2,4,6-三氯酚的UV/H2O2光化学降解
高乃云1,祝淑敏1,马艳1,戎文磊2,周圣东2,陆纳新2
(1. 同济大学 污染控制与资源化研究国家重点实验室,上海,200092;
2. 无锡自来水公司,江苏 无锡,214031 )
摘要:采用UV/H2O2工艺降解水中2,4,6-三氯酚(2,4,6-TCP),研究H2O2投加量、pH、阴离子、阳离子、叔丁醇和腐殖酸对降解效果的影响,并利用LC-HESI-MS-MS探讨UV/H2O2降解2,4,6-TCP的降解机理。研究结果表明:UV/H2O2降解2,4,6-TCP的过程符合拟一级反应动力学。随着H2O2投加量的增加,2,4,6-TCP的去除率和反应速率增加,当H2O2投加量为10 mmol/L时,反应速率常数K达到0.109 4 min-1。酸性条件更利于UV/H2O2降解2,4,6-TCP。水中各种离子的存在对2,4,6-TCP的光解速率有较大的影响,其中阴离子CO32-对反应均存在明显的抑制作用,阳离子Fe3+促进效果显著。2,4,6-TCP的UV/H2O2反应速率随叔丁醇浓度的增加而下降,腐殖酸在低浓度时促进反应进行,在高浓度时,2,4,6-TCP的降解受到抑制。水中2,4,6-三氯酚在UV/H2O2作用下主要发生脱氯反应, 生成二氯邻二苯酚或二氯对二苯酚,未得到彻底矿化。
关键词:2,4,6-三氯酚;UV/H2O2;降解;反应动力学
中图分类号:TU 991.2 文献标志码:A 文章编号:1672-7207(2013)03-1262-07
UV/H2O2 photochemical degradation of 2,4,6-trichlorophenol
GAO Naiyun1, ZHU Shumin1, MA Yan1, RONG Wenlei2, ZHOU Shengdong2, LU Naxin2
(1. State Key Laboratory of Pollution Control and Resource Reuse, Tongji University, Shanghai 200092, China;
2. Wuxi Water Supply General Company, Wuxi 214031, China)
Abstract: The degradation of 2,4,6-trichlorophenol (2,4,6-TCP) by UV/H2O2 in aqueous solutions was investigated. The effects of H2O2 dosage, pH, cations, anions, tert-butanol and humic acid on the removal of 2,4,6-TCP were evaluated and the mechanism for oxidation of 2,4,6-TCP by UV/H2O2 was analyzed by using LC-HESI-MS-MS. The results indicate that the UV-H2O2 degradation of 2,4,6-TCP well follows pseudo first order kinetics. The removal of 2,4,6-TCP and reaction rate can be enhanced by increasing H2O2 dosage. When the dosage of H2O2 is 10 mmol/L, the reaction rate constant K reaches 0.109 4 min-1. The pH value of the solution greatly influences the 2,4,6-TCP degradation and the degradation performs well in acid condition. Irons in water have a significant influence on the 2,4,6-TCP degradation by UV/H2O2. CO32- inhibits the 2,4,6-TCP degradation significantly, however, Fe3+ exhibits an obvious promoting effects on 2,4,6-TCP degradation. A majority of 2,4,6-TCP transforms into dichlorocatechol or dichlorohydroquinone without complete mineralization.
