
Kinetic study on pressure leaching of high iron sphalerite concentrate
XIE Ke-qiang(谢克强)1, YANG Xian-wan(杨显万)1, WANG Ji-kun(王吉坤)2,
YAN Jiang-feng(阎江峰)2, SHEN Qing-feng(沈庆峰)2
1. Faculty of Materials and Metallurgical Engineering, Kunming University of Science and Technology,Kunming 650093, China;
2. Yunnan Metallurgical General Company, Kunming 650031, China
Received 1 April 2006; accepted 16 July 2006
Abstract: The kinetics of pressure leaching high iron sphalerite concentrate was studied. The effects of agitation rate, temperature, oxygen partial pressure, initial acid concentration, particle size, iron content in the concentrate and concentration of Fe2+ added into the solution on the leaching rate of zinc were examined. The experiment results indicate that if the agitation rate is greater than 600 r/min, its influence on Zn leaching rate is not substantial. A suitable rise in temperature can facilitate the leaching reaction, and the temperature should be controlled at 140-150 ℃. The increase trend of Zn leaching rate becomes slow when pressure is greater than 1.2 MPa, so the pressure is controlled at 1.2-1.4 MPa. Under the conditions of this study, Zn leaching rate decreases with a rise in the initial sulfuric acid concentration; and Zn leaching rate increases with a rise of iron content in the concentrate and Fe2+ concentration in the solution. Moreover, the experiment demonstrates that the leaching process follows the surface chemical reaction control kinetic law of “shrinking of unreacted core”. The activation energy for pressure leaching high iron sphalerite concentrate is calculated, and a mathematical model for this pressure leaching is obtained. The model is promising to guide the practical operation of pressure leaching high iron sphalerite concentrate.
Key words: high iron sphalerite concentrate; pressure leaching; kinetics; activation energy; mathematical model
1 Introduction
A substantial portion of zinc sulphide ore resources is high iron sphalerite [(Zn, Fe)S], which is widely spread in China, and in Yunnan Province the zinc reserves in the form of high iron sphalerite amounts to 7 million tons and accounts for 1/3 of the proved zinc resources[1-2].
Up to now lots of researches on acid pressure leaching sphalerite concentrate have been carried out by use of thermodynamics, kinetics and the electrochemistry methods[3-15]. The kinetics on leaching sphalerite with FeCl3 in microwave field[16] and the mechanism of influence of ferric iron on electrogenerative leaching of sulfide minerals with FeCl3[17] have also been studied, but most of the reported researches on the kinetics of acid pressure leaching sphalerite concentrate are about low iron (w(Fe)<10%) sphalerite concentrate and only a few are about high iron sphalerite concentrate. Therefore, investigation into the reaction kinetics and mechanisms of pressure leaching high iron sphalerite concentrate and mathematical modeling of this leaching process will help taking effective measures to facilitate the leaching reactions, enhance the productivity and reduce the operation costs. This has the potential to guide the production operation of pressure leaching high iron sphalerite concentrate.
2 Experimental
2.1 Principe
The main chemical reactions in acid pressure leaching high iron sphalerite concentrate were reported in Refs.[8,11-13]. At temperatures below 180 ℃, sphalerite was oxidized according to the following reaction equation[13]:
ZnS+H2SO4+1/2O2→ZnSO4+S0+H2O (1)
The oxidation processes of pyrite and pyrrhotite are similar[13]:
2FeS2+7O2+2H2O→2H2SO4+2FeSO4 (2)
FeS+H2SO4+1/2O2→FeSO4+H2O+S0 (3)
With a rise in temperature, the elemental sulphur produced was oxidized according to the following equation[13]:
2S0+2H2O+3O2→2H2SO4 (4)
The sulphur acid formed facilitates the dissolution of the base metal in high iron sphalerite concentrate, which leads to the direct oxidation of the sphalerite[13]:
ZnS+2O2→ZnSO4 (5)
With a change in the retention time, temperature, oxygen concentration, oxygen partial pressure and initial zinc contents in the concentrate, the reactions above are interrelated and interact with each other[13].
2.2 Starting materials
The materials used in this research were the high iron sphalerite concentrate from two mines of Yunnan Province, China. The concentrates were dried and screened, and the portions of 75-80 μm were used as the samples for the kinetic research experiment. The chemical compositions of these samples are listed in Table 1.
Table 1 Chemical compositions of samples (mass fraction, %)
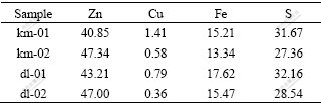
The main mineralogical phases in samples km-01, km-02 and dl-01 are sphalerite (ZnS), pyrrhotite (Fe1-xS), and a small quantity of pyrite, and those in sample dl-02 are sphalerite (ZnS), pyrite (FeS2) and a small quantity of pyrrhotite.
2.3 Equipment
The leaching experiments were conducted in a 2 L autoclave. The autoclave vessel, sampling tube, thermo- couple sheaths and magnetic stirring device were all made of titanium. During experiment sampling for analysis can be done through the sampling valve at regular intervals.
2.4 Method of experiment and analysis
In kinetic study, the starting material was screened, and in every experiment 5 g of sample was added into 1 L of dilute sulfuric acid solution. The ratio of liquid to solid was 200?1, and the acid concentration was 50 g/L that is about 20 times as large as the stoichiometric value. The oxygen used was industrially pure.
In the experiment 20-30 mL of solution was sampled every time, and its volume was measured accurately after it was cooled to room temperature. Then the sample was sent to Kunming Metallurgical Institute for examination and analysis. The influence of the sampling was taken into account when the experiment results were calculated.
3 Results and discussion
3.1 Effect of particle size on Zn leaching rate
The effect of particle size on leaching high iron sphalerite concentrate is examined under the conditions that temperature is 140 ℃, initial acid concentration is 50 g/L, pressure is 1.4MPa and some surfactant is added. The results are shown in Fig.1.
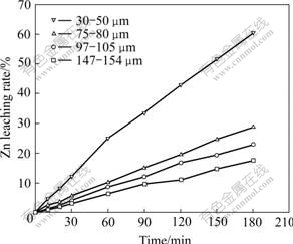
Fig.1 Effect of particle size on Zn leaching rate
Fig.1 indicates that the influence of particle size on Zn leaching rate is remarkable. Zn leaching rate is raised markedly when the particle size is less than 50 μm. In order to examine the Zn leaching kinetics thoroughly, the samples of 75-80 μm are used for the subsequent experiment.
3.2 Effect of agitation on Zn leaching rate
The effect of agitation on leaching high iron sphalerite concentrate is examined under the same conditions. The results are shown in Fig.2, and it can be seen that Zn leaching rate increases with a rise in agitation rate, but if the agitation rate is greater than 600 r/min its influence on Zn leaching rate is not substantial. Therefore, the agitation rate is fixed at 600 r/min for the subsequent experiments.
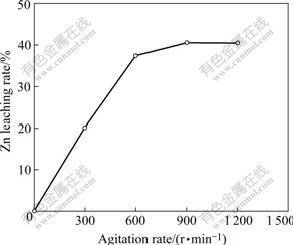
Fig.2 Effect of agitation rate on Zn leaching rate
3.3 Effect of temperature on Zn leaching rate
The experimental results are shown in Fig.3. Obviously, a rise in temperature can facilitate the leaching reaction.
