文章编号: 1004-0609(2006)09-1634-07
浸矿菌Leptospirillum ferrooxidans
氧化Fe2+的电化学动力学
李宏煦1, 苍大强1, 董清海2, 王淀佐2
(1. 北京科技大学 冶金与生态工程学院, 教育部生态与循环冶金重点实验室, 北京 100083;
2. 北京有色金属研究总院, 北京 100088)
摘 要: 研究Leptospirillum ferrooxidans溶液氧化还原电位φ与Fe2+氧化的内在联系, 得到φ随溶液ln[c(Fe3+)/c(Fe2+)]的增加呈线性增长关系; 研究Fe2+、 细菌浓度、 温度变化时Fe2+氧化反应速率的变化规律。 在给定条件下, 当溶液中Fe2+浓度较低时, 随着Fe2+浓度的上升, Fe2+的氧化速率加快, 而当Fe2+浓度在5kg/m3以上时, Fe2+浓度的增加不但不会促使氧化速率加快, 反而会抑制Fe2+的氧化; 细菌浓度的增加, 氧化速率随之上升, 当细菌浓度在1.25×108cells/dm3以上时, 氧化速率随细菌浓度的增加有较大幅度的增长; 当温度在20~35℃范围时, 温度的升高会加快细菌氧化Fe2+的速率, 当温度再升高, 则会抑制Fe2+的氧化。 通过一系列电化学与生物化学分析, 导出了Leptospirillum ferrooxidan生长动力学方程, 并计算出活化能Ea、 频率因子K0、 表观饱和常数Km和单元附着系数K′i等动力学参数的值; 动力学模型能很好说明实验结果。
关键词: Leptospirillum ferrooxidans; 细菌浸出; 生物冶金; 混合电位; 动力学; 电化学 中图分类号: TF803 21; Q939 99
文献标识码: A
Electrochemical growth kinetics of Leptospirillum ferrooxidans
through oxidation of Fe2+
LI Hong-xu1, CANG Da-qiang1, DONG Qing-hai2, WANG Dian-zuo2
(1. Ecology and Recycling Metallurgy Laboratory of National Education Ministry,
School of Metallurgy and Ecology Engineering, University of Science and Technology Beijing,
Beijing 100083, China;
2. General Research Institute of Nonferrous Metals, Beijing 100083, China)
Abstract: The relatively relationship between solution rexdox potential and the ferrous iron oxidation rate was established, and it shows that the φ value increases linearly with ln[c(Fe3+)/c(Fe2+)]. All the ferrous ion, the bacteria cells concentration and temperature have apparent effect on the ferrous oxidation rate. When the concentration of ferrous is less than 5kg/m3 the oxidation of ferrous ion increases, however the increase of ferrous ion concentration up to 5kg/m3 would give inhibition of oxidation rate, as well as at the temperature range of 20℃ to 35℃, the increased temperature can enhance the ferrous ion oxidation, but when the temperature is above 40℃, the growth of bacteria inhibis and the oxidation decreases relatively. The increase of cells concentration of bacteria enhances the oxidation apparently especially when the concentration of cells increases to above 1.25×108cells/dm3. Based on the ferrous ion oxidation and redox potential the growth kinetics models were established and the value of activation energy (Ea), frequency factor (K0), saturation constant (Km), and substrate inhibition coefficient (Ki) were determined. The kinetics model is well in coincidence to the experiments results.
