温度对复杂氧化铜矿中不同赋存状态铜矿物浸出行为的影响
来源期刊:中国有色金属学报(英文版)2019年第10期
论文作者:王改荣 刘媛媛 佟琳琳 金哲男 陈国宝 杨洪英
文章页码:2192 - 2201
关键词:复杂氧化铜矿;赋存状态;温度;浸出行为;铜矿物
Key words:complex copper oxide ores; occurrence state; temperature; leaching behavior; copper minerals
摘 要:采用XRD、光学显微镜、SEM-EDS等物相分析手段,研究温度对复杂氧化铜矿中不同赋存状态铜矿物浸出行为的影响。结果表明:常温下易浸氧化铜矿物完全被浸出,而结合态铜矿物丝毫未被浸出;微温(40 °C)下主要是类质同象铜的浸出;中温(60 °C)时吸附态铜的浸出速率明显加快;达80 °C时,类质同象铜完全浸出,但仍有11.2%的吸附态铜未被浸出。在整个浸出过程中,长石-石英-铜-铁胶体中的铜未被溶解。不同铜矿物的浸出速率大小如下:孔雀石、假孔雀石>硅孔雀石>含铜绿泥石>含铜白云母>含铜黑云母>含铜褐铁矿>长石-石英-铜-铁胶结体。
Abstract: The effect of temperature on leaching behavior of copper minerals with different occurrence states in complex copper oxide ores was carried out by phase analysis means of XRD, optical microscopy and SEM-EDS. The results indicated that at ambient temperature, the easily leached copper oxide minerals were completely dissolved, while the bonded copper minerals were insoluble. At lukewarm temperature of 40 °C, it was mainly the dissolution of copper in isomorphism state. With increasing temperature to 60 °C, the copper leaching rate in the adsorbed state was significantly accelerated. In addition, when the temperature increased to 80 °C, the isomorphic copper was completely leached, leaving 11.2% adsorbed copper un-leached. However, the copper in feldspar-quartz-copper-iron colloid state was not dissolved throughout the leaching process. Overall, the leaching rates of copper in different copper minerals decreased in the order: malachite, pseudo-malachite > chrysocolla > copper-bearing chlorite > copper-bearing muscovite > copper-bearing biotite > copper-bearing limonite > feldspar-quartz-copper-iron colloid.
Trans. Nonferrous Met. Soc. China 29(2019) 2192-2201
Gai-rong WANG1,2, Yuan-yuan LIU3, Lin-lin TONG1,2, Zhe-nan JIN1,2, Guo-bao CHEN1,2, Hong-ying YANG1,2
1. Key Laboratory for Ecological Metallurgy of Multimetallic Mineral (Ministry of Education), Northeastern University, Shenyang 110819, China;
2. School of Metallurgy, Northeastern University, Shenyang 110819, China;
3. CNMC Luanshya Copper Mines Plc (CLM), Independence Avenue, Luanshya City, 90456, Zambia
Received 1 February 2019; accepted 18 July 2019
Abstract: The effect of temperature on leaching behavior of copper minerals with different occurrence states in complex copper oxide ores was carried out by phase analysis means of XRD, optical microscopy and SEM-EDS. The results indicated that at ambient temperature, the easily leached copper oxide minerals were completely dissolved, while the bonded copper minerals were insoluble. At lukewarm temperature of 40 °C, it was mainly the dissolution of copper in isomorphism state. With increasing temperature to 60 °C, the copper leaching rate in the adsorbed state was significantly accelerated. In addition, when the temperature increased to 80 °C, the isomorphic copper was completely leached, leaving 11.2% adsorbed copper un-leached. However, the copper in feldspar-quartz-copper-iron colloid state was not dissolved throughout the leaching process. Overall, the leaching rates of copper in different copper minerals decreased in the order: malachite, pseudo-malachite > chrysocolla > copper-bearing chlorite > copper-bearing muscovite > copper-bearing biotite > copper-bearing limonite > feldspar-quartz-copper-iron colloid.
