
Processing parameters for Cu nanopowders prepared by anodic arc plasma
WEI Zhi-qiang(魏智强)1, 2, XIA Tian-dong(夏天东)2, MA Jun(马 军)1,
FENG Wang-jun(冯旺军)1, DAI Jian-feng(戴剑锋)1, WANG Qing(王 青)1, YAN Peng-xun(闫鹏勋)3
1. School of Science, Lanzhou University of Technology, Lanzhou 730050, China;
2. State Key Laboratory of Gansu Advanced Non-ferrous Metal Materials,Lanzhou University of Technology, Lanzhou 730050, China;
3. School of Physical Science and Technology, Lanzhou University, Lanzhou 730000, China
Received 14 May 2006; accepted 22 September 2006
Abstract: Copper nanopowders were successfully prepared by anodic arc discharging plasma method with home-made experimental apparatus. The effects of various processing parameters on the particle size of Cu nanopowders were investigated in the process, and the optimum processing parameters were obtained. In addition, the morphology, crystal structure, particle size distribution of the nanopowders were characterized via X-ray diffraction(XRD), transmission electron microscopy(TEM) and the corresponding selected area electron diffraction(SAED). The experimental results show that the crystal structure of the samples is the same fcc structure as that of the bulk materials. The processing parameters play a major role in controlling the particle size. The particle size increases with the increase of the arc current or gas pressure.
Key words: Cu nanopowders; particle size; processing parameters; anodic arc plasma
1 Introduction
In the latest decade, the research for metal nanopowders has attracted significant interest because the intriguing physical and chemical properties of nanopowders essentially differ from those of bulk material[1-4]. The extensive scientific efforts have been taken to investigate the dependency of those properties on particle size. Metal nanopowders are used as conductive materials, catalysts, high-dense magnetic recording materials, activation and sintering materials, etc[5-7]. All these applications require the powders consisting of monodisperse particles with desired physical and chemical properties, which strongly depend on the production method. Many techniques have been developed to prepare metal nanopowders, such as chemical vapor deposition[8], spray pyrolysis[9], hydrothermal reaction[10], laser ablation[11], flame processing[12], vapor deposition[13], microwave- induced plasma synthesis[14], and sol-gel method[15]. Anodic arc plasma technique has been successfully applied for the production of metal nanopowders in the past[16-17], which has been proven to be suitable for production of metal nanopowders with ultrafine particle size, higher purity, narrow size distribution, single phase and spherical shape. Especially, the physical and chemical properties of the nanopowders by this method can be easily improved by varying the processing parameters, such as the type of inert gases, the ambient gas pressure, the arc current intensity, and the water- cooling condition. It is important to study the dependence of the particle size on the various parameters, because it offers the possibility of obtaining nanopowders with pre-established physical and chemical properties by adjusting the processing parameters.
In this paper, copper nanopowders were successfully prepared by anodic arc discharging plasma method with home-made experimental apparatus. The samples were characterized via X-ray diffraction(XRD), transmission electron microscopy(TEM) and the corresponding selected area electron diffraction(SAED). Moreover, the effect of various processing parameters on the particle size of Cu nanopowders was investigated in the process, and the optimum processing parameters were obtained.
2 Experimental
The metal nanopowders were prepared by anodic arc discharging plasma technique with home-made experimental apparatus which was described elsewhere [17]. In the process, the vacuum chamber was pumped to 10-3 Pa, then backfilled with inert gas to about 103 Pa. The electric arc in the inert environment was automatically ignited between the tungsten electrode and the water-cooled nozzle by the high frequency initiator. Under the gas pressure and electric discharge current heating, the ionized gases were driven through the nozzle outlet and a plasma jet formed[18-19]. The bulk metal Cu was heated and melted and then metal atoms were detached from the metal surface when the plasma jet heating energy exceeded the metal superficial energy, and evaporated into free atom state. Above the evaporation source, there was a region filled with supersaturated metal vapor, where the metal atoms diffused around and collided each other to decrease the nuclei formation energy. When the metal vapor was supersaturated, a new phase was nucleated homogeneously out of the aerosol systems. The droplets were rapidly cooled and the primary particles formed by an aggregation growth mechanism[20-21]. The free inert gas convection transported the particles out of this nucleation and growth region to the inner walls of the cylinder. The loose nanopowders could be obtained after a period of passivation and stabilization with working gas.