Key words: Leptospirillum ferrooxidans; bioleaching; biohydrometallurgy; redox mixed potential; kinetics; electrochemistry
生物冶金是一门将微生物学、 冶金学等学科结合起来的矿物资源生态化提取新技术[1], 在实际的硫化矿堆浸或搅拌浸矿过程中, 起作用的有Acidthiobacillus ferrooxidans、 Leptospirillum ferrooxidans等多种菌种[2, 3]。 以往对浸矿菌的研究主要集中在Acidthiobacillus ferrooxidans上, 而对于其他菌种的研究相对较少[4-7]; 与Acidthiobacillus ferrooxidans既氧化Fe2+、 又氧化元素硫而生长有所不同, Leptospirillum ferrooxidans是专门以氧化Fe2+而完成代谢的浸矿菌种[8]。 细菌氧化Fe2+具有非常重要的意义: 一方面, 作为能源Fe2+的氧化会为细菌的生长提供足够的能量; 另一方面, Fe2+的氧化会产生大量Fe3+, Fe3+会作为氧化剂氧化浸出硫化矿, 硫化矿氧化产生的Fe2+又会被细菌氧化为Fe3+以供浸矿, 如此反复, 这一作用又称细菌浸矿的间接作用。 随着细菌的生长, 浸矿体系的细菌数量会大大增加, Fe2+的氧化速率亦会不同, 浸出体系的氧化电位也会随之发生较大变化, 这必然对硫化矿的浸出产生较大影响[9-11]。 一般认为, 溶液电位越高, 越有利于硫化矿物的氧化分解, 但溶液电位受c(Fe3+)/c(Fe2+)变化的直接影响, 同时与细菌生长和温度等环境相关[11-14]。 有研究报道在搅拌细菌浸出的中后期, 起主要作用的不是Acidthiobacillus ferrooxidans, 而是Leptospirillum ferrooxidans[15, 16]。 尽管关于Leptospirillum ferrooxidans的生长及其对浸矿的影响研究有些报道[20, 21], 但从细菌生长与溶液电位变化的关系、 基质离子浓度、 细菌浓度、 温度与氧化速率关系入手, 定量研究Fe2+氧化和细菌生长的电化学动力学关系的并不多见。 本文作者将利用溶液中Fe3+/ Fe2+电对电位的变化, 较系统地研究Leptospirillum ferrooxidans的生长及Fe2+氧化的动力学。
1 实验
1.1 Leptospirillum ferrooxidans的分离与纯化
浸矿菌株Leptospirillum ferrooxidans由酸性矿坑水中分离、 纯化得到, 并根据实验需要驯化。 Leptospirillum ferrooxidans菌培养基用标准9K培养基, 其组成为: 3.0g/L (NH4)2SO4; 0.1g/L KCL; 0.5g/L K2HPO4; 0.5g/L MgSO4·7H2O; 0.01g/L Ca(NO2)3; 9g/L Fe2+; 以上试剂均为分析纯, 培养基用去离子水配制, 培养基pH根据需要用6mol/L H2SO4调节。
1.2 Leptospirillum ferrooxidans的培养
细菌培养采用动态培养方式。 将转接数次具有较高活性的纯Leptospirillum ferrooxidans菌株接入500mL含有200mL 9K培养基的摇瓶中, 放于摇床中动态培养, 培养液pH维持在2, 培养期间保持温度恒定, 摇瓶转速200r/min, 培养过程用软管给培养液充气, 保证氧的供应, 使其不为细菌生长过程动力学限制因素。
1.3 分析与溶液混合电位的测量
溶液中细菌的数量由显微计数法测定, Fe2+采用滴定法测得, 溶液中总铁则通过原子吸收法测定。 溶液混合电位测量时, 将铂片电极与参比电极(Ag/AgCl)插入细菌培养液中, 尽量使参比电极靠近铂片电极, 菌液混合电位通过pH电位计测量。
2 结果与讨论
2.1 细菌培养液中的混合电位
