
Electrodeposition of dixanthogen(TETD) on pyrite surface
LI Wei-zhong(黎维中), QIN Wen-qing(覃文庆), SUN Wei(孙 伟), QIU Guan-zhou(邱冠周)
School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China
Received 20 July 2006; accepted 28 October 2006
Abstract: The electrochemical reaction of xanthate on the surface of pyrite was studied using cyclic voltammogrametry, chronopotentiometry and rotating-disc electrode measurements. Experimental results demonstrate that the first step in the reaction is electrochemical adsorption of xanthate ion, and then the adsorbed ion associates with a xanthate ion from the solution and forms a dixanthogen on the pyrite electrode surface. The diffusion coefficient of butyl xanthate on pyrite electrode surface can be determined to be about 1.09×10-6 cm2/s. Using the galvanostatic technique, the kinetic parameters of oxidation of the butyl xanthate ion on the pyrite surface are calculated as Ja=200 μA/cm2, β= 0.203 and J0=27.1 μA/cm2.
Key words: electrochemical reaction; pyrrhotite; xanthate
1 Introduction
In the past decade, much progress has been made in understanding the reactions of sulfide mineral surfaces with xanthate reagents. It is widely recognized that the reactions in a flotation system involve electron transfer. Many electrochemical techniques have been employed to study the reaction mechanism of sulfide minerals (such as pyrite, galena and chalcopyrite) with xanthate reagents[1]. As well known, flotation of pyrite is an electrochemical process, and adsorption of collectors on the surface of mineral results from the electron transfer between mineral surface and oxidation-reduction composition in pulp. These investigations indicate that the oxidation of both the mineral and the collector plays an important role in the flotation process. It is generally believed that the reactions produce the hydrophobic particle surfaces required in flotation, and the dominant hydrophobic reactions are electrochemical in nature. Many distinct anode processes can produce hydrophobic products, such as the chemical adsorption reaction of xanthate ions, and the oxidation reaction of the xanthate ion to its disulfide compound. It is important in flotation to identify the different anodic reactions coupled to a cathodic process that is generally represented by the reduction of oxygen[2-4].
Pyrite(FeS2 ) is the most abundant sulfide mineral in crust of earth and an important raw material of chemical engineering process. The surface chemistry and surface reactivity of FeS2 have been extensively studied, particularly their importance in the processing of sulfide ores[5-9]. The electrochemical reaction on the surface of pyrite during flotation has been studied well, but there are only a few references available on the oxidation chemical kinetics and mechanism of the reaction on its surface[10-14].
In this study, the electrochemical behavior of the pyrite electrode in a xanthate solution was investigated. Some advanced electrochemical techniques were employed to research the electrodeposit of dixanthogen on the pyrite surface.
2 Experimental
The pyrite used in this investigation consisted of 58.20% (mass fraction) Fe, 36.20%S, 3.1%SiO2 and was from Mengzi Mine of Yunnan Province, China. Sections cut from the highly mineralized pyrite were fashioned into the form of electrodes for electrochemical measurement.The cut section of mineral was mounted on the tip of a perspex tubule of d 7 mm using epoxy resin and the exposed outer surface was well polished. The exposed surface area of the electrode was about 1 cm2. The reference and auxiliary electrodes were saturated calomel electrode(SCE) and graphite rods, respectively. All potential in this study were quoted in volts, with respect to a standard hydrogen electrode (SHE).
The electrochemical measurements were performed with a conventional three-electrode cell using a electronics potentiostat/galvanostat model EG&G 273A and a model 636 Electrode Rotator. Both were operated by a computer. The EG&G corrosion measurement system (Princeton Applied Research Model 352) and Analysis Software (Model 270) were used in the experiment.
The studies were carried out using the pyrite as electrode in a 0.5 mol/L KCl solution in the presence of butyl xanthate.
3 Results and discussion
3.1 Two stages of electrochemical reaction on pyrite surface in butyl xanthate solution
While the potentiostat applies a constant current on the pyrite electrode for a specified duration, the overall consumed charge Q can be calculated by the following equation:
Q=Qθ+Qc (1)
where Qc is the charge consumed by the diffusion of xanthate ion on the pyrite surface, and Qθ is the charge consumed by the electrochemical adsorption of xanthate ion.
According to the Sand formula[15]:
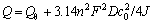 (2)
(2)
where n is the number of transfer electrons in reaction, F is the Farady constant, D is the diffusion coefficient of xanthate, c0 is the initial concentration of butyl xanthate ion, and J is the current density.
If the electrochemical adsorption of butyl xanthate ion on the pyrite surface did not occur, the charge consumed by the electrochemical adsorption of xanthate ion (Qθ) would be zero.