Key words: complex copper oxide ores; occurrence state; temperature; leaching behavior; copper minerals
1 Introduction
With the continuous depletion of high-grade copper sulfide ores, there is a need to win metals from the abundant complex copper oxide ores [1-3]. These ores have the characteristics of low copper grade, high oxidation rate, high combined rate, fine distribution granularity as well as extremely complex copper phase composition [4,5], which makes them difficult to utilize and become a refractory ore resource. In recent years, the treatment of complex copper oxide ores has been an important and difficult problem for the mining and metallurgical researchers. Hydrometallurgical process is a good choice for its environmental friendliness [6], including sulfuric acid leaching and ammonia leaching [7]. Compared with ammonia leaching [8], sulfuric acid leaching has more application prospect because of its advantages of high copper leaching rate, low environmental pollution, and low energy consumption.
Temperature is an important factor affecting the kinetics of leaching in hydrometallurgical processes. It is generally considered that higher temperatures are used to increase the leaching rate [9-11]. LEE et al [12] studied the extraction kinetics of heavy copper-containing sludge, and found that both in nitric acid and citric acid solutions, the copper saturation concentrations and the apparent rate coefficient increased with the extraction temperature [12]. The extraction rates of Cu and Zn from sewage sludge by citric acid were also found to be increased with increasing temperature [13]. In addition, the influence of seawater and discard brine on the dissolution of copper ores and copper concentrate was studied by LILIAN [14]. It was indicated that regardless of the chloride solution used in the leaching test stirred at room temperature, the chalcopyrite dissolution did not exceed 4%, but increased to 90% when the temperature reached 50 °C. However, the effect of temperature on the leaching behavior of copper minerals with different occurrence states in complex copper oxide ores was seldom studied.
It is a typical complex copper oxide ore from Mulyashy Copper Mine in Luanshya, Zambia. The process mineralogy of the ores showed that there were four occurrence states of the copper [15]. The copper in mineral state mainly existed in the form of malachite, chrysocolla and pseudo-malachite; isomorphic copper existed in the form of biotite, chlorite and muscovite; adsorbed copper existed in the form of limonite, and colloid co-precipitated copper existed in the cemented body of feldspar-quartz-copper-iron. Thus, the occurrence state of copper-bearing minerals was extremely complicated. At present, most studies focus on the leaching technology or copper leaching efficiency of this complex copper ore [16-18]. However, there is a lack of details in the leaching process analysis of copper-bearing minerals from the perspective of mineral phase. In this work, the leaching process of complex copper oxide ores with sulfuric acid at different temperatures was carried out in order to analyze the leaching behavior of copper minerals. It was found that the leaching order of copper in different occurrence states was significantly varied. This study will provide the theoretical value and significant guidance for the leaching of copper-containing minerals for industrial application in this field.
2 Experimental
2.1 Materials
The samples used in this study were complex copper oxide ores from Mulyashy Copper Mine in Luanshya, Zambia, where the copper sulfide was a trace amount (0.5%). Table 1 presents the chemical composition of the ores in which the copper content was 1.85%. Figure 1 shows the X-ray powder diffraction (XRD) analysis. As indicated in Fig. 1, the minerals in the ores contained quartz, biotite, potassium feldspar, muscovite, plagioclase and tremolite. Owing to the dispersed distribution of copper in many minerals, there was no characteristic spectrum associated with copper minerals detected by XRD. Table 2 presents the phase analysis of the ores, which showed that the copper minerals contained malachite, chrysocolla, pseudo- malachite, copper-bearing biotite, copper-bearing chlorite, copper-bearing muscovite and copper-bearing limonite. In addition, Fig. 2 presents the microscopic images of copper-bearing minerals. The sulfuric acid used in this work was of analytical grade.
Table 1 Chemical composition of copper ores (wt.%)


Fig. 1 XRD pattern of copper ores
Table 2 Phase analysis results of copper ores (wt.%)

2.2 Characterization
The chemical composition of complex copper oxide ores with different occurrence states of copper minerals was analyzed by XRF (X'pertPRO). XRD (PW3040/60) was used to measure the phase composition of the sample to preliminarily determine the existence of copper minerals. The raw ores and leaching slags at different temperatures were mixed with resins to prepare thin sections (two pieces of each temperature). In addition, the sections were examined and analyzed using optical microscope (LEICA-DMLP) and scanning electron microscope (SEM) (SHIMADZU SSX-550) equipped with an EDS detector.