The crystal structure and grain sizes of Cu nanopowders were analyzed by Japan Rigaku D/max-2400 X-ray diffractometer using monochromatic high-intensity Cu Kα radiation (λ=0.154 056 nm, 40 kV, 100 mA), at a scanning speed of 2 (?)/min from 30 to 100? (2θ). The average grain size of the particles was estimated from X-ray line broadening measurements according to Scherrer formula. The particle size and morphology shape were investigated by transmission electron microscopy(TEM) and the corresponding selected area electron diffraction(SAED) attached to a Japan JEOL JEM-1200EX microscope with an accelerating voltage of 80 kV.
3 Results and discussion
3.1 Influence of processing parameters on nano- powders
In our experiments, some metal nanopowders are prepared. Plenty of evidences are available to show that various process parameters (such as the type of inert gases, ambient gas pressure, arc current intensity, starting raw materials, water-cooling condition) have much effects on the properties of final powder, and the properties can be easily improved by varying the processing parameters. Two series of measurements were conducted by varying arc current and gas pressure. For each series, one parameter was varied systematically and the particle size was measured, while the other parameters kept constant. The relations between the particle size and the process parameters (gas pressure and arc current) were investigated carefully. The referential experimental conditions were obtained and summarized in Table 1.
Table 1 Referential process parameters of preparing metal nanopowders by anodic arc plasma

Fig.1 shows the influence of arc current on the size of Cu nanopowders in argon atmosphere under the pressure of 1.2 kPa. From the curve, it is easily found that the increase of arc current results in the increase of particle size. A possible explanation to this trend is related to the increase of system power at higher arc current. Since it can be expected that the effective gas temperature increases with increasing arc current, this leads to higher flow velocities. On one hand, the higher velocity leads to a reduced growth time of the particles. According to the aggregation growth theory, the time is inversely proportional to particle size, so the particle size increases with increasing arc current. On the other hand, higher arc current leads to a higher arc temperature, which means that more metal atoms detach from the metal surface and evaporate into atom vapour, and naturally more basic nuclei form as well, which are favorable for the formation of nanopowders. So the particle size of the powder increases with the increase of arc current when other factors are constant.
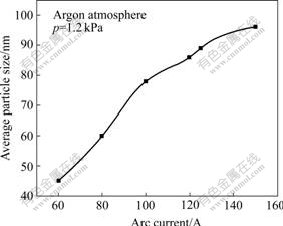
Fig.1 Variation of average particle size with arc current
In argon atmosphere at the same arc current, the influence of gas pressure on the particle size of Cu nanopowders is presented in Fig.2. As it is shown, a clear tendency of particle size increasing with increasing gas pressure can be deduced, which can be explained by using the same methodology. The increase in the pressure is expected to increase the initial particle number and the collision frequency among particles, and then the nucleation and growth rates of the basic nuclei increase as well. So the particle size increases linearly with the increase of the pressure for same type of gas.

