盐酸-磷酸分解钼酸钙的动力学
来源期刊:中国有色金属学报(英文版)2019年第4期
论文作者:张文娟 王成彦 马保中
文章页码:859 - 867
关键词:钼酸钙;浸出动力学;磷酸;盐酸
Key words:calcium molybdate; leaching kinetics; phosphoric acid; hydrochloric acid
摘 要:钼酸钙是钼的一次资源,也是钼湿法和火法冶金过程中重要的中间产物。采用盐酸-磷酸混酸浸出钼酸钙以提取钼,为了了解浸出过程的机理,通过对浸出过程5个主要影响因素的研究,进行浸出过程动力学分析。结果表明:钼酸钙在该体系中的分解速率不受搅拌速度的影响,而受盐酸浓度、温度和粒径的影响比较显著;随着盐酸浓度增加和反应温度上升,浸出速率增加;随着粒径增大,反应速率降低。钼酸钙在该体系中的反应受收缩核模型的化学反应控制,所得反应的表观活化能为70.879 kJ/mol,并建立描述反应过程的半经验动力学方程。
Abstract: Calcium molybdate (CaMoO4) is the main component of powellite and is a predominant intermediate in the pyrometallurgical and hydrometallurgical process of molybdenum. The extraction of Mo from CaMoO4 by a combination of phosphoric acid and hydrochloric acid was investigated. For further understanding of the leaching mechanism, the effects of five key factors were studied to describe the leaching kinetics. The results indicated that the dissolution rate of CaMoO4 was independent of the stirring speed. Mo extraction significantly increased with increasing HCl concentration and temperature, but decreased with increasing particle size. A shrinking core model with surface chemical reaction was found to withstand the dissolution of CaMoO4. The apparent activation energy was calculated to be 70.879 kJ/mol, and a semi-empirical equation was derived for the rate of reaction.
Trans. Nonferrous Met. Soc. China 29(2019) 859-867
Wen-juan ZHANG, Cheng-yan WANG, Bao-zhong MA
School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China
Received 14 May 2018; accepted 10 December 2018
Abstract: Calcium molybdate (CaMoO4) is the main component of powellite and is a predominant intermediate in the pyrometallurgical and hydrometallurgical process of molybdenum. The extraction of Mo from CaMoO4 by a combination of phosphoric acid and hydrochloric acid was investigated. For further understanding of the leaching mechanism, the effects of five key factors were studied to describe the leaching kinetics. The results indicated that the dissolution rate of CaMoO4 was independent of the stirring speed. Mo extraction significantly increased with increasing HCl concentration and temperature, but decreased with increasing particle size. A shrinking core model with surface chemical reaction was found to withstand the dissolution of CaMoO4. The apparent activation energy was calculated to be 70.879 kJ/mol, and a semi-empirical equation was derived for the rate of reaction.
Key words: calcium molybdate; leaching kinetics; phosphoric acid; hydrochloric acid
1 Introduction
CaMoO4 is the main component of powellite, which is considered as a primary Mo resource. In general, powellite occurs in the oxidation zone of molybdenite deposits or co-exists with tungsten minerals as isomorphic impurities in nature [1,2]. Furthermore, CaMoO4 appears naturally as an important intermediate, generated in pyrometallurgical and hydrometallurgical processes of extracting Mo: (1) roasting molybdenite or Ni-Mo ore in the presence of lime [3,4]; (2) extracting Mo from the alkaline leachate [5]; (3) recycling Mo from scraps and spent catalysts [6,7]; (4) treating waste liquors containing Mo with calcium salts [8].
SINGH et al [9] obtained CaMoO4 by roasting low-grade MoS2 with limestone to protect the environment against SO2 pollution. WANG et al [10] processed a low-grade Ni-Mo ore by pyrometallurgical pretreatment, in which all Mo-bearing compounds were converted to CaMoO4. DOUGLAS et al [11] patented a process to recover Mo, W and V from alloy scraps, in which a mixed solid comprising CaMoO4, CaWO4 and CaO×nV2O5 was produced. Experimental studies by WANG et al [12] showed that a CaMoO4 product of 99.2% purity was prepared through the decomposition of leaching residue of Ni-Mo ore in sodium hydroxide medium, followed by the addition of CaCl2 in leaching liquor. NGUYEN and LEE [13] obtained CaMoO4 powders with uniform morphology by recovering Mo from spent hydrodesulphurization catalysts.