Leptospirillum ferrooxidans是专门以氧化Fe2+而完成代谢的浸矿菌种, Fe2+氧化响应其生长可用细胞外的电化学反应式来标度, 其过程可用下面3式表示
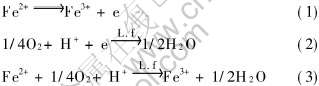
若忽略其它因素而只考虑铁离子的氧化还原对细菌培养液中混合电位的影响, 则溶液混合电位可用下式表示

图1所示为在不同温度下, 调整不同的c(Fe3+)和c(Fe2+), 计算所得的无菌溶液电位与ln[c(Fe3+)/c(Fe2+)]的关系。 由图可知, 在不同的温度下, 二者呈线性关系, 即式(4)成立。 且从直线的斜率可得φ0的值, 大约为695mV, 尽管低于氧化还原对Fe3+/Fe2+的标准理论值771mV, 但与Pesic等[19]的实验值(687mV)接近。 由式(4)可知, 随着细菌的生长, Fe2+逐步被氧化成Fe3+, 细菌数量随之增加, 培养液的电位随之正向上升。 在一定温度下, 氧化还原电对Fe3+/Fe2+的φ0值是一定的, 若量得φ值, 培养液中的总铁浓度已知, 且不考虑细菌生长后期黄钾铁钒生成的影响时, 便可知细菌生长过程中培养液Fe2+离子浓度的变化状况。 这为在不同物理化学条件下进一步研究细菌生长过程氧化Fe2+的动力学提供了可能。
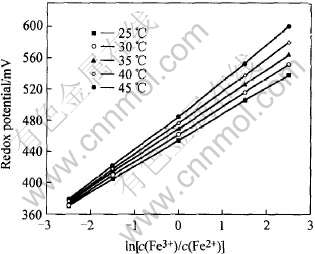
图1 混合电位与ln[c(Fe3+)/c(Fe2+)]的关系
Fig.1 Relationship between mixed potential and ln[c(Fe3+)/c(Fe2+)
2.2 不同初始Fe2+时有菌溶液电位的变化
当不加入Leptospirillum ferrooxidans菌时, 溶液的电位基本保持不变, 即在常温下, Fe2+的化学氧化是很微弱的。 而当加入细菌后, 在不同细菌浓度下溶液电位不同程度呈上升趋势。 图2所示是不同初始Fe2+浓度, 当接入细菌浓度在4.5×108cells/dm3时, 溶液混合电位与时间的关系, 此时保持温度为35℃。 由图可知, 随着细菌的生长繁殖, Fe2+逐步被氧化为Fe3+, Fe3+/Fe2+电位上升。 在较高的初始Fe2+浓度时, 尽管在该培养液下细菌会有较快的生长速度, 此时有较多的Fe2+被氧化, 但c(Fe3+)/c(Fe2+)比仍然不大, 故初始Fe2+浓度在30.0kg/m3时培养液的电位较初始浓度0.25kg/m3时小。 可见溶液的电位随时间的变化还不能直接用来评判Fe2+的细菌氧化速率及细菌生长动力学的规律, 要掌握其规律, 需进一步研究Fe2+氧化速率与溶液Fe2+浓度的关系。
2.3 Fe2+浓度与Fe2+氧化速率的关系
图3所示为温度恒定为35℃时, 不同细菌浓度下Fe2+氧化速率与溶液Fe2+浓度的关系。 从图
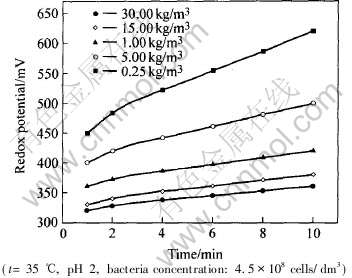
图2 不同初始Fe2+浓度时溶液电位随时间的变化
Fig.2 Variation of mixed potential of culture solution of different initial Fe2+ concentration with growth time
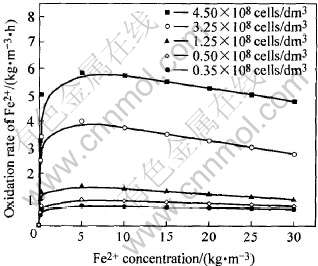
图3 温度35℃、 不同细菌浓度下Fe2+氧化速率与
溶液Fe2+浓度的关系
Fig.3 Relationship between Fe2+ oxidation rate and its concentration under different bacteria concentration at 35℃
中可以看出, 随着细菌浓度的上升, Fe2+的氧化速率加快, 如在相同的Fe2+下, 细菌浓度为4.50×108cells/mL时, Fe2+的氧化速率明显快于细菌浓度为0.35×108cells/mL时。 同时, 在每一细菌浓度下, 氧化速率随Fe2+浓度变化有一从小到大再逐步变小的过程, 并经过有一峰值的最大氧化速率的过程, 这和图2结果相同, 说明Fe2+大时, 溶液电位不一定最大。