Fig.2 Variation of average particle size with gas pressure
3.2 Factors of controlling particle size
In order to understand how particle characteristics could be improved, we must first identify the physical variables determining the particle nucleation and growth in the process, then relate these variables to the experimentally controllable process parameters such as evaporation temperature, chamber pressure, type of gas and chamber geometry. The nucleation and the growth of the particles are determined by the local cooling rate, C, the residence time distribution, t, and the density, N, in the nucleation and growth zone. The local cooling rate significantly influences the homogeneous nucleation. With coagulation, the final particle size distribution depends on the particle number density and the residence time distribution. The smaller N, the less the extent of coagulation is, and the smaller the final particle size will be. The lower the average residence time, the faster the particle are cooled to the temperature below which coalescence is no longer possible. The width of the particle size distribution is primarily determined by the width of the residence time distribution. A broad residence time distribution means that particles in a given volume exhibit large age differences, i.e. they form at different time, and grow over different residence time. Because of the large age differences, the particle sizes also vary greatly. Coagulation will lead to a further broadening of the particle distribution. In summary, under the conditions of the dilution of the inert gas, i.e. small N, and a high average cooling rate, i.e. short average residence time, small particles can be produced.
By setting the evaporation temperature, T, the number of the atoms evaporated and the number density can be controlled. Increasing temperature will increase the number density of the atoms. Therefore, in the process the temperature can not be set too high, since the coagulation rate, defined as dN/dt, increases proportionally to N2, and rather large particles will be resulted.
According to the Kelvin-equation r*=2σVm/ [kTln(p/p0)], where r* is the radius of the embryos, σ is the surface free energy between the liquid and its vapor phase per unit surface area, k is the Boltzmann constant, T is the absolute temperature, p/p0 is the supersaturation ratio, Vm is the molecular volume, p and p0 respectively stand for vapor pressure and balance pressure on micron-nuclei surface. From the equation, larger p/p0 helps to reduce the radius of critical crystal nuclei. Hence, more and smaller nuclei become stable. Thus more (increase in number density) and small particles are formed.
The gas pressure, p, influences the cooling rate and average residence time by determining the mean free path 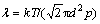 . Increasing p will decrease λ. On one hand, this effect is beneficial, since it increases C and, thus, should lead to smaller particles. On the other hand, this effect is offset by the concurrent increase in the average residence time which promotes the growth of particles. In fact, the latter seems to be dominant, as an increase is found in average particle size with increasing p. Therefore, the gas pressure can not be set too low, since λ becomes too large for cooling the atoms sufficiently to obtain the supersaturation, which is necessary for homogeneous nucleation to occur in the gas phase.
. Increasing p will decrease λ. On one hand, this effect is beneficial, since it increases C and, thus, should lead to smaller particles. On the other hand, this effect is offset by the concurrent increase in the average residence time which promotes the growth of particles. In fact, the latter seems to be dominant, as an increase is found in average particle size with increasing p. Therefore, the gas pressure can not be set too low, since λ becomes too large for cooling the atoms sufficiently to obtain the supersaturation, which is necessary for homogeneous nucleation to occur in the gas phase.
The initial particle size in synthesis process is in the range of several nanometers. Therefore, we can describe their motions at this moment according to Brown motion law. Obviously, the interactions among particles will result in particle condensation. According to gas-molecular kinetics, the collision frequency can be written as f=4(πkT/m)1/2dN2, where N is particle concentration, m is particle mass, d is particle diameter, and k is Boltsmann constant. The retention time of particles must be effectively controlled and the initial concentration of particles must be reduced in order to limit particle growth resulted from collision and agglomeration of powders.
3.3 Characterization of nanopowders
Fig.3(a) shows the representative transmission electron microscopy (TEM) micrograph of Cu nano- powders prepared at argon atmosphere, pressure of 1 kPa and electric current of 100 A. A small amount of the powder was dispersed in isopropanol and ultrasonicated, deposited on copper grids with holey carbon coated film. The samples were placed in a vacuum oven to dry at ambient temperature before examination. It can be seen from the graph that all of the particles are in spherical shape, small particles aggregate into larger particles because of their extremely small dimensions and high surface energy, and most of the particles appear fairly uniform in size, with ultrafine particle size and smooth surface.