2.3 Leaching experiment

Fig. 2 Microscopic images of copper-bearing minerals in ores
The leachable property of the copper ores was detected using orthogonal array method. Based on the previous leaching tests, it was identified that temperature (A), sample size (B), leaching time (C), stirring speed (D), and H2SO4 concentration (E) could affect the leaching process. Each of the five parameters has four levels with an orthogonal array 16 (45). The experimental parameters and their levels are given in Table 3. The experimental procedure is presented as follows: Firstly, 30 g raw ore was added into a 250 mL three-neck beaker with 150 mL sulfuric acid of certain concentrations. The leaching process was carried out with a constant temperature water-bath heating and dynamic stirring. After leaching for a certain time, the solution was filtered and the slag was washed, dried, and finally analyzed by atomic absorption spectroscopy (AAS) to determine the copper contents.
The copper leaching rates (R) of copper minerals at different temperatures were calculated using Eq. (1):
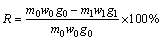 (1)
(1)
where w0, w1 (wt.%) are the fractions of copper minerals in the raw ores and the leaching slags at different temperatures; g0, g1 (wt.%) are the copper contents of copper minerals in the raw ores and the leaching slags at different temperatures; m0, m1 (g) are the mass of raw ores and the leaching slags at different temperatures.
The fractions (wt.%) of the copper minerals in raw ores and leaching slags at different temperatures were obtained by the study of process mineralogy using microscopy and SEM-EDS. The copper contents (wt.%) of the minerals in raw ores and leaching slags at different temperatures were achieved by EDS statistics. In this statistical process, 40 particles of each copper-containing mineral were collected from 40 thin sections at different particle sizes.
3 Results and discussion
3.1 Effect of factors on leaching
The copper leaching rates and range analysis results of the L16 (45) experiment are shown in Table 4. It can be seen that the copper leaching rate of experiment No. 13 was the highest about 96.02%, while experiment No. 1 was the lowest of 47.69%. In addition, the range values of the factors (A), (B), (C), (D) and (E) were 21.53%, 11.04%, 9.17%, 7.59% and 10.61%, respectively. Therefore, the order of factors influencing the leaching was as follows: temperature > sample size > H2SO4 concentration > leaching time > stirring speed, which indicated that temperature has the greatest effect on the leaching.
Figure 3 presents the effect of each factor on the leaching rate of copper. It should be noted that the graph was only used to show the trend of each factor, rather than predicting other values that were not tested experimentally. From Fig. 3, it can be seen that as the temperature increased from 25 to 80 °C, the leaching rate of copper increased remarkably. As higher temperature can increase the speed of molecular motion and the collisions between the minerals and H+ [19], the increase in the temperature causes an elevation in the dissolution rate [20]. A decrease in particle size less than 74 μm accounting for 40% to 60% led to a significant increase in the leaching rate of copper. Since the leaching kinetics was more rapid for the finer particle size with greater available surface area for the reaction to take place [2,21].
Table 3 Experimental parameters and their levels

Table 4 Leaching results of L16 (45) experiment


Fig. 3 Effect of each factor on leaching rate of copper
However, this trend tended to decrease when the particle size less than 74 μm accounting for 60% to 100%. Based on the grinding energy consumption, the sample particle size less than 74 μm accounting for 80% was considered. The leaching rate of copper also increased with increasing time, and 120 min was the best. Furthermore, with an increase in stirring rate from 200 to 300 r/min, the leaching rate increased significantly, but remained almost constant at 500 r/min. Therefore, the optimal stirring speed was considered to be 300 r/min. When the H2SO4 concentration increased from 0.2 to 1.2 mol/L, the leaching rate was raised from 78.55% to 89.16%. The phenomenon was attributed to the acid concentration effect on increasing the H+ activity that resulted in the further dissolution of copper. However, the upward trend decreased as the H2SO4 concentration increased from 1.2 to 1.7 mol/L. Considering the corrosion of equipment, 1.2 mol/L was the optimum.
3.2 Effect of temperature on leaching
As indicated above, the temperature had the greatest influence on the leaching rate of copper. Therefore, the effect of temperature on the leaching behavior of copper minerals with different occurrence states in complex copper oxide ores was carried out at different temperatures of ambient temperature (AT, 25 °C), lukewarm temperature (LT, 40 °C) and medium temperatures (MT1, 60 °C and MT2, 80 °C) at the optimal conditions mentioned above. The leaching conditions were as follows: 30 g ores, sample size less than 74 μm accounting for 80%, sulfuric acid of 1.2 mol/L, leaching time of 120 min, solid-liquid ratio of 0.2 g/mL, stirring speed of 300 r/min. Table 5 shows the leaching results of copper contents in copper-bearing minerals at different leaching temperatures.