这一结果可用如下动力学反应方程解释。 假设每一细菌含有一定量的酶(E)来催化氧化Fe2+, 可供细菌生长的Fe2+浓度为S, 生成的Fe3+浓度为P, 则作为细菌生长的基质Fe2+的浓度和细菌氧化速率的关系可用如下几个步骤表示:
细菌氧化酶与Fe2+结合

结合后Fe2+被氧化为Fe3+
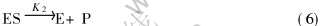
反应中间体ES与S生成非反应中间物ES2

由上述反应过程可以看出, 当细菌氧化酶和Fe2+接触时, 氧化酶通过反应(5)和铁离子结合, 此时, 氧化酶尽可能地与Fe2+结合, 接着通过反应(6)将Fe2+氧化成Fe3+同时释放出氧化物酶, 氧化物酶再结合Fe2+离子, 进一步进行氧化反应, 如此反复; 当Fe2+浓度较小时, Fe2+浓度的增加会加快反应(5)和(6)的进行, 即此时Fe2+浓度的增加会促进Fe2+的氧化反应; 随着Fe2+的增加和反应的进行, 反应(5)和(6)会达到一个动态平衡, 此时如反应式(3), 多余的反应基质S会和ES进一步结合为ES2, 此时, Fe2+的增加不仅不会促进Fe2+的氧化, 反而会降低氧化速率, 直到反应(7)达到动态平衡。 这就是图2和3中表现出的Fe2+浓度上升, 而氧化速率与电位反而下降的原因。
而Fe2+氧化速率可用修正的Michalis-Menten方程表示为[4, 5]

反应达到平衡的条件是初始氧化酶和基质Fe2+浓度的比值E0/S0足够小, 若基质Fe2+浓度和氧化酶浓度相比不足够大时, 反应达不到平衡, 则上述假设不成立。 对于细菌生长基质Fe2+对硫化矿浸矿菌的阻抑作用也有不同报道, Kelly和Jones对浸矿菌Thiobacillus ferrooxidans生长的研究结果认为, 当Fe2+浓度在5.6kg/m3以下时, 其浓度的上升会增加氧化速率, 而大于该浓度, 浓度的上升会对细菌的生长起阻抑作用[5]。
2.4 细菌浓度对Fe2+氧化速率的影响
细菌浓度对Fe2+氧化速率的影响在图3中已得到反应。 由图3可知, 在一定的温度下, 随着细菌浓度的增加, 同Fe2+浓度时的Fe2+氧化速率随之上升, 当细菌浓度由0.35×108cells/dm3增加到4.50×108cells/dm3时, Fe2+的最大氧化速率(氧化速率峰值)由0.65kg/(m3·h)提高到5.85kg/(m3·h), 且当细菌浓度增加到1.25×108cells/dm3以上时, 氧化速率随细菌浓度的增加有较大幅度的增长。 可见, 要使Fe2+的氧化速率提高, 需要保证相对较高的细菌浓度。
根据方程(8)对实验数据进行处理, 并对图3中的数据进行非线性回归, 可以得到细菌生长的动力学系数, 如表1所示。
表1 不同细菌浓度下细菌氧化Fe2+的动力学速率常数
Table 1 Kinetics rate constant of bacteria oxidizing Fe2+ under different bacteria concentrations
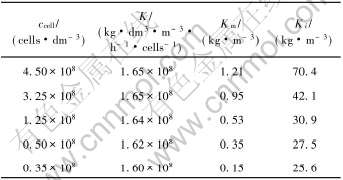
由表1数据可知, 细菌浓度对反应速率常数K的影响不大, 保持在1.60×108~1.65×108kg·m-3·h-1·cells-1·dm3之间, 基本恒定; 而对饱和常数Km和基质阻抑系数Ki影响较大, 当细菌浓度上升时, Km和Ki的值均有所增大, 且以Ki的增长最为明显。 当细菌浓度由0.35×108cells/dm3增加到4.50×108cells/dm3时, Km由0.31kg/m3增加到1.21kg/m3, Ki由25.6kg/m3增加到70.4kg/m3, 说明细菌的浓度的上升会影响吸附与氧化反应的平衡。