Key words: 2,4,6- trichlorophenol; UV/H2O2; degradation; reaction kinetics
2,4,6-三氯酚(2,4,6-TCP)是一种氯酚类化合物,广泛应用于油漆、医药、农药、木材、纸浆等制造业,该物质易溶于水,25 ℃溶解度为0.434 g/L[1],能广泛存在于各类水体中,并通过食物链富集。有研究发现:三氯酚具有三致效应和遗传毒性[2],对人类健康造成巨大威胁,因此,近年来,水中2,4,6-TCP的去除已成为环境领域的热点之一。2,4,6-TCP具有稳定的C—Cl键,Cl原子和羟基之间的独特位置关系使2,4,6-TCP结构稳定,不易被生物降解[3],传统工艺和生物作用不能对其达到较好的去除效果。去除三氯酚的方法主要分为物理和化学2类,物理方法包括活性炭吸附[4]、膜分离技术[5]等,该方法只是将水中的三氯酚从水相转移至另一相,并没有彻底降解污染物,容易造成二次污染;化学方法,尤其是各种高级氧化(AOP)技术,如超声辐照[6]、臭氧氧化[7]、紫外光解等,能有效去除水中三氯酚,此外,多种AOP技术的联用可大大提高氯酚类物质的去除效果[8]。本文作者分析了UV/H2O2工艺对2,4,6-TCP的去除效果和降解机理,以期为实际应对水源水2,4,6-TCP污染提供有效的理论依据和技术指导。
1 材料与方法
1.1 试验材料
2,4,6-TCP(纯度>98%) 购自美国Sigma-Aldrich公司,用去离子水配制成质量浓度为100 mg/L的储备液,使用时根据需要进行稀释。双氧水(分析纯,质量分数为30%)购自阿拉丁(Aladdin)试剂集团有限公司。调节反应液pH所用溶液采用浓盐酸和NaOH固体(分析纯)配制。流动相甲醇、乙腈为HPLC级(Sigma-Aldrich),冰醋酸为优级纯。试验所用其他药剂均为分析纯,购于国药集团化学试剂有限公司(SCRC)。
1.2 试验方法与装置
采用UV/H2O2工艺去除水中2,4,6-TCP,分别进行不同H2O2投加量、不同反应pH、不同阴离子(Cl-,SO42-,NO3-,HCO3-,CO32-)、不同阳离子(Mg2+,Ca2+,Mn2+,Fe3+)以及投加叔丁醇、腐植酸的对照试验,在不同时刻取样,并在水样中加入适量0.1 mol/L的Na2S2O3溶液终止反应。利用高效液相色谱仪对水样中2,4,6-TCP浓度进行测定。自制反应装置见图1,所用紫外灯管为Philips公司生产,功率为75 W,额定工作电压为220 V,紫外灯主波长为254 nm,光强为142 μW/cm2。
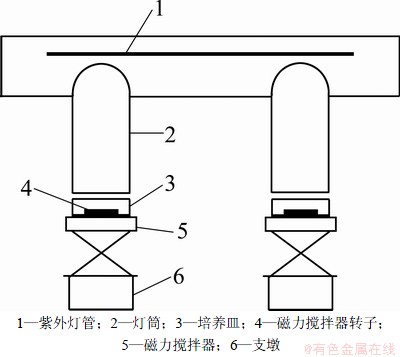
图1 反应装置示意图
Fig.1 Schematic description of reactor
1.3 2,4,6-TCP分析方法
2,4,6-TCP浓度采用高效液相色谱仪(岛津LC-2010AHT)测定,色谱柱采用shim-pack VP-ODS色谱柱(长度×直径为250 mm×4.6 mm),流动相中甲醇与水(水中含1%乙酸)的体积比为V(甲醇):V(水)=80:20,流动相流速为1.0 mL/min,色谱柱柱温40 ℃,检测波长289 nm。
