O. Chemical engineering kinetics[M]. 3rd ed. New York: Wiley, 1972: 359?368
[16] SOHN H Y, WADSWORTH M E. 提取冶金速率过程[M]. 郑蒂基, 译. 北京: 冶金工业出版社, 1984: 1?46.
[17] SOHN H Y, WADSWORTH M E. Rate process of extractive metallurgy[M]. ZHENG Di-ji, transl. Beijing: Metallurgical Industry Press, 1984: 1?46.
[18] 陈甘棠. 化学反应工程[M]. 北京: 化学工业出版社, 1990: 172?174.
CHEN Gan-tang. Chemical reaction engineering[M]. Beijing: Chemical Industry Press, 1990: 172?174.
[19] 李洪桂. 湿法冶金学[M]. 长沙: 中南大学出版社, 2002: 69?118.
LI Hong-gui. Hydrometallurgy[M]. Changsha: Central South University Press, 2002: 69?118.
基金项目:国家自然科学基金资助项目(50574084);河南师范大学博士科研启动课题资助项目(0706)
收稿日期:2008-03-19;修订日期:2008-06-10
通讯作者:刘玉民,副教授,博士;电话:13639636179;E-mail: ymliu2007@163.com
(编辑 何学锋)