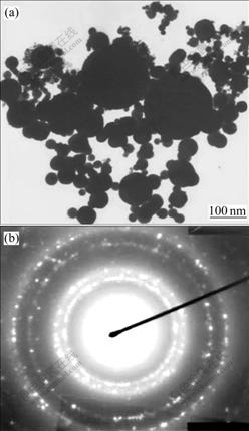
Fig.3 TEM micrograph (a) and SAED pattern (b) of Cu nanopowders
From the TEM data, the particle size histograms can be drawn and the mean size of the particles is determined. Fig.4 shows the particle size distribution of Cu nanopowders. It can be seen that the particle sizes range from 20 nm to 100 nm, and the median diameter (taken as average particle diameter) is about 67 nm, being deduced from the images, which shows a relatively narrow size distribution.
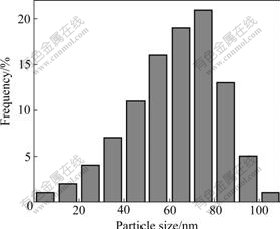
Fig.4 Particle size distribution of Cu nanopowders
Fig.3(b) shows the corresponding selected-area electron diffraction (SAED) pattern. It can be indexed from the reflection of FCC structure, and this result is also confirmed by means of X-ray diffraction. Tropism of the particles and the small particles cause the widening of diffraction rings. The rings are made up of many diffraction spots, which indicates that the nanopowders are polycrystalline. Electron diffraction reveals that each particle is composed of many small crystal nuclei, which is convincing proof that the particles grow in an aggregation model.
Fig.5 shows the typical X-ray diffraction(XRD) pattern for the specimen. Five broad peaks with 2θ values of 43.44?, 50.56?, 74.24?, 90.04? and 95.24? correspond to (111), (200), (220), (311), (222) planes of bulk Cu respectively, which can be assigned to FCC Cu. The XRD spectrum does not reveal any other phase except for the characteristic peaks of copper, which shows that the physical phases of copper nanopowders synthesized in this work have high purity.
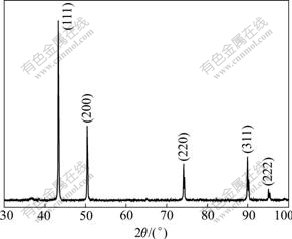
Fig.5 XRD pattern of Cu nanopowders
From the full width at half maximum, the average crystalline size can be estimated in conjunction with Scherrer equation: d=Kλ/Bcosθ, where d is the crystallite size; K=0.89 is the Scherrer constant related to the shape and index (hkl) of the crystals; λ is the wavelength of the X-ray (Cu Kα, 0.149 54 nm); θ is the diffraction angle; and B is the corrected half-width of the diffraction peak (in radians) given by 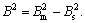 Bm is the measured half-width and Bs is the half-width of a standard sample with a known crystal size greater than 100 nm. The effect of geometric (instrumental) broadening on the reflection peaks was calibrated. The average crystallite size is 63 nm, which is well consistent with the average particle diameter obtained from the TEM image of Fig.3(a).
Bm is the measured half-width and Bs is the half-width of a standard sample with a known crystal size greater than 100 nm. The effect of geometric (instrumental) broadening on the reflection peaks was calibrated. The average crystallite size is 63 nm, which is well consistent with the average particle diameter obtained from the TEM image of Fig.3(a).
4 Conclusions
Copper nanopowders are successfully prepared by means of anodic arc discharging plasma method with home-made experimental apparatus. The nanopowders possess uniform size, weakly agglomerated and spherical shape. The crystalline structure of the samples has the same FCC structure as that of the bulk materials. The properties of Cu nanopowders are affected by many factors, such as the type of inert gases, the pressure of ambient gas, the arc current intensity, and water-cooling condition. The gas pressure and the arc current play a major role in controlling the particle size of Cu nanopowders. The particle size increases with the increase of the arc current or gas pressure. The optimal processing parameters are obtained.
References
[1] GLEITER H. Nanocrystalline materials [J]. Prog Mater Sci, 1990, 33(4): 223-315.