3.2.1 Leaching at ambient temperature
At AT, the copper leaching rate of the ores was 69.35%. It was detected by microscope and SEM-EDS that the easily leached copper oxide minerals such as malachite and pseudo-malachite were completely dissolved except for a few chrysocolla [22]. As can be seen in Table 5, almost no copper was leached from the bonded copper minerals, such as copper-bearing biotite, copper-bearing chlorite, copper-bearing muscovite, copper-bearing limonite and colloid of feldspar-quartz- copper-iron. LIU et al [4] noted that all of the copper minerals leached in the solution, copper silicates exhibited the poorest propensity to leach. Figure 4 shows the copper leaching rates of bonded copper minerals at different temperatures.
3.2.2 Leaching at lukewarm temperature
82.04% of the copper leaching rate was achieved at LT. It was found from Table 5 that at this temperature, the un-leached chrysocolla at AT was dissolved completely. Figure 4 shows that 67.57% of isomorphic copper minerals were leached, including 56.52% copper- bearing biotite, 66.80% copper-bearing muscovite and all copper-bearing chlorite. Moreover, about 20.75% of the copper adsorbed by limonite was also leached.
Table 5 Copper contents of copper-bearing minerals at different temperatures (wt.%)


Fig. 4 Copper leaching rates of bonded copper minerals at different temperatures
Figure 5 presents the SEM-EDS images of morphologies and elemental characteristics of the copper minerals at LT. Figures 5(a1, a2) correspond to single copper-bearing biotite and single copper-free biotite. Furthermore, Fig. 5(b1) corresponds to the associated copper-bearing biotite. It was observed that there were no differences in morphologies between the copper- bearing biotite and copper-free biotite, they were both relatively intact with the acid leaching at LT. However, much of the EDS data showed that the copper contents of biotite and muscovite decreased from 3.55% and 0.6% to 1.22% and 0.2%, respectively, indicating that some copper in isomorphism state could be leached out at LT. In addition, Fig. 5(b2) corresponds to copper-adsorbed limonite associated with copper-bearing biotite. It was found that the sign of copper-adsorbed limonite eroded by acid was not obvious [23]. From EDS analysis, the copper content of copper-adsorbed limonite decreased from 2.94% to 2.32%, showing that the copper in the adsorbed state cannot be leached effectively at LT.
3.2.3 Leaching at medium temperature
The leaching experiments of the complex copper ores with sulfuric acid were carried out at medium temperatures (MT1, 60 °C and MT2, 80 °C). The leaching behaviors between copper minerals with different occurrence states were obviously different.
The leaching rate of copper was 87.84% at MT1. The easily leached copper oxide minerals were dissolved totally, 86.34% of isomorphic copper minerals were also leached, including 78.88% copper-bearing biotite, 95.14% copper-bearing muscovite and all copper-bearing chlorite. In addition, 45.64% of copper adsorbed by limonite was also leached (Fig. 4).
The SEM-EDS images of morphologies and elemental characteristics of copper minerals at MT1 are shown in Fig. 6. Figures 6(a1) and (b2) represent single copper-bearing biotite and associated biotite, respectively. It was observed that the copper-bearing biotite minerals were obviously eroded by acid, reflected by the widening of the distances between the cleavage cracks as well as the disjunction cracks as compared to Fig. 5(a1) and (b1). The reason may be that the biotite structure was collapsed at this leaching conditions, and the similar behavior was also observed in other studies [24,25]. The dissolution morphology of the copper-bearing muscovite was the same as the copper-bearing biotite. Besides, the EDS results showed that the copper contents of biotite and muscovite decreased significantly from 3.55% and 0.6% to 0.59% and 0.03%. However, there were no differences in the morphologies and elemental characteristics between the copper-free biotites in Fig. 6(b1) and Fig. 5(a2). The result indicated that the leaching effect of copper-bearing mica minerals is better than that of copper-free mica minerals. In addition, it was inferred from the obvious appearance of micropores and holes in Fig. 6(b) that the copper-adsorbed limonite Fig. 6(b3) was significantly etched by acid solution, and the copper content decreased apparently from 2.94% to 1.59%.