细菌浓度与Km变化关系如图4所示。 由图可知Km随细菌浓度的变化呈线性增长关系。 假设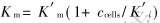 , 则可将式(8)变换为
, 则可将式(8)变换为
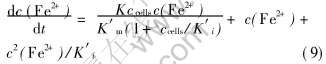
该反应速率方程能较好符合图4中的直线, 可较好说明细菌氧化Fe2+的反应动力学趋势, 从直线的斜率和截距可得到两动力学参数K′m和K′i的近似值分别为0.07kg/m3和2.70cells/dm3。
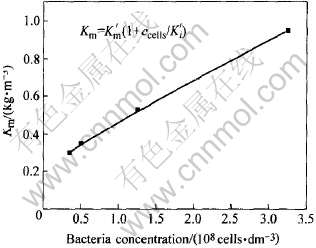
图4 细菌浓度与饱和系数Km的关系
Fig.4 Relationship between bacteria concentration and saturation coefficient
分析数据可知, 随着细菌浓度的上升, Ki随之增大, 可以认为, 细菌浓度的增加会降低基质的组抑效应, 即细菌浓度上升会增加细菌表面氧化物酶的吸附点, 会在较高的Fe2+浓度下达到动态平衡。 以Ki的导数随细菌浓度变化做图, 如图5所示, 1/Ki与细菌浓度的关系可表示为
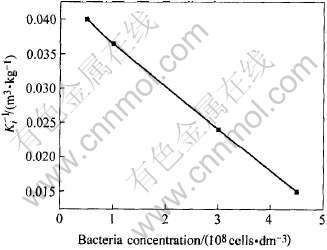
图5 细菌浓度与1/Ki的关系
Fig.5 Relationship between bacteria concentration and K-1i
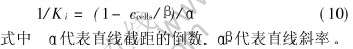
根据图中直线的斜率和截距, 计算出α值为27.1kg/m3, β值为8.0×108cells/dm3。
2.5 温度对Fe2+氧化速率的影响
不同温度下Fe2+氧化速率与其浓度的关系如图6所示。 由图中曲线可知, 当温度在20~35℃范围时, 温度的升高会加快细菌氧化Fe2+的速率, 当温度再升高, 则会抑制Fe2+的氧化, 如温度达到40℃时, 氧化速率曲线位于35和30℃氧化速率曲线之下。 同样图中曲线显示了Fe2+的阻抑效应, 起始随着Fe2+浓度的增大, 氧化速率加快, 当达到最大值后, 氧化速率随着Fe2+浓度的上升反而呈下降趋势, 这同前面结果一致。
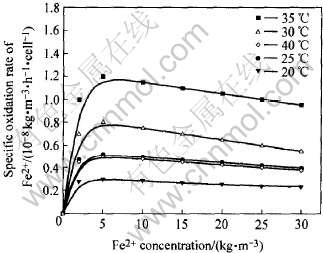
图6 温度对细菌氧化Fe2+速率的影响
Fig.6 Influence of temperature on oxidation rate of Fe2+ by bacteria
通过实验数据和应用方程式(9)可以确定不同温度下速率常数K的值。 反应热力学温度的升高会得到较高的K值。 根据化学和酶反应标准Arrhenius方程, 反应速率常数K与温度的关系为
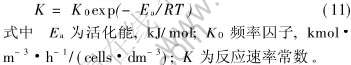
取对数, 对lnK与1/T做图, 得一直线如图7所示。 根据直线的斜率和截距, 得到活化能Ea为73.5kJ/mol, 频率因子K0值为6492kmol·m-3·h-1·cells-1·dm3。 应用同样的方法, Ahonen等[20]研究过T.ferrooxidans生长的活化能, 其值一般在33至96kJ/mol, 本次对Leptospirillum ferrooxidans活化能的结果在其范围内, 可供参考。
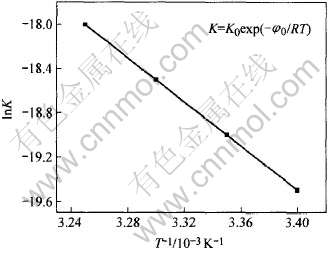
图7 lnK与1/T的关系
Fig.7 Relationship between lnK and T-1
将式(10)、 (11)代入式(9)可得细菌氧化Fe2+而生长的变换形式的动力学方程式:
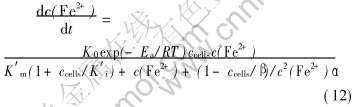
式中的K0、 Ea、 K′m、 K′i、 α、 β等动力学参数的值已在前面分别得到, 代入式(12)便得到了量化的Leptospirillum ferrooxidans氧化Fe2+而生长的动力学方程, 并能很好地说明实验结果。