The CaMoO4 obtained from the above processes is usually treated by a hydrometallurgical process to produce molybdate compounds. The leaching of CaMoO4 is a subject attracting great attentions, and various reagents have been adopted in CaMoO4 decomposition. According to the study of ILHAN et al [14], the leaching reaction of CaMoO4 in H2C2O4 solution was processed in two steps. SINGH et al [9] extracted Mo from a low grade molybdenite concentrate by roasting with slaked lime, ensued leaching of the calcine (CaMoO4) with dilute sulfuric acid, recovering 99% of Mo. ZHANG et al [15] reported a process for leaching a mixed solid of CaMoO4 and CaWO4 in HCl solution containing H2O2, with the extraction of Mo and W reaching 97.7% and 99.4%, respectively. XIA et al [16] studied the thermodynamics of decomposition of CaMoO4 by sodium carbonate [16], and PAN et al [17] investigated the alkaline pressure leaching of powellite. Generally, the alkaline leaching process was usually conducted at elevated temperature and comparatively large amounts of reagents to obtain high yields. By contrast, the acid leaching process can be carried out at atmosphere pressure and demonstrate more advantages with the development of a direct Mo extraction process under acidic conditions.
During acid decomposition, Mo can form the soluble heteropoly acid with impurities phosphorous, arsenic and silicon, having a 1:6-1:12 molar ratio of impurities to Mo [18], such that part of molybdenum enters into solution while the rest is left as solid in the residue. Furthermore, molybdenum in solution exists in two forms: cation and heteropoly acid anion, influencing the subsequent extraction effect. However, this implies that if adding a small amount of phosphorous, CaMoO4 might be completely leached. Actually, there is no doubt about that. So far, many leaching studies have been conducted to understand the decomposition behavior and mechanism of CaWO4 under acid leaching conditions in the presence of phosphorous [19-21]. Considering the isomorphous structure of CaMoO4 with CaWO4, it can be inferred that the leaching of Mo resembles that of tungsten. Nevertheless, few studies have reported on the Mo leaching from synthetic CaMoO4 or powellite using acid medium with phosphorous. The objective of this study is to determine the reaction kinetics for CaMoO4 dissolution in HCl solution with H3PO4 as a chelating agent. Leaching experiments were conducted with different stirring speeds, leaching temperatures, HCl concentrations, H3PO4 concentrations and particle sizes. Results from experiments are used to clarify the dissolution mechanism of CaMoO4 in the mixed acid and a mathematical model of the leaching kinetics is also established. Furthermore, a novel process for Mo recovery from different resources is proposed.
2 Experimental
2.1 Materials
CaMoO4 was prepared by sedimentation of sodium molybdate and calcium chloride in the aqueous solution. Since the product after drying is a fine powder, it was subjected to melting at 1823 K to obtain a dense solid. The mass was finely ground and sieved to different size fractions (106-180, 75-106, 48-75 and 38-48 μm). The phase composition was determined using XRD with Cu Kα as source. The XRD pattern (Fig. 1) showed that peaks of the sample were in perfect agreement with CaMoO4 peaks given in the PDF card 07-0212.

Fig. 1 XRD pattern of prepared sample
In the present investigation, all the aqueous solution was prepared using analytical reagents and de-ionized water.
2.2 Procedure
The leaching experiments were carried out using a 500 mL round-bottom flask in thermostatic water bath. The reactor was loaded with 250 mL of acid solution with a desired concentration. When the temperature of the acid solution met the target operating temperature, 1 g of sample was added into the reaction vessel with a certain stirring speed. 1 mL of solution was periodically withdrawn for analysis. For keeping the volume of the solution constant, 1 mL of fresh lixiviant was re-added after each sampling. The concentration of Mo was analyzed by using the ICP-AES instrument, and then the leaching rate was calculated.