The value of Q can be verified using galvanostatic technique. Table 1 lists the results of galvanostatic experiments for a pyrite electrode at different currents.
Table 1 Results of galvanostatic experiments
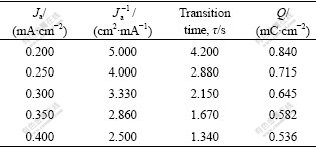
The Q value is linearly related with 1/J. Based on the results in Table 1, the plot of the consumed charge Q as a function of the reciprocal of current density (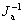 ) is drawn in Fig.1. From this figure, we calculate the charge consumed by the electrochemical adsorption of butyl xanthate ion (Qθ) to be equal to 0.242 mC/cm2, not zero, which means that an electrochemical adsorption of butyl xanthate ion takes place on the pyrite surface. The electrochemical absorption reaction of butyl xanthate ion on pyrite surface can be written as
) is drawn in Fig.1. From this figure, we calculate the charge consumed by the electrochemical adsorption of butyl xanthate ion (Qθ) to be equal to 0.242 mC/cm2, not zero, which means that an electrochemical adsorption of butyl xanthate ion takes place on the pyrite surface. The electrochemical absorption reaction of butyl xanthate ion on pyrite surface can be written as
X-→X(ads)+e (3)
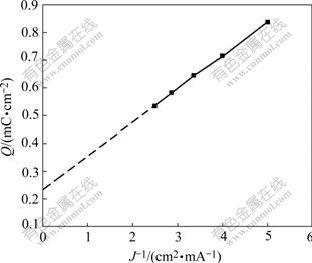
Fig.1 Relationship between overall charge and current density in galvanostatic experiment ([BX]=10-4mol/L, pH=9.18, 25 ℃)
Fig.2 shows the voltamograms in pH 9.18 buffer for a pyrite electrode starting from -0.4V (vs SHE) in the positive direction. There is an anodic peak appeared at 0.1 V, which indicates that an electrochemical reaction occurs. The possible reaction is the oxidation of butyl xanthate ion on the pyrite surface:
2X-→X2+2e (4)
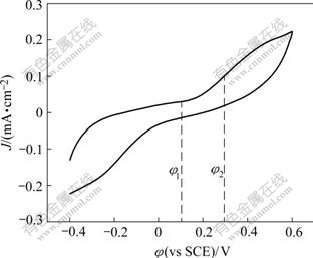
Fig.2 Voltammogram curves of pyrite electrode in presence of xanthate ([BX]=10-4mol/L, pH=9.18, 25 ℃)
φ=-0.128-0.059log[X-], [X-]=10-4mol/L, φ=0.108 V. So we can think that the oxidation of butyl xanthate ion on the pyrite surface is a consecutive charge transfer reaction. The electrochemical reaction can be divided into two stages: the first step is electrochemical adsorption of butyl xanthate ion, and then the adsorbed ion associates with a butyl xanthate ion from the solution and forms a dixanthogen on the sulfide mineral.
The oxidation process of xanthate ion is as
X(ads)+X-1(aq)→X2(ads)+e (5)
3.2 Determination of diffusion coefficient of butyl xanthate on pyrite surface
In potentiostatic experiment, the potentiostat applies a constant potential for a special duration and monitors the resulting current density, which can be used to investigate electrode process and to determine the diffusion coefficient of butyl xanthate. From Fig.2, there is a space of 0.70 V between the anodic shoulder and cathodic shoulder, and we can determine the production of dixanthogen(BX2) on pyrite surface is an irreversible reaction.
Fig.3 shows the plot of current density versus time for pyrite electrode in butyl xanthate solution in response to potentiostatic step of 0.10 V. The relationship between current density and time can be ascertained by the following equation:
J-1=-4.16×10-6+1.57×10-2t0.5 (6)
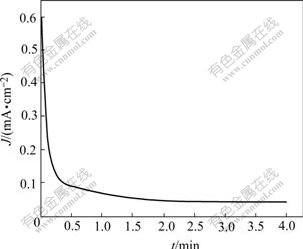
Fig.3 Current and time changes in response to potentiostatic step for pyrite electrode ([BX-]=10-4mol/L, pH=9.18, 25 ℃)
From an electrochemical reaction in the electrolyte solution, the suppositions are given as follows. 1) Diffusion of reactant obeys the second Fick law; the current caused by diffusion of reactant is in direct proportion to the superficial area of electrode surface; 2) The electrode process is controlled by diffusion of reactant; and 3) The electromigration and convection are neglected.