3 结论
1) 溶液氧化还原电位φ与浸矿菌Leptospirillum ferrooxidan的生长及Fe2+的氧化速率有内在的联系, 研究认为溶液混合电位φ随溶液ln[c(Fe3+)/c(Fe2+)]的变化呈线性增长关系。
2) 当溶液温度恒定时, 一定的细菌浓度下, 溶液中Fe2+浓度较低时, 如温度35℃, c(Fe2+)在5kg/m3以下时, 随着Fe2+浓度的上升, Fe2+的氧化速率加快, 而当c(Fe2+)在5kg/m3以上时, 随着Fe2+浓度的上升, Fe2+的氧化速率下降, 即经过一最大氧化速率值后, Fe2+浓度的增加不但不会促使氧化速率加快, 反而对Fe2+的氧化有抑制作用。 这是由于溶液中的Fe2+与细菌表面的氧化物酶结合过程存在竞争吸附关系, 当达到Fe2+与氧化物酶的结合及Fe2+的氧化、 生成的Fe3+脱附的动态平衡后, Fe2+的增加会出现阻抑作用。
3) 在一定的温度下, 随着细菌浓度的增加, 氧化速率随之上升, 当细菌浓度由0.35×108cells/dm3增加到4.50×108cells/dm3时, Fe2+的最大氧化速率(氧化速率峰值)由0.65提高到5.85kg·m-3·h-1, 且当细菌浓度增加到1.25×108cells/dm3以上时, 氧化速率随细菌浓度的增加有较大幅度的增长。 当温度在20~35℃范围时, 温度的升高会促进细菌氧化Fe2+的速率, 当温度再大, 则会抑制Fe2+的氧化, 如温度达到40℃时, 氧化速率曲线在35和30℃氧化速率曲线之下。
4) 通过一系列电化学与生物化学分析, 导出了Leptospirillum ferrooxidan生长动力学方程, 计算出了动力学模型中活化能、 频率因子等各参数值。
REFERENCES
[1]Fernando Acevedo. Present and future of bioleaching in developing countries[J]. EJB Electronic Journal of Biotechnology ISSN, 2002, 5(2): 196-199.
[2]李宏煦, 王淀佐. 生物冶金中的微生物及其作用[J]. 有色金属, 2003, 55(2): 60-63.
LI Hong-xu, WANG Dian-zuo. Review of investigation on microoganism behaviors in ore bioleaching[J]. Nonferrous Metals, 2003, 55(2): 60-63.
[3]Suzuki I. Microbial leaching of metals from sulfide minerals[J]. Biotechnology Advances, 2001, 19: 119-132.
[4]Lang D S, Lawson F. Kinetics of the liquid-phase oxidation of acid ferrous sulfate by the bacterium Thiobacillus ferrooxidans[J]. Biotechnol Bioeng, 1970, 12: 29-50.
[5]Eccleston M, Kelly D P. Oxidation kinetics and chemostat growth kinetics of Thiobacillus ferrooxidans on tetrathionate and thiosulfate[J]. J Bacteriol, 1978, 134: 718-727.
[6]Dispirito A A, Tuovine O H. Kineticies of uranous and ferrous iron oxidation by Thiobacillus ferrooxidans[J]. Arch Microbiol, 1982, 133: 33-37.