3 Result and discussion
3.1 Leaching chemistry of molybdenum in solution
To improve understanding of the chemical reaction during the leaching process of CaMoO4, a thermodynamic analysis with the aid of lg c-pH diagrams was carried out. The predominance area and species distribution diagrams were calculated with the data collected from relevant references under the conditions of [CaMoO4(s)]T = 0.1 mol/L and [P]T=0.1 mol/L, as shown in Fig. 2 [22-24]. In the diagram, CaMoO4(s) was observed in the pH range from 2.7 to 7.8. At 0≤pH≤2.7, there was no solid phase. In this region, CaMoO4(s) was completely dissolved, and the thermo- dynamic calculation indicated that heteropoly compound species  and
and 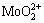 became predominant with the decrease in pH value, while
became predominant with the decrease in pH value, while  predominates with the increasing pH value in turn. This indicated that the reaction was fundamentally different from the leaching without phosphorus. When the pH value was greater than 5.7, CaMoO4(s) started to be decomposed and Ca5(PO4)3OH(s) was formed. Further- more, as the pH value was increased to 7.8, CaMoO4(s) was completely transformed into Ca5(PO4)3OH(s). Thermodynamic analysis confirmed that CaMoO4(s) can be decomposed with the solution containing phosphorus, as the solution pH value is a key factor.
predominates with the increasing pH value in turn. This indicated that the reaction was fundamentally different from the leaching without phosphorus. When the pH value was greater than 5.7, CaMoO4(s) started to be decomposed and Ca5(PO4)3OH(s) was formed. Further- more, as the pH value was increased to 7.8, CaMoO4(s) was completely transformed into Ca5(PO4)3OH(s). Thermodynamic analysis confirmed that CaMoO4(s) can be decomposed with the solution containing phosphorus, as the solution pH value is a key factor.

Fig. 2 lg C-pH (a, b) and Mo soluble species distribution (c, d) diagrams of Ca-Mo-P-H2O system ([CaMoO4(s)]T=0.1 mol/L and [P]T=0.1 mol/L)
3.2 Effect of parameters
According to the general rules of the fluid-solid heterogeneous reaction kinetics, temperature, HCl concentration, H3PO4 concentration, stirring speed, and particle size are key factors influencing the dissolution process. Thus, these five parameters were selected to be investigated in this study, as shown in Fig. 3. While the effect of one factor was studied, the other factors were kept constant.
The effect of stirring speed on the dissolution of CaMoO4 was studied in the range of 200-400 r/min. Figure 3(a) shows that leaching rate was practically independent of stirring speed. The phenomenon indicates that the mass transfer was not significantly affected by the fluid film around the solid particles and the leaching of Mo was under chemical reaction control. Hence, an intermediate rate of 300 r/min was applied for all the subsequent experiments to avoid film diffusion as a control mechanism and assure independence of this variable.
Figure 3(b) illustrates the change in Mo extraction as a function of temperature in the range of 313-343 K. It is illustrated that the extraction of Mo gradually increases with rising leaching temperature. Mo extraction after 20 min dissolution increases from 20.94% to 92.66%, as the leaching temperature increases from 313 to 343 K. This increase is due to the increase in the reaction velocity constant k with the development of reaction temperature.
The influence of the HCl concentration on Mo extraction is presented in Fig. 3(c). Dissolution curves show that the Mo extraction is strongly dependent on the HCl concentration. Moreover, the leaching rate increases with increasing HCl concentration, as the Mo extraction at 75 min improves from 54.34% to 91.56% when the HCl concentration increases from 1 to 3 mol/L.
Similar experiments were performed to investigate the effect of H3PO4 concentration in the range of 0.1- 0.5 mol/L. Results in Fig. 3(d) show that the variations in H3PO4 concentration have a moderate effect on Mo extraction in the investigated range.

Fig. 3 Effect of different parameters on Mo extraction
Figure 3(e) depicts the influence of particle size on CaMoO4 dissolution, and a significant enhancing effect with decrease in particle size is observed. The Mo extraction at 55 min reaches 63.39% for 106-180 μm particles and 85.94% for 38-48 μm particles, respectively. The results also indicate that tinier particle size has a significant accelerative effect on the dissolution rate, due to the increase in specific solid surface area and particle-solution contact with decreasing particle size.
3.3 Kinetic analysis
In a fluid-solid heterogeneous reaction system, the reaction rate may be controlled by the chemical reaction at the surface of the solid particles, diffusion through the fluid film and/or diffusion through the product layer. As mentioned in Fig. 3(a), the diffusion through the liquid film fails to behave as the rate-controlling step under a string speed of 200 r/min or higher. Furthermore, no solid product is obtained in this leaching system and all the particles are dissolved at the end. Therefore, no solid layer wraps the unreacted core as the reaction progresses. Consequently, the leaching process is assumed to be controlled by the surface chemical reaction. Assuming that the sample particles have spherical geometry and gradually shrink during leaching (shown in Fig. 4), the core-shrinking model can be used to describe the kinetics of the leaching process [25,26]. The kinetic parameters were determined and a quantitative measurement of the leaching mechanism was established by analyzing the Mo extraction and further calculation against the shrinking core model.