Fig. 5 SEM images (a, b) of copper minerals at LT and corresponding EDS results (a1, a2, b1, b2) of points 1-4

Fig. 6 SEM images (a, b) of copper minerals at MT1 and corresponding EDS results (a1, b1, b2, b3) of points 1-4
The leaching rate of copper was 94.03% at MT2. At this temperature, the easily leached copper oxide minerals were leached. Besides, the bonded copper minerals of isomorphism state such as copper-bearing biotite, copper-bearing muscovite and copper-bearing chlorite were completely dissolved. However, the leaching rate of copper in the adsorbed state was 88.8%, leaving 11.2% of the copper adsorbed by limonite un-leached (Fig. 4).
Figure 7 shows the SEM-EDS images of the morphologies and elemental characteristics of copper minerals at MT2. The SEM-EDS results revealed that there was no copper remained throughout the biotite and muscovite minerals after sulfuric acid leaching at MT2. It was also noticed that some biotite and muscovite were seriously corroded by acid, as observed from the obvious widening of the distances between the cleavage cracks and disjunction cracks in Region 1 (Fig. 7(a)) and Region 3 (Fig. 7(b)). In addition, Figs. 7(a1, b1) indicated that the contents of Mg, Al, K and Fe in this kind of mica minerals were greatly reduced, which can be deduced that these minerals were copper-bearing mica minerals. However, the shapes of some mica minerals were relatively intact, and the content of Fe was almost un-changed from Fig. 7(a2), due to its poor insulation performance [26], which inferred that the minerals were copper-free mica. Overall, it was further indicated that the leaching effect of copper-bearing mica minerals was better than that of copper-free mica minerals. The reason may be that the stability of mica minerals with copper isomorphism is lower than that of copper-free mica minerals, which allows the elements to dissolve more easily [27].
At MT2, the seriously dissolved cavities and cracks in Region 4 indicated that the dissolution effect of copper-adsorbed limonite was accelerated significantly. Figure 7(b2) showed that the copper content of limonite was significantly reduced by 2.94% to 0.33%. However, as can be seen in Table 5, the copper in colloid co-precipitation state was not leached throughout the leaching experiment.
3.3 Comparison of leaching behaviors of copper minerals at different temperatures
It can be seen from Table 5 that at AT, the easily leached copper oxide minerals such as malachite and pseudo-malachite were completely dissolved, except for a few chrysocolla. As the copper from chrysocolla was leached, it was thought to leave behind an impervious layer of hydrated silica that hindered further leaching of copper [28-30]. This result was consistent with the leaching experiment by SUN et al [31], which showed that the copper oxidized minerals can be dissolved in sulfuric acid at room temperature. Nevertheless, the bonded copper minerals were not leached at this temperature. Additionally, with the increase in leaching temperature, the bonded copper minerals were gradually dissolved. Figure 8 shows the copper contents of bonded copper minerals at different temperatures.

Fig. 7 SEM images (a, b) of copper minerals at MT2 and corresponding EDS results (a1, a2, b1, b2) of points 1-4
It was noticeable from Fig. 8 that at LT, the leaching rates of isomorphic copper minerals such as copper- bearing biotite and copper-bearing muscovite were significantly accelerated. The leaching effect of copper- bearing chlorite appeared was the most significant, which was completely dissolved out. As known, most copper-bearing chlorite was altered from copper-bearing biotite along with the dissociation and cleavage cracks [32]. During the alteration process, the layered structure of copper-bearing chlorite could be changed, which led to the improvement of copper leaching [33]. However, a poor effect on the dissolution of copper adsorbed by limonite was observed at LT.
It was propitious for the leaching of bonded copper minerals at MT. At MT1, large numbers of isomorphic copper minerals were dissolved. The copper contents in biotite and muscovite were reduced from the original 3.55% and 0.6% to 0.59% and 0.03%, respectively. And the copper content in copper-adsorbed limonite was also decreased significantly from 2.94% to 1.59%. Moreover, it was shown that at MT2, the isomorphic copper minerals were completely leached, while 0.33% of the copper content remained in the copper-adsorbed limonite, indicating that the copper in the adsorbed state was more difficult to be leached than that in isomorphism state. However, the copper in colloid co-precipitation of feldspar-quartz-copper-iron was not leached throughout the leaching process. It was suggested that the un-leached bonded copper at MT2 needs some strengthening means such as mechanical activation, microwave or ultrasound for further dissolving.