利用高效液相色谱(Waters e2695 Separation Module)/质谱(Thermo Finnigan TSQ Quantum型)对UV/H2O2工艺降解2,4,6-TCP的生成产物进行分析。色谱柱为C18 柱( Thermo Scientific Hypersil GOLD, 长度×直径为100 mm×2.1 mm),流动相为乙腈(CH3CN)(A相)与0.1%(质量分数)的甲酸(HCOOH)溶液(B相),采用梯度洗脱模式,0~5 min,流动相体积比维持在V(A):V(B)=5:95,之后10 min A相不断增加直至V(A):V(B)=90:10,并维持3 min,最后5 min,流动相体积比返回至V(A):V(B)=5:95,检测时间共23 min。流动相流速300 μL/min,柱温40 ℃,进样量为10 μL。质谱电离源为加热型电喷雾电离负源(H-ESI),电喷雾电压为3.5 kV,鞘气(N2)压力为0.28 MPa,辅助气(N2) 压力为0.07 MPa,离子传输毛细管温度为300 ℃。
2 结果与讨论
2.1 H2O2投加量的影响
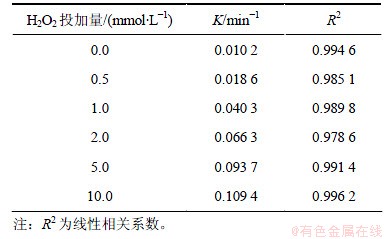
2,4,6-TCP初始质量浓度为5.0 mg/L,通过投加浓度分别为0,0.5,1.0,2.0,5.0,10.0 mmol/L的H2O2,研究H2O2投加量对UV/H2O2降解2,4,6-TCP的影响。图2所示为不同H2O2投加量条件下2,4,6-TCP的光降解拟一级动力学曲线,图中ρ0和ρ分别代表水中2,4,6-TCP的初始和不同时刻的质量浓度。由图2可知:UV/H2O2对2,4,6-TCP的降解过程符合拟一级反应动力学模型。不同H2O2投加量条件下2,4,6-TCP的光降解拟一级动力学参数见表1。结果表明,随着H2O2投加量增大,2,4,6-TCP的降解速率迅速升高,当H2O2 投加量为10 mmol/L时,反应速率常数K达到0.109 4 min-1,反应45 min后,2,4,6-TCP基本降解完全。这是由于H2O2在UV辐射下产生具有强氧化能力的·OH,增大H2O2投加量导致更多·OH产生,从而提高反应速率。但有研究指出:过量的H2O2对·OH存在捕获作用[9],具体过程见式(1),生成的HO2·氧化性弱于·OH[10],会导致2,4,6-TCP降解速率降低。本试验条件下没有出现抑制现象,说明试验投加的H2O2未过量,实际工艺中H2O2的最佳投量须根据具体客观工艺条件进行试验确定。
H2O2+ ·OH→ HO2· +H2O (1)
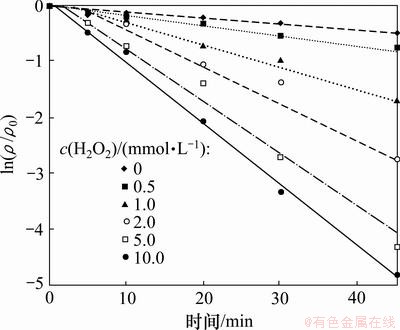
图2 H2O2投加量对UV/H2O2降解2,4,6-TCP的影响
Fig.2 Effect of dosage of H2O2 on degradation of 2,4,6-TCP by UV/H2O2 process
表1 不同H2O2投加量下2,4,6-TCP降解的拟一级动力学模型拟合参数
Table 1 Degradation parameters of kinetics models (pseudo first order) under different dosages of H2O2
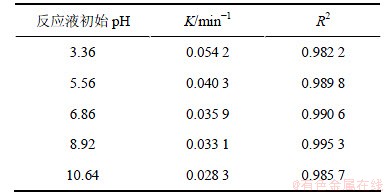
2.2 pH的影响