Fig. 4 SEM images of raw material (a), residues after leaching for 10 min (b) and for 25 min (c) (temperature 333 K, particle size 106-180 μm, C(HCl)=2.0 mol/L, C(H3PO4)=0.1 mol/L)
In a leaching system under surface chemical reaction, the reacted fraction at any time can be obtained from Eq. (1):
 (1)
(1)
where k is the apparent rate constant, kr is the surface chemical reaction rate constant, r0 is the initial radius of particle, C is the concentration of the reactant, n is the empirical reaction order, ρ is the density of the solid, and t is the reaction time.
According to Eq. (1), plots of 1-(1-x)1/3 versus reaction time are depicted in Fig. 5, and values of the correlation coefficient (R2) are illustrated in Table 1. There is a good fit between the experimental data and Eq. (1), suggesting that the leaching kinetics can be exactly described by the shrinking core model with chemical control. Moreover, the apparent rate constant k is calculated as the slope of the straight line.
The influence of the leaching temperature on the kinetics can be characterized by the activation energy. Generally, an activation energy of greater than 40 kJ/mol indicates that the chemical reaction controls the leaching rate, while that lower than 20 kJ/mol implies a diffusion-controlled process. Using the apparent rate constants obtained in Fig. 5(a), the Arrhenius plot of ln k vs 1/T is illustrated in Fig. 6(a). According to the Arrhenius equation (Eq. (2)), the activation energy for the leaching reaction is calculated to be 70.879 kJ/mol (>40 kJ/mol), which clearly confirms that the chemical reaction is most likely to be the controlled step in this leaching process.
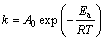 (2)
(2)
where k is the apparent rate constant, A0 is the pre-exponential factor, Ea is the apparent activation energy, R is the universal gas constant and T is the absolute temperature.
The results indicate that CaMoO4 dissolution is positively affected by HCl concentration and moderately affected by H3PO4 concentration. To determine the order of reaction with respect to HCl and H3PO4 concentration, the data from Fig. 3(c) and Fig. 3(d) were applied to this kinetic model. The k values for each acid concentration were determined from the slopes of the straight lines presented in Fig. 5(b) and Fig. 5(c). From the k values and acid concentrations, plots of ln k versus ln C(HCl) and ln C(H3PO4) were obtained. As seen in Fig. 6(b) and Fig. 6(c), the empirical reaction orders for the concentration of HCl and H3PO4 are 0.861 and 0.113.
From the variation in the kinetics model at various particle sizes, the values of apparent rate constant were determined. A plot of ln k as a function ln r according to Eq. (1) yields a linear relationship in Fig. 6(d) with a correlation coefficient of 0.983, supporting the proposed shrinking-sphere chemical controlled mechanism.

Fig. 5 Plots of 1-(1-x)1/3 versus time for different parameters
Table 1 Parameters of 1-(1-x)1/3 versus time for all experimental data

Calculations show that activation energy and the order of reaction values with respect to HCl concentration, H3PO4 concentration and particle size validate the shrinking core model as a chemical reaction process. Thus, the apparent rate constant can be clearly presented by Eq. (3):
 (3)
(3)
According to Fig. 5(a), the Arrhenius equation is as follows:
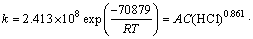
 (4)
(4)
Substituting C(HCl) of 2.0 mol/L, C(H3PO4) of 0.1 mol/L and r0 of 143 μm, gives A amount of 4.768×108. Consequently, the kinetics expression used to describe the leaching process of CaMoO4 in HCl-H3PO4 solution can be presented as
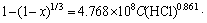
 (5)
(5)
3.4 Proposed process for molybdenum values recovering
CaMoO4 is obtained from pyrometallurgical and/or hydrometallurgical processes of various molybdenum values, such as Ni-Mo ore, molybdenite, spent catalysts and powellite. The results of the present work demonstrated that the extraction of Mo from CaMoO4 with HCl-H3PO4 solution was feasible on the bench scale. The kinetics analysis revealed the reaction mechanism of CaMoO4 in the mixed acid and also contributed to industrialized design. Based on the results and previous researches, a simplified flowsheet for recovery Mo from different Mo-bearing resources is proposed (Fig. 7).