Fig. 8 Copper contents of bonded copper minerals at different temperatures
Figure 9 presents the leaching behavior of Si, Al, Ca and Mg, as well as the acid consumption at different temperatures. It can be seen that with an increase in the temperature, the leaching rates of Si, Al, Ca and Mg raised gradually, while the growing trend about Si was not significant. It was found that most of Si existed in quartz, feldspar, mica, chlorite and chrysocolla in the copper ores. The order of dissolution rate of rock-forming minerals in acid studied by Swedish scholars was biotite > chlorite > hornblende > feldspar > quartz [34]. In this study, the quartz, feldspar and hornblende were difficult to be decomposed, in contrast, chrysocolla and part of mica and chlorite can be leached with the generation of hydrated silica, resulting in a relatively low leaching rate of Si. Al mainly existed in the minerals of feldspar, mica and chlorite; Mg was mainly found in the minerals of amphibole, mica and chlorite. With increasing temperature, the mica and chlorite were gradually dissolved, leading to the increase of leaching rates of Al and Mg. Furthermore, the leaching rate of Ca was lower than that of Al and Mg, due to the fact that the Ca was mostly found in the insoluble amphibole and a few calcite. In addition, it was shown from Fig. 9 that with an increase of temperature, the acid consumption was enhanced but a relatively low value appeared, because the main minerals such as quartz, feldspar, amphibole and copper-free mica in the ores did not consume much sulfuric acid, while the contents of acid-consuming minerals such as malachite, chrysocolla, calcite, copper-bearing chlorite and copper-bearing mica were relatively low.

Fig. 9 Leaching behaviors of Si, Al, Ca and Mg, as well as acid consumption at different temperatures
The above analyses summed up that the order of copper leaching rates in different occurrence states was as follows: mineral copper > isomorphic copper > adsorbed copper > colloidal copper, and corresponding to the copper minerals: malachite, pseudo-malachite > chrysocolla > copper-bearing chlorite > copper-bearing muscovite > copper-bearing biotite > copper-bearing limonite > feldspar-quartz-copper-iron colloid.
4 Conclusions
(1) The order of factors affecting the leaching was as follows: temperature > sample size > H2SO4 concentration > leaching time > stirring speed.
(2) At ambient temperature, the easily leached copper oxide minerals such as malachite and pseudo- malachite were completely dissolved except for a few chrysocolla, while the bonded copper minerals were not leached at all.
(3) Increasing temperature was favorable to the leaching of bonded copper minerals. At lukewarm temperature of 40 °C, the dissolution of copper was mainly in isomorphism state, which was not obvious in the adsorbed state. With an increase in temperature to 60 °C, the leaching rate of copper in the adsorbed state was significantly accelerated. Moreover, at 80 °C, the isomorphic copper was completely leached, but leaving 11.2% adsorbed copper un-leached.
(4) The leaching effect of copper-bearing mica minerals was better than that of copper-free mica minerals.
(5) The leaching rates of copper in different occurrence states decreased in the order: mineral copper > isomorphic copper > adsorbed copper > colloidal copper, corresponding to the leaching of copper minerals: malachite, pseudo-malachite > chrysocolla > copper-bearing chlorite > copper-bearing muscovite > copper-bearing biotite > copper-bearing limonite > feldspar quartz copper-iron colloid.
References
[1] YIN Sheng-hua, WANG Lei-ming, WU Ai-xiang, FREE M L, KABWE E. Enhancement of copper recovery by acid leaching of high-mud copper oxides: A case study at Yangla Copper Mine, China [J]. Journal of Cleaner Production, 2018, 202: 321-331.
[2] LIU Mei-lin, WEN Jian-kang, TAN Gui-kuan, LIU Guo-liang, WU Biao. Experimental studies and pilot plant tests for acid leaching of low-grade copper oxide ores at the Tuwu Copper Mine [J]. Hydrometallurgy, 2016, 165: 227-232.
[3] DENG Jiu-shuai, WEN Shu-ming, DENG Jian-ying,WU Dan-dan. Extracting copper from copper oxide ore by a zwitterionic reagent and dissolution kinetics [J]. International Journal of Minerals, Metallurgy, and Materials, 2015, 22(3): 241-248.
[4] LIU Wei, TANG Mo-tang, TANG Chao-bo, HE Jing, YANG Sheng-hai, YANG Jian-guang. Dissolution kinetics of low grade complex copper ore in ammonia-ammonium chloride solution [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 910-917.