The boundary conditions are determined as follows. 1) The concentration of butyl xanthate in the system is equal to the initial concentration, ie. cX(x, 0)=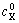 ; 2) The concentration of butyl xanthate at indefinite position away from the electrode is equal to the initial concentration, which is described by cX(∞, t)=
; 2) The concentration of butyl xanthate at indefinite position away from the electrode is equal to the initial concentration, which is described by cX(∞, t)=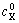 ; and 3) The concentration of butyl xanthate on the surface of electrode is zero, expressed by cX(0, t)=0.
; and 3) The concentration of butyl xanthate on the surface of electrode is zero, expressed by cX(0, t)=0.
The current density caused by diffusion can be given by
J=nFD[dcX(x, t)/dx]x=0 (7)
According to the second Fick law, the following equation can be given:
cX(x, t)=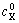 erf([x/2(Dt)0.5] (8)
erf([x/2(Dt)0.5] (8)
J=nF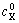 (D/πt)0.5 (9)
(D/πt)0.5 (9)
J-1=(nF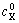 )-1(πt/D)0.5 (11)
)-1(πt/D)0.5 (11)
where D is the diffusion coefficient of butyl xanthate, 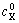 is the initial concentration of butyl xanthate, t is the reaction time, n is the number of transfer electrons in reaction, F is the Farady constant, and π is 3.141 6.
is the initial concentration of butyl xanthate, t is the reaction time, n is the number of transfer electrons in reaction, F is the Farady constant, and π is 3.141 6.
From Eqn.(5), the intercept is -4.16×10-6. It is small enough, so the relationship between current density and time in Eqn.(5) can be simplified as follows:
J-1=1.57×10-2t0.5 (12)
According to the Eqns.(10) and (11), the diffusion coefficient of butyl xanthate on pyrite electrode surface can be determined, and it is about 1.09×10-6 cm2/s.
3.3 Kinetics of electrochemical reaction on pyrite electrode surface
The oxidation of butyl xanthate ion on the pyrite electrode surface is an irreversible reaction, so there are suppositions given as follows. 1) The electromigration and convection are neglected; and 2) The variations in the concentration of xanthate are caused by diffusion.
The relationship between over potential of anodic reaction and the reaction time can be ascertained by the following equation:
η=(8.314T/nβF)ln(Ja/J0)-(8.314T/nβF)ln[1-(t/τ)1/2] (12)
where η is the over potential of the anodic reaction, T is the temperature, Ja is the galvanostatic current density, J0 is the exchange current density, τ is the transition time, t is the time of reaction, and β is the transmission coefficient.
In chronopotentiometry experiment, the potentiostat applies a constant current (200 μA/cm2) for a specified duration and monitors the resulting potential of pyrite electrode. Fig.4 shows the resulting curve of chronopotentiometry in butyl xanthate solution. From this plot, the transition time (τ) of the oxidation of xanthate ion on the pyrite surface can be determined as 4.73 s.
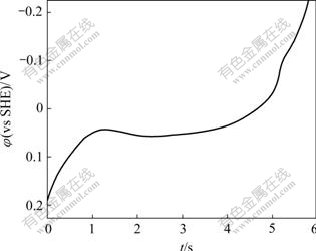
Fig.4 Potential and time changes in response to galvanostatic step for pyrite electrode ([BX]=10-4mol/L, pH=9.18, 25 ℃)
The oxidation of butyl xanthate ions on a pyrite surface is given in the following reaction:
2X-→X2+2e
The thermodynamic potential(φ) of this reaction can be calculated by the Nernst equilibrium:
φ=φ0-0.059log[X-], [X-]=10-4mol/L (13)
η=φt-φ
where φt is the value of the potential at time t.
As the function of log[1-(t/4.73)0.5], the change of the over potential(η) of the oxidation of butyl xanthate ion on pyrite surface can be plotted as Fig.5.
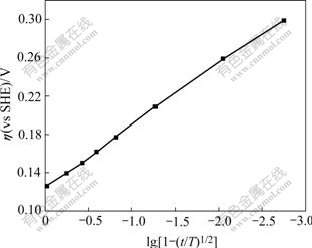
Fig.5 Relationship between over potential of pyrrhotite electrode and log[1-(t/τ)1/2] ([BX]=10-4mol/L, pH=9.18, 25 ℃)
From Fig.5, it can be seen that the over potential is linearly related to the log[1-(t/τ)1/2], and the relationship can be represented by following equation:
η=0.126-0.063log[1- (t/4.73)1/2] (14)
According to the Eqns.(12) and (14), because the value of R, T, F and τ are certain, the electrochemical kinetic parameters can be calculated as
Ja = 200 μA/cm2, β=0.203, J0=27.1 μA/cm2
4 Conclusions
1) The first step in the electrodeposit of butyl xanthate ion on the pyrite surface is the electrochemical adsorption of xanthate ion, and then the adsorbed ion associates with a xanthate ion from the solution and forms dixanthogen on the sulfide mineral.