Fig. 6 Plots of ln k as function of 1/T (a), ln C(HCl) (b), ln C(H3PO4) (c) and ln r (d)

Fig. 7 Proposed flowsheet for Mo recovery from different resources
4 Conclusions
(1) CaMoO4 can be effectively decomposed in the form of heteropoly compound through leaching with HCl solutions in the presence of phosphorus at room temperature, because the solid H2MoO4 is not produced. Results from the experiments conform well to thermodynamic analysis.
(2) Under conditions where the Mo dissolution reaction was unlimited by mass transfer, the measured rate was not a function of stirring speed, but it was considerably dependent on temperature, HCl concentration, H3PO4 concentration and particle size.
(3) The experimental data accurately agreed with the shrinking core model, with surface chemical reaction as the rate-controlling step. The activation energy for the temperature range of 313-343 K was calculated to be 70.879 kJ/mol and the following kinetic equation was established as


References
[1] LASHEEN T A, EL-AHMADY M E, HASSIB H B, HELAL A S. Molybdenum metallurgy review: Hydrometallurgical routes to recovery of molybdenum from ores and mineral raw materials [J]. Mineral Processing and Extractive Metallurgy Review, 2015, 36(3): 145-173.
[2] LI Hong-gui. Production of high purity APT from scheelite and complex tungsten raw materials with a high Mo content [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(2): 366-369.
[3] JUNEJA J M, SINGH S, BOSE D K. Investigations on the extraction of molybdenum and rhenium values from low grade molybdenite concentrate [J]. Hydrometallurgy, 1996, 41(2-3): 201-209.
[4] GAN Min, FAN Xiao-hui, CHEN Xu-ling, WU Cheng-qin, JI Zhi-yun, WANG Song-rong, WANG Guo-jing, QIU Guan-zhou, JIANG Tao. Reaction mechanisms of low-grade molybdenum concentrate during calcification roasting process [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(11): 3015-3023.
[5] ZHANG Qi-xiu, ZHAO Qin-sheng. Tungsten and molybdenum metallurgy [M]. Beijing: Metallurgical Industry Press, 2007. (in Chinese )
[6] LUO L, KEJUN L, SHIBAYAMA A, YEN W, FUJITA T, SHINDO O, KATAI A. Recovery of tungsten and vanadium from tungsten alloy scrap [J]. Hydrometallurgy, 2004, 72(1): 1-8.
[7] ZENG Li, CHENG Chu-yong. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts: Part I: Metallurgical processes [J]. Hydrometallurgy, 2009, 98(1): 1-9.
[8] SWINKELS P L J, van der WEIJDEN R D, AJAH A N, ARIFIN Y, LOE H L, MANIK M H, SIRISKI I, REUTER M A. Conceptual process design as a prerequisite for solving environmental problems: A case study of molybdenum removal and recovery from wastewater [J]. Minerals Engineering, 2004, 17(2): 205-215.
[9] SINGH S, CHETTY M K, JUNEJA J M, SEHRA J C, GUPTA C K. Studies on the processing of a low grade molybdenite concentrate by lime roasting [J]. Minerals Engineering, 1988, 1(4): 337-342.
[10] WANG Ming-yu, WANG Xue-wen, LIU Wan-li. A novel technology of molybdenum extraction from low grade Ni-Mo ore [J]. Hydrometallurgy, 2009, 97(1): 126-130.
[11] DOUGLAS D A, MENASHI J, RAPPAS A S. Process for recovering chromium, vanadium, molybdenum and tungsten values from a feed material: US 4298581 [P]. 1981-11-3.
[12] WANG Ming-shuang, WEI Chang, FAN Gang, DENG Zhi-gan, WANG Si-fu, WU Jun. Molybdenum recovery from oxygen pressure water leaching residue of Ni-Mo ore [J]. Rare Metals, 2013, 32(2): 208-212.
[13] NGUYEN T H, LEE M S. Development of a hydrometallurgical process for the recovery of calcium molybdate and cobalt oxalate powders from spent hydrodesulphurization (HDS) catalyst [J]. Journal of Cleaner Production, 2015, 90: 388-396.