配制相同浓度的2,4,6-TCP反应液,初始质量浓度为5.0 mg/L,H2O2投加量为1 mmol/L,研究反应液不同pH(3.36,5.56,6.86,8.92,10.64)对UV/H2O2降解2,4,6-TCP的影响。试验结果如图3和表2所示。结果表明,随着反应液pH增加,反应速率降低。这是由于H2O2在碱性条件下易电离,不稳定,主要以HO2-形式存在,可以大量消耗·OH,有研究表明[9],HO2-消耗·OH的速度比H2O2要高2个数量级,具体过程见式(2)和(3),·OH的减少使得2,4,6-TCP降解速率降低。而酸性条件下H+的存在能阻止H2O2分解,氧化效率较高。
H2O2 +OH-→HO2-+H2O (2)
HO2-+·OH→ H2O+O2- (3)
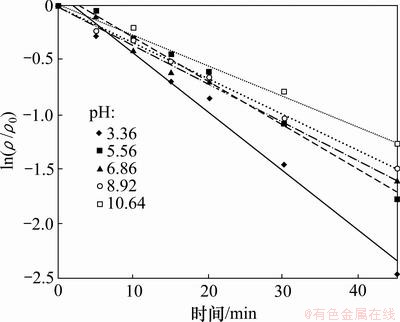
图3 pH对UV/H2O2降解2,4,6-TCP的影响
Fig.3 Effect of pH on degradation of 2,4,6-TCP by UV/H2O2 process
表2 不同pH条件下2,4,6-TCP降解的拟一级动力学模型拟合参数
Table 2 Degradation parameters of kinetics models (pseudo first order) under different pH
2.3 阴离子的影响
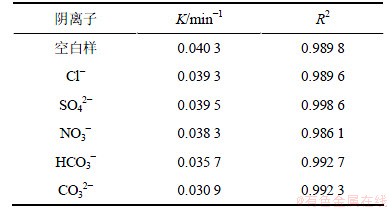
2,4,6-TCP初始质量浓度为5.0 mg/L,H2O2投加量为1 mmol/L,溶液中分别投加浓度均为1 mmol/L的Cl-,NO3-,SO42-,HCO3-和CO32-,试验结果如图4和表3所示。结果表明,5种阴离子对2,4,6-TCP的UV/H2O2降解均体现抑制作用,其中 CO32-的抑制作用最为明显。CO32-和HCO3-都是·OH的清除剂,HCO3-与·OH反应生成的CO3-·也会消耗H2O2,具体过程见反应式(4)~(7),从而降低UV/H2O2工艺的氧化效率。有研究发现,CO32-与·OH的反应速率远远高于比HCO3-,从而CO32-对UV/H2O2体系降解2,4,6-TCP的抑制作用大于HCO3-[11]。NO3-本身对不饱和键具有亲电作用,紫外照射时可光解产生·OH,但同时因其在紫外光区有较强的吸收作用(内在惰性滤层作用),妨碍光线有效通过溶液,降低了H2O2光解产生·OH的效率,通常NO3-的惰性滤层作用比产生·OH的作用强[12]。另有研究表明,NO3-对UV/H2O2工艺的影响与其在溶液中的浓度有很大关系[13],本试验中NO3-浓度对UV/H2O2降解整体表现为抑制作用。水中Cl-和SO42-对反应的影响较复杂,在UV照射下可能产生一定量的·OH,同时也会消耗一部分·OH,离子浓度在一定范围内可以提高反应速率,超过范围则对反应速率用抑制作用[11],在本试验条件下,1 mmol/L的Cl-和SO42-对反应略用抑制作用。
CO32-+·OH→CO3-·+OH- (4)
HCO3-+·OH→HCO3·+OH- (5)
HCO3·→CO3-·+H+ (6)
CO3-·+H2O2→HO2·+HCO3- (7)
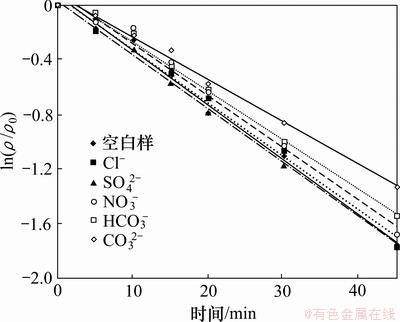
图4 不同阴离子对UV/H2O2降解2,4,6-TCP的影响
Fig.4 Effect of anions on degradation of 2,4,6-TCP by UV/H2O2 process