[5] HAN Jun-wei, LIU Wei, XUE Kai, QIN Wen-qing, JIAO Fen, ZHU Lin. Influence of NH4HF2 activation on leaching of low-grade complex copper ore in NH3-NH4Cl solution [J]. Separation and Purification Technology, 2017, 181: 29-36.
[6] ZHAO Zhong-wei, ZHANG You-xin, CHEN Xing-yu, CHEN Ai-liang, HUO Guang-sheng. Effect of mechanical activation on the leaching kinetics of pyrrhotite [J]. Hydrometallurgy, 2009, 99(1): 105-108.
[7] ZHANG Feng-hua, SONG Bao-xu. Efficient recovery of a complex refractory copper oxide ore [J]. Mining and Metallurgical Engineering, 2014, 34(6): 26-28.
[8] WEN Sheng-lai. Overview on flotation method of low-grade oxidized copper ore and its bioleaching technology [J]. Modern Mining, 2010, 26(2): 57-59.
[9] SMITH J M. Chemical engineering kinetics [M]. New York: McGraw-Hill, 1956.
[10] SILVAS F P C, CORREA M M J, CALDAS M P K, MORAES V T D, ESPINOSA D C R, TENORIA J A S. Printed circuit board recycling: Physical processing and copper extraction by selective leaching [J]. Waste Management, 2015, 46: 503-510.
[11] SUN Z H I, XIAO Y, SIETSMA J, AGTERHUIS H, VISSER G, YANG Y. Selective copper recovery from complex mixtures of end-of-life electronic products with ammonia-based solution [J]. Hydrometallurgy, 2015, 152: 91-99.
[12] LEE I H, WANG Y J, CHERN J M. Extraction kinetics of heavy metal-containing sludge [J]. Journal of Hazardous Materials, 2005, 123(1): 112-119.
[13] VEEKEN A H M, HAMELERS H V M. Removal of heavy metals from sewage sludge by extraction with organic acids [J]. Water Science & Technology, 1998, 40(1): 129-136.
[14] LILIAN V Y, VICTOR Q R. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate [J]. Hydrometallurgy, 2018, 180: 88-95.
[15] WANG Gai-rong, YANG Hong-ying, TONG Lin-lin, LIU Yuan-yuan. Research on process mineralogy of oxidized copper ore in Luanshya, Zambia [J]. Journal of Northeastern University (Natural Science), 2019, 40(3): 350-355. (in Chinese)
[16] FENG Qi-cheng, WEN Shu-ming, CHEN Ci-yun, ZHAO He-fei, WANG Yi-jie, LV Chao. Extraction of copper from a refractory copper oxide ore by catalytic oxidation acid leaching [J]. Advanced Materials Research, 2013, 734-737: 941-944.
[17] SHAYESTEHFAR M, NASAB S K, MOHAMMADALIZADEH H. Mineralogy, petrology, and chemistry studies to evaluate oxide copper ores for heap leaching in Sarcheshmeh copper mine, Kerman, Iran [J]. Journal of Hazardous Materials, 2008, 154: 602-612.
[18] WU Ai-xiang, YIN Sheng-hua, YANG Bao-hua, WANG Jun, QIU Guan-zhou. Study on preferential flow in dump leaching of low-grade ores [J]. Hydrometallurgy, 2007, 87: 124-132.
[19] CHEN Xiang-yang, LAN Xin-zhe, ZHANG Qiu-li, MA Hong-zhou, ZHOU Jun. Leaching vanadium by high concentration sulfuric acid from stone coal [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s123-s126.
[20] BIINGOL D, CANBAZOGLU M. Dissolution kinetics of malachite in sulphuric acid [J]. Hydrometallurgy, 2004, 72(1): 159-165.
[21] LI Yu-biao, WANG Bing, XIAO Qing, CLEMENT L, ZHANG Qi-wu. The mechanisms of improved chalcopyrite leaching due to mechanical activation [J]. Hydrometallurgy, 2017, 173: 149-155.
[22] TANDA B C, EKSTEEN J J, ORABY E A. An investigation into the leaching behaviour of copper oxide minerals in aqueous alkaline glycine solutions [J]. Hydrometallurgy, 2017, 167: 153-162.