[7]Nagpal S. A structured model for Thiobacillus ferrooxidans growth on ferrous iron[J]. Biotechnology and Bioengineering, 1997, 53: 310-319.
[8]Mignone C F, Donati E R. ATP requirements for growth and maintenance of iron-oxidizing bacteria[J]. Journal Biochemical Engineering, 2004, 18: 211-216.
[9]李宏煦, 王淀佐, 陈景河. 细菌浸矿的间接作用分析[J]. 有色金属, 2003, 55(4): 98- 100.
LI Hong-xu, WANG Dian-zuo, CHEN Jing-he. Discussion on indirect mechanism of bioleaching[J]. Nonferrous Metals, 2003, 55(4): 98-100.
[10]李宏煦, 王淀佐, 陈景河, 等. 细菌浸矿作用分析[J]. 有色金属, 2003, 55(3): 68-71.
LI Hong-xu, WANG Dian-zuo, CHEN Jing-he, et al. Description of microbe effects on sulfide mineral bioleaching[J]. Nonferrous Metals, 2003, 55(3): 68-71.
[11]李宏煦, 王淀佐. 硫化矿细菌浸出过程的电化学(Ⅱ)[J]. 矿冶, 2003, 12(2): 45-48.
LI Hong-xu, WANG Dian-zuo. Electrochemistry of sulfide bioleaching(Ⅱ)[J]. Mining and Metallurgy, 2003, 12(2): 45-48.
[12]李宏煦, 王淀佐. 硫化矿细菌浸出过程的电化学(Ⅰ)[J]. 矿冶, 2003, 12(1): 49-51.
LI Hong-xu, WANG Dian-zuo. Electrochemistry of sulfide bioleaching(Ⅰ)[J]. Mining and Metallurgy, 2003, 12(1): 49-51.
[13]LI Hong-xu, WANG Dian-zuo, QIU Guan-zhou, et al. Growth kinetics of Thiobacillus ferrooxidans in bioelectrochemical cell[J]. J Cent Technol, 2004, 11(1): 36-40.
[14]李宏煦, 王淀佐, 胡岳华, 等. Fe2+在T.f菌修饰粉末微电极表面氧化的电化学[J]. 中国有色金属学报, 2002, 12(6): 1263-1267.
LI Hong-xu, WANG Dian-zuo, HU Yue-hua, et al. Electrochemistry on oxidation of Fe2+ on Thiobacillus ferrooxidans modified powder microelectrode[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(6): 1263-1267.
[15]Falco L, Pogliani C, Curutchet G, et al. A comparison of bioleaching of covellite using pure cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a mixed culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans[J]. Hydrometallurgy, 2003, 71: 31-36.
[16]Boon M, Brasser H J, Hansford G S, et al. Comparison of the oxidation kinetics of different pyrites in the presence of Thiobacillus ferrooxidans or Leptospirillum ferrooxidans[J]. Hydrometallurgy, 1999, 53: 57-72.
[17]Breed A W, Hansford G S. Effect of pH on ferrous-iron oxidation kinetics of Leptospirillum ferrooxidans in continuous culture[J]. Biochemical Engineering, 1999, 13: 193-201.
[18]Dempers C J N, Breed A W, Hansford G S. The kinetics of ferrous-iron oxidation by Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans: effect of cell maintenance[J]. Biochemical Engineering, 2003, 16: 337-346.
[19]Pesic B, Oilver D J. An electrochemical method of measuring the oxidation rate of ferrous to ferric iron with oxygen in the presence of Thiobacillus ferrooxidans[J]. Biotechnology and Bioengineering, 1989, 33: 428-439.
[20]Ahoned L, Tuovinen O. Microbial oxidation of ferrous iron at low temperature[J]. Appl Environ Microbiol, 1989, 55: 312-316.
基金项目: 国家自然科学基金资助项目(50204001); 国家重点基础研究发展计划资助项目(2004CB619205-2)
收稿日期: 2005-09-19; 修订日期: 2006-03-10
通讯作者: 李宏煦, 博士, 副教授; 电话: 010-62332786; E-mail: lihongxu2001@126.com
(编辑 龙怀中)