表3 不同阴离子条件下2,4,6-TCP降解的拟一级动力学模型拟合参数
Table 3 Degradation parameters of kinetics models (pseudo first order) in presence of different anions
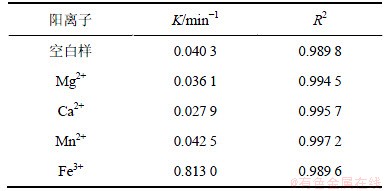
2.4 阳离子的影响
2,4,6-TCP初始质量浓度为5.0 mg/L,H2O2投加量为1 mmol/L,向反应液中分别投加浓度均为1 mmol/L的Mg2+,Ca2+,Mn2+,Fe3+,研究不同阳离子对UV/H2O2降解2,4,6-TCP的影响,试验结果如图5和表4所示。由图5可知:Mg2+,Ca2+和Mn2+对UV/ H2O2降解2,4,6-TCP影响不明显,Mg2+和Ca2+对反应呈抑制作用,Mn2+对反应略有促进。Fe3+的加入则明显促进了降解。这可能是因为Fe3+在H2O2体系中产生类Fenton反应,同时UV辐照会提高类Fenton反应的氧化能力,促进·OH的生成,具体过程见反应式(8)和(9),从而提高2,4,6-TCP的去除率。
Fe3++H2O2→Fe2++HO2·+H+ (8)
Fe2++H2O2→Fe3++OH-+·OH (9)
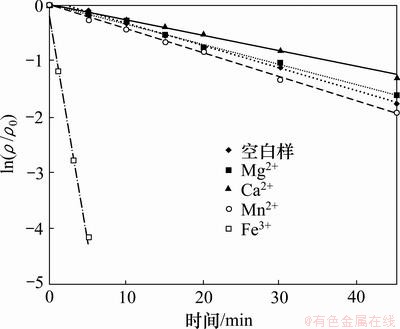
图5 水中阳离子对UV/H2O2降解2,4,6-TCP的影响
Fig.5 Effect of cations on degradation of 2,4,6-TCP by UV/H2O2 process
表4 不同阳离子条件下UV/H2O2降解2,4,6-TCP的拟一级动力学模型拟合参数
Table 4 Degradation parameters of kinetics models (pseudo first order) in presence of different cations
2.5 叔丁醇的影响
2,4,6-TCP初始质量浓度为5.0 mg/L,H2O2投加量为5 mmol/L,叔丁醇投加量分别为0,1,2,3 mmol/L,研究投加不同浓度的叔丁醇对UV/H2O2降解2,4,6-TCP的影响,试验结果如图6和表5所示。结果表明,叔丁醇对UV/H2O2降解2,4,6-TCP有明显抑制作用,叔丁醇投加量越大,抑制效果越明显。这主要是由于叔丁醇作为一种有效的·OH 抑制剂,对·OH 有强烈的捕捉作用,高级氧化反应过程中,叔丁醇优先与·OH 发生反应,生成了具有高度选择性和惰性的中间产物,从而终止了自由基链反应,阻碍了·OH 与有机物的反应,导致2,4,6-TCP降解反应速率大幅度降低[14]。
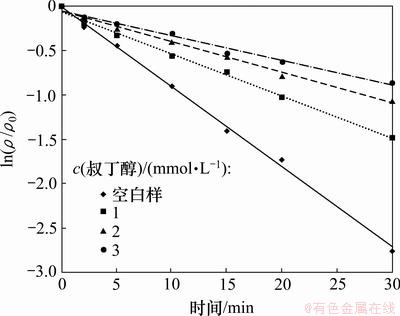
图6 投加叔丁醇对UV/ H2O2降解2,4,6-TCP的影响
Fig.6 Effect of concentration of tert-butanol on degradation of 2,4,6-TCP by UV/H2O2 process
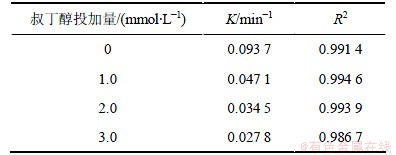
表5 不同叔丁醇投加量条件下UV/H2O2降解2,4,6-TCP的拟一级动力学模型拟合参数