[23] RUBISOV D H, KROWINKEL J M, PAPANGELAKIS V G. Sulphuric acid pressure leaching of laterites-universal kinetics of nickel dissolution for limonites and limonitic/saprolitic blends [J]. Hydrometallurgy, 2000, 58(1): 1-11.
[24] OZDEMIR M, CETISLI H. Extraction kinetics of alunite in sulfuric acid and hydrochloric acid [J]. Hydrometallurgy, 2005, 76(3-4): 217-224.
[25] OZDEMIR M, CAKiR D, KiPCAK l. Magnesium recovery from magnesite tailings by acid leaching and production of magnesium chloride hexahydrate from leaching solution by evaporation [J]. International Journal of Mineral Processing, 2009, 93(2): 209-212.
[26] LUO Zheng, YANG Jing, MA Hong-wen, LIU Mei-tang, MA Xi. Recovery of magnesium and potassium from biotite by sulfuric acid leaching and alkali precipitation with ammonia [J]. Hydrometallurgy, 2015, 157: 188-93.
[27] CHEN Fan-rong, EWING R C. U6+ structural entropy of compounds and its influence on thermodynamic stability: Application in geological disposal of nuclear waste [J]. Scientia Sinica (Terrae), 2002, 32(8): 644-652.
[28] COOPER R M, PARKINSON G M, NEWMAN O M G. The precipitation of silica from acidic zinc leach liquors [C]// GUPTA B S, IBRAHIM S. Mixing and Crystallization. Dordrecht, Netherlands: Springer, 2000: 163-176.
[29] SUN M S. The nature of chrysocolla from inspiration mine Arizona [J]. American Mineralogist, 1963, 48: 649-658.
[30] NICOL M J, AKILAN C. The kinetics of the dissolution of chrysocolla in acid solutions [J]. Hydrometallurgy, 2018, 178: 7-11.
[31] SUN Xi-liang, CHEN Bai-zhen, YANG Xi-yun, LIU You-yuan. Technological conditions and kinetics of leaching copper from complex copper oxide ore [J]. Journal of Central South University, 2009, 16(6): 936-941.
[32] WU Ying. Optics and micro-shape characters in the process of biotite transforming to chlorite [J]. Inner Mongolia Petrochemical Industry, 2009(10): 5-7.
[33] VEEIER D R, FENNY J M. TEM study of the biotite [34] SNALL S, LILJEFORS T. Leachability of major elements from minerals in strong acids [J]. Journal of Geochemical Exploration, 2000, 71(1): 1-12. 王改荣1,2,刘媛媛3,佟琳琳1,2,金哲男1,2,陈国宝1,2,杨洪英1,2 1. 东北大学 多金属共生矿生态化冶金教育部重点实验室,沈阳 110819; 2. 东北大学 冶金学院,沈阳 110819; 3. CNMC Luanshya Copper Mines Plc (CLM), Independence Avenue, Luanshya City, 90456, Zambia 摘 要:采用XRD、光学显微镜、SEM-EDS等物相分析手段,研究温度对复杂氧化铜矿中不同赋存状态铜矿物浸出行为的影响。结果表明:常温下易浸氧化铜矿物完全被浸出,而结合态铜矿物丝毫未被浸出;微温(40 °C)下主要是类质同象铜的浸出;中温(60 °C)时吸附态铜的浸出速率明显加快;达80 °C时,类质同象铜完全浸出,但仍有11.2%的吸附态铜未被浸出。在整个浸出过程中,长石-石英-铜-铁胶体中的铜未被溶解。不同铜矿物的浸出速率大小如下:孔雀石、假孔雀石>硅孔雀石>含铜绿泥石>含铜白云母>含铜黑云母>含铜褐铁矿>长石-石英-铜-铁胶结体。 关键词:复杂氧化铜矿;赋存状态;温度;浸出行为;铜矿物 (Edited by Xiang-qun LI) Foundation item: Project (U1608254) supported by the Special Fund for the National Natural Science Foundation of China; Projects (ZJKY2017(B)KFJJ01, ZJKY2017(B)KFJJ02) supported by Zijin Mining Group Co., Ltd., China Corresponding author: Hong-ying YANG; Tel: +86-13889803669; E-mail: yanghy@smm.neu.edu.cn DOI: 10.1016/S1003-6326(19)65125-3温度对复杂氧化铜矿中不同赋存状态铜矿物浸出行为的影响