[14] ILHAN S, KALPAKLI A O, KAHRUMAN C, YUSUFOGLU I.The use of Langmuir–Hinshelwood mechanism to explain the kinetics of calcium molybdate leaching in oxalic acid solution [J]. Hydrometallurgy, 2012, 127: 91-98.
[15] ZHANG Wen-juan, LI Jiang-tao, ZHAO Zhong-wei, HUANG Shao-bo, CHEN Xing-yu, HU Kai-long. Recovery and separation of W and Mo from high-molybdenum synthetic scheelite in HCl solutions containing H2O2 [J]. Hydrometallurgy, 2015, 155: 1-5.
[16] XIA Wen-tang, ZHAO Zhong-wei, LI Hong-Gui. Thermodynamic analysis on sodium carbonate decomposition of calcium molybdenum [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(3): 622-625.
[17] PAN Mao-sen, ZHU Yun. Experimental study on leaching molybdenum at high-pressure from calcium molybdate [J]. China Molybdenum Industry, 2005, 29(6): 19-21. (in Chinese)
[18] ZHANG Wen-juan, YANG Jin-hong, ZHAO Zhong-wei, WANG Wen-qiang, LI Jiang-tao. Coordination leaching of tungsten from scheelite concentrate with phosphorus in nitric acid [J]. Journal of Central South University, 2016, 23(6): 1312-1317.
[19] XUIN G H, YU D Y, SU Y F. Leaching of scheelite by hydrochloric acid in the presence of phosphate[J]. Hydrometallurgy, 1986, 16(1): 27-40.
[20] LI Jiang-tao, ZHAO Zhong-wei. Kinetics of scheelite concentrate digestion with sulfuric acid in the presence of phosphoric acid [J]. Hydrometallurgy, 2016, 163: 55-60.
[21] KAHRUMAN C, YUSUFOGLU I. Leaching kinetics of synthetic CaWO4 in HCl solutions containing H3PO4 as chelating agent [J]. Hydrometallurgy, 2006, 81(3-4): 182-189.
[22] PETTERSSON L, ANDERSSON I, OHMAN L O. Multicomponent polyanions. 35. A 31P NMR study of aqueous molybdophosphates [J]. Acta Chem Scand, 1985, 39: 53-58.
[23] PETTERSSON L, ANDERSSON I, OHMAN L O. Multicomponent polyanions. 39. Speciation in the aqueous H+-MoO42--HPO42- system as deduced from a combined Emf-phosphorus-31P NMR study [J]. Inorganic Chemistry, 1986, 25(26): 4726-4733.
[24] SPEIGHT J G. Lange’s handbook of chemistry [M]. New York: McGraw-Hill, 2005.
[25] YANG Jin-hong, HE Li-hua, LIU Xu-heng, DING Wen-tao, SONG Yun-feng, ZHAO Zhong-wei. Comparative kinetic analysis of conventional and ultrasound-assisted leaching of scheelite by sodium carbonate[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(4): 775-782.
[26] YANG Hong-ying, LI Xue-jiao, TONG Lin-lin, JIN Zhe-nan, YIN Lu, CHEN Guo-bao. Leaching kinetics of selenium from copper anode slimes by nitric acid-sulfuric acid mixture [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(1): 186-192.
张文娟,王成彦,马保中
北京科技大学 冶金与生态工程学院,北京 100083
摘 要:钼酸钙是钼的一次资源,也是钼湿法和火法冶金过程中重要的中间产物。采用盐酸-磷酸混酸浸出钼酸钙以提取钼,为了了解浸出过程的机理,通过对浸出过程5个主要影响因素的研究,进行浸出过程动力学分析。结果表明:钼酸钙在该体系中的分解速率不受搅拌速度的影响,而受盐酸浓度、温度和粒径的影响比较显著;随着盐酸浓度增加和反应温度上升,浸出速率增加;随着粒径增大,反应速率降低。钼酸钙在该体系中的反应受收缩核模型的化学反应控制,所得反应的表观活化能为70.879 kJ/mol,并建立描述反应过程的半经验动力学方程。
关键词:钼酸钙;浸出动力学;磷酸;盐酸
(Edited by Bing YANG)
Foundation item: Project (2017M610766) supported by China Postdoctoral Science Foundation; Project (FRF-BD-17-010A) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: Bao-zhong MA; Tel: +86-10-62333170; E-mail: mabaozhong@ustb.edu.cn
DOI: 10.1016/S1003-6326(19)64996-4