Table 5 Degradation parameters of kinetics models (pseudo first order) under different concentrations of tert-butanol
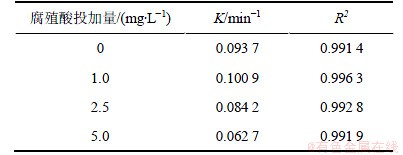
2.6 腐殖酸的影响
腐殖酸是水体中有机物的主要组成部分,分子结构复杂,会对有机物的氧化过程产生复杂的影响。试验通过投加不同浓度的腐殖酸,研究腐殖酸对UV/H2O2降解2,4,6-TCP的影响。试验中采用的2,4,6-TCP初始质量浓度为5.0 mg/L,H2O2投加量为5 mmol/L,腐殖酸投加量分别为0,1.0,2.5,5.0 mg/L,试验结果如图7和表6所示。结果表明,低质量浓度的腐殖酸(1 mg/L)有利于2,4,6-TCP的降解,而随着腐殖酸投加量的增加,2,4,6-TCP的降解速率降低。腐殖酸在光化学作用下会生成水合电子,可与水中溶解氧反应生成超氧负离子自由基(O2·),进而结合水体中的氧离子生成H2O2,促进光降解反应的进行[15]。当腐殖酸投加量变大时,反应液的色度相应增加,影响光降解效率,同时腐殖酸更易捕获水中·OH,与2,4,6-TCP产生竞争效应,使UV/H2O2对2,4,6-TCP降解过程受到抑制。
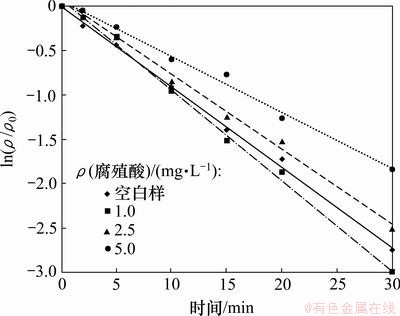
图7 投加腐殖酸对UV/H2O2降解2,4,6-TCP的影响
Fig.7 Effect of concentration of humic acid on degradation of 2,4,6-TCP by UV/H2O2 process
表6 不同腐殖酸投加量条件下UV/H2O2降解2,4,6-TCP的拟一级动力学模型拟合参数
Table 6 Degradation parameters of kinetics models (pseudo first order) under different concentrations of humic acid
2.7 UV/H2O2降解2,4,6-TCP的产物分析
利用LC-HESI-MS-MS对UV/H2O2降解2,4,6-TCP的产物进行分析,并对反应氧化途径进行分析推测。表7所示为UV/H2O2工艺降解2,4,6-TCP的主要产物。结果表明,UV/H2O2降解水中2,4,6-TCP生成的产物主要是质核比为177,179,181的物质。2,4,6-TCP的质核比由于氯原子存在同位素而呈现为195,197,199,主要产物的质荷比较2,4,6-TCP的少18,由此推断:UV/H2O2降解2,4,6-TCP 主要是通过氧化脱氯完成。UV/H2O2体系中,生成的羟基自由基·OH 攻击2,4,6-TCP苯环上的一个C—Cl,氯原子被氧化脱去生成氯离子,而2,4,6-TCP则形成正电离子,可以和水中的氢氧根结合,生成二氯对苯二酚或二氯邻苯二酚,具体过程见图8。相关研究也表明,光催化氧化2,4,6-TCP 时,主要发生氧化脱氯过程[16]。从2,4,6-TCP的产物分析可知:UV/H2O2工艺可以去除水中2,4,6-TCP,但反应不彻底,无法对其完全降解,达到彻底矿化。
表7 LC-HESI-MS-MS检测UV/H2O2降解2,4,6-TCP主要中间产物(负离子模式)