[2] CHEN Y J, CAO M S, TIAN Q. A novel preparation and surface decorated approach for α-Fe nanoparticles by chemical vapor-liquid reaction at low temperature [J]. Materials Letters, 2004, 58: 1481-1484.
[3] ZHANG W W, CAO Q Q. Structural, morphological, and magnetic study of nanocrystalline cobalt-nickel-copper particles [J]. Journal of Colloid and Interface Science, 2003, 257: 237-243.
[4] ZHANG L D, MOU J M. Nanomaterials and Nanostructure [M]. Beijing: Science Press, 2001. (in Chinese)
[5] GLEITER H. Materials with ultrafine microstructures: retrospectives and perspectives [J]. Nanostruct Mater, 1992, 1(1): 1-20.
[6] CHEN B J, SUN X W, XU C X. Growth and characterization of zinc oxide nano/micro-fibers by thermal chemical reactions and vapor transport deposition in air [J]. Physica E, 2004, 21: 103-107.
[7] CHEN D H, CHEN D R. Hydrothermal synthesis and characterization of octahedral nickel ferrite particles [J]. Powder Technology, 2003, 133: 247-250.
[8] CAO M S, DENG Q G. Synthesis of nitride-iron nanometer powder by thermal chemical vapor-phase reaction method [J]. Journal of Inorganic Chemistry, 1996, 12(1): 88-91.
[9] KARTHIKEYAN J, BERNDT C C, TIKKANEN J. Plasma spray synthesis of nanomaterial powders and deposits [J]. Materials Science and Engineering A, 1997, 238: 275-286.
[10] ZHENG H G, LANG J H. ZENG J H. Preparation of nickel nanopowders in ethanol-watersystem (EWS) [J]. Materials Research Bulletin, 2001, 36: 947-952.
[11] GAERTNER G F, MIQUEL P F. Particle generation by laser ablation from solid targets in gas flows [J]. Nanostruct Mater, 1994, 4(5): 559-568.
[12] KATZ J L, MIQUEL P F. Synthesis and applications of oxides and mixed oxides produced by a flame process [J]. Nanostruct Mater, 1994, 4(5): 551-557.
[13] GUNTHER B, KUMPMANN A. Ultrafine oxide powders prepared by inert gas evaporation [J]. Nanostruct Mater, 1992, 1(1): 27-30.
[14] VOLLATH D, SICKAFUS K E. Synthesis of nanosized ceramic oxide powders by microwave plasma reactions [J]. Nanostruct Mater, 1992, 1(5): 427-437.
[15] CHEN D H, HE X R. Synthesis of nickel ferrite nanoparticles by sol-gel method [J]. Materials Research Bulletin, 2001, 36: 1369-1377.
[16] WEI Z Q,XIA T D. Study on the growth mechanism of Cu nanopowders prepared by anodic arc plasma [J]. Trans Nonferrous Met Soc China, 2006, 16(2): 168-172.
[17] WEI Z Q, YAN P X. Preparation and characterization of Ni nanopowders prepared by anodic arc plasma [J]. Trans Nonferrous Met Soc China, 2005, 15(1): 51-56.
[18] ANDRE J, KWAN W. Arc-discharge ion sources for heavy ion fusion [J]. Nuclear Instruments and Methods in Physics Research, 2001, A464: 569-575.
[19] IOAN B. Nanoparticle production by plasma [J]. Matel Sci Eng B, 1999, 68: 5-9.
[20] KAITO C. Coalescence growth mechanism of smoke particles [J]. Jpn J Appl Phys, 1985, 24: 261-264.
[21] SCOTT J H, MAJETICH S A. Morphology, structure, and growth of nanoparticles produced in a carbon arc [J]. Phys Rev B, 1995, 52: 12564-12571.
Foundation item: Project(3ZS042-B25-017) supported by the Natural Science Foundation of Gansu Province, China
Corresponding author: WEI Zhi-qiang; Tel: +86-931-2975732; E-mail: zqwei7411@163.com
(Edited by YUAN Sai-qian)