2) The diffusion coefficient of butyl xanthate on pyrite electrode surface can be determined to be about 1.09×10-6 cm2/s.
3) The oxidation of the butyl xanthate ion on the pyrite surface produces dixanthogen. The kinetic parameters are calculated as Ja=200 μA/cm2, β=0.203, J0=27.1 μA/cm2.
3) For an effective flotation separation of pyrite from polysulfide minerals containing Pb, Cu, Zn, etc, it is important to control the formation of dixanthogen on the pyrite surface.
References
[1] KELSALL G H, YIN Q, VAUGHAN D J. Electrochemical oxidation of pyrite (FeS2) in aqueous electrolytes [J]. Journal of Electroanalytical Chemistry, 1999, 471(2): 116-125.
[2] FINKELSTEIN N P. The activation of sulphide minerals for flotation: a review [J]. International Journal of Mineral Processing, 1997, 52(2/3): 81-120.
[3] PATRICK R A D, ENGLAND K E R, CHARNOCK J M. Copper activation of sphalerite and its reaction with xanthate in relation to flotation: An X-ray absorption spectroscopy (reflection extended X-ray absorption fine structure) investigation [J]. International Journal of Mineral Processing, 1999, 55(4): 247-265.
[4] ZHANG Qin, HU Yu-hua, GU Guo-hua, NIE Zhen-yuan. Electrochemical flotation of ethyl xanthate-pyrrhotite system [J]. Trans Nonferrous Met Soc China, 2004, 14(6): 1174-1179.
[5] HUNG A, MUSCAT J. Density functional theory studies of pyrite FeS2 (111) and (210) surfaces [J]. Surface Science, 2002, 520(12): 111-119.
[6] OPAHLE I, KOEPERNIK K, ESCHRIG H. Full potential band structure calculation of iron pyrite [J]. Computational Materials Science, 2000, 17(24): 206-210.
[7] EDELBRO R, SANDSTR J M A, PAUL J. Full potential calculations on the electron band structures of sphalerite, pyrite and chalcopyrite [J]. Applied Surface Science, 2003, 206(14): 300-313.
[8] QIN Wen-qing, QIU Guan-zhou. Electrodeposition of dixanthogen on surface of pyrrhotite electrode [J]. Trans Nonferrous Met Soc China, 2000, 10(Special Issue): 61-63.
[9] QIN Wen-qing, LI Quan, QIU Guan-zhou, XU Ben-jun. Electrochemical oxidation of pyrrhotute in aqueous solution [J]. Trans Nonferrous Met Soc China, 2005, 15(4): 922-925.
[10] WANG Hui-xiang, WANG Dian-zuo, LI Bo-dan. Improved methods to determine the electrochemical Peltier heat using a thermistor I: Improved heat-sensor electrodes and lumped-heat-capacity analysis[J]. Journal of Electroanlytical Chemistry, 1995, 392(Issues 1/2): 21-25.
[11] WANG Hui-xiang, WANG Dian-zuo, LI Bo-dan. Improved methods to determine the electrochemical Peltier heat using a thermistor Ⅱ: Extreme and optimization methods [J]. Journal of Electroanlytical Chemistry, 1995, 392(Issues 1/2): 13-19.
[12] SONG S, LOPEZ-VALDIVIESO A, OJEDA-ESCAMILLA M C. Electrophoretic mobility study of the adsorption of alkyl xanthate ions on galena and sphalerite [J]. Journal of Colloid and Interface Science, 2001, 237(1): 70-75.
[13] SUN Wei, HU Yue-hua, QIU Guan-zhou, QIN Wen-qing. Oxygen adsorption on pyrite (100) surface by density functional theory [J]. Journal of Central South University of Technology, 2004, 11(4): 385-390.
[14] BUSWELL A M, BRADSHAW D J, HARRIS P J, EKMEKCI Z. The use of electrochemical measurements in the flotation of a platinum group minerals (PGM) bearing ore [J]. Minerals Engineering, 2002, 15(6): 395-404.
[15] MACDONALD D D. Transient Techniques in Electrochemistry [M]. New York: Plenum Press, 1977.
Foundation item: Project(50204013) supported by the National Natural Science Foundation of China
Corresponding author: QIN Wen-qing; Tel: +86-731-8879622; E-mail: QWQ@mail.csu.edu.cn
(Edited by YANG Bing)