Table 7 Main fragment ions of reaction intermediates of 2,4,6-TCP degraded by UV/H2O2 process identified by LC-HESI-MS-MS (negative ion detection mode)
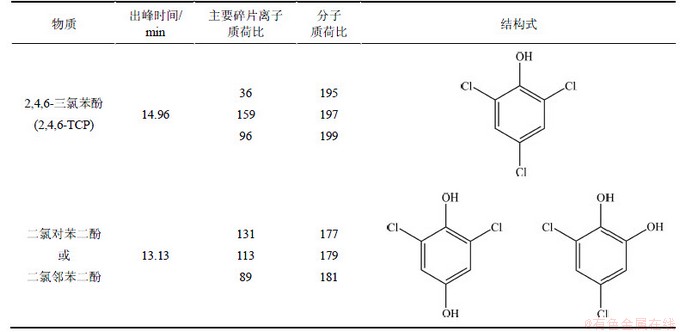
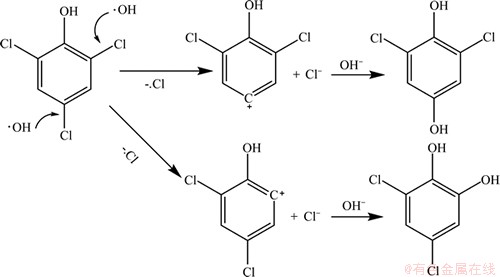
图8 UV/H2O2降解2,4,6-TCP的反应途径推断
Fig.8 Proposed 2,4,6-TCP degradation pathway by UV/H2O2 process
3 结论
(1) UV/H2O2工艺能有效地降解2,4,6-TCP,降解效果受H2O2投加量、pH、不同阴离子、阳离子的影响。在相同条件下,随着H2O2投加量的增大,2,4,6-TCP降解速率增大;反应液pH降低,2,4,6-TCP降解效果提高;Cl-,NO3-,SO42-,HCO3-和CO32-对2,4,6-TCP的降解存在抑制作用,其中CO32-影响最大。常见阳离子中,Mg2+和Ca2+对2,4,6-TCP的UV/H2O2降解反应有抑制作用,Mn2+和Fe3+的加入对降解反应有正向促进作用,其中Fe3+的促进效果显著。叔丁醇对2,4,6-TCP的UV/H2O2降解有抑制作用,其浓度越大抑制效果越明显。腐殖酸对2,4,6-TCP降解的影响较复杂。腐殖酸在低浓度时,促进2,4,6-TCP降解反应的进行;高浓度时,2,4,6-TCP的降解受到抑制。
(2) UV/H2O2降解2,4,6-TCP 主要是通过氧化脱氯完成。在羟基自由基·OH作用下,2,4,6-TCP上的一个氯原子被氧化脱去并被羟基取代,生成二氯对苯二酚或二氯邻苯二酚,无法得到彻底矿化,需要在UV/H2O2工艺基础上进一步优化改善技术,使2,4,6-TCP矿化完全。
参考文献:
[1] Czaplicka M. Sources and transformations of chlorophenols in the natural environment[J]. Science of the Total Environment, 2004, 322(1/2/3): 21-29.
[2] Collins J J, Bodner K, Aylwad L L, et al. Mortality rates among trichlorophenol workers with exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin[J]. American Journal of Epidemiology, 2009, 170(4): 501-506.
[3] Wang J L, Qian Y. Microbial degradation of 4-chlorophenol by microorganisms entrapped in carrageenan-chitosan gels[J]. Chemosphere, 1999, 38(13): 3109-3117.
[4] 詹健, 朱冬梅, 刘振中. 表面改性活性炭去除水中2,4,6-三氯酚的实验[J]. 重庆大学学报, 2011, 34(7): 120-124.
ZHAN Jian, ZHU Dongmei, LIU Zhenzhong. The experiment on surface modification activated carbon for the removal of 2,4,6-trichlorophenol from water[J]. Journal of Chongqing University, 2011, 34(7): 120-124.
[5] 孙亚锡, 沙布, 王晓东, 等. 膜生物反应器去除原水中微量2,4,6-三氯酚的研究[J]. 水处理技术, 2007, 33(12): 42-45.
SUN Yaxi, Sagbo O, WANG Xiaodong, et al. Removal of trace from surface water by membrane bioreactor [J]. Technology of Water Treatment, 2011, 34(7): 120-124.
[6] Pandit A B, Gogate P R, Mujumdar S. Ultrasonic degradation of 2:4:6 trichlorophenol in presence of TiO2 catalyst[J]. Ultrasonics Sonochemistry, 2007, 33(12): 42-45.
[7] Huang W J, Fang G C, Wang C C. A nanometer-ZnO catalyst to enhance the ozonation of 2,4,6-trichlorophenol in water[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 260(1/2/3): 45-51.
[8] Trapido M, Hirvonen A, Veressinina Y, et al. Ozonation, ozone/UV and UV/H2O2 degradation of chlorophenols[J]. Ozone-Science & Engineering, 1997, 19(1): 75-96.
[9] Crittenden J C, Hu S M, Hand D W, et al. A kinetic model for UV/H2O2 process in a completely mixed batch reactor[J]. Water Research, 1999, 33(10): 2315-2328.
[10] Perez M, Torrades F, Garcia-Hortal J A, et al. Removal of organic contaminants in paper pulp treatment effluents under Fenton and photo-Fenton conditions[J]. Applied Catalysis B-Environmental, 2002, 36(1): 63-74.
[11] Liao C H, Gurol M D. Chemical oxidation by photolytic decomposition of hydrogen-peroxide[J]. Environmental Science and Technology, 1995, 29(12): 3007-3014.
[12] Du Y X, Zhou M H, Lei L C. The role of oxygen in the degradation of p-chlorophenol by Fenton system[J]. Journal of Hazardous Materials, 2007, 139(1): 21-29.
[13] 张文兵, 肖贤明, 傅家谟, 等. 溶液中阴离子对UV/H2O2降解4-硝基酚的影响[J]. 中国环境科学, 2002, 22(4): 108-115.
ZHANG Wenbing, XIAO Xianming, FU Jiamo, et al. Effect of anions in aqueous solution on the degradation of 4-nitrophenol by UV/H2O2 process[J]. China Environmental Science, 2002, 22(4): 108-115.
[14] Ma J, Graham N J D. Degradation of atrazine by manganese- catalysed ozonation: Influence of radical scavengers[J]. Water Research, 2000, 34(15): 3822-3828.
[15] 潘晶, 郝林, 张阳, 等. 溶液中阴离子和腐殖酸对UV/H2O2降解2,4-二氯酚的影响[J]. 环境污染与防治, 2007, 29(7): 487-494.
Pan Jing, HAO Lin, ZHANG Yang, et al. Effects of anions and humic acid on UV/H2O2 oxidation of 2,4-DCP[J]. Environmental Pollution and Control, 2007, 29(7): 487-494.
[16] Konstantinos C C, Eleni S, Maria L, et al. Mechanism of catalytic degradation of 2,4,6-trichlorophenol by a Fe-porphyrin catalyst[J]. Applied Catalysis B: Environmental, 2011, 101(3/4): 417-424.
(编辑 赵俊)
收稿日期:2012-03-21;修回日期:2012-05-28
基金项目:国家科技重大专项(2008ZX07421-002);住房和城乡建设部研究开发项目(2009-K7-4)
通信作者:马艳(1986-),女,江苏宜兴人,博士研究生,从事水处理理论与技术研究;电话:021-65988099;E-mail: my041203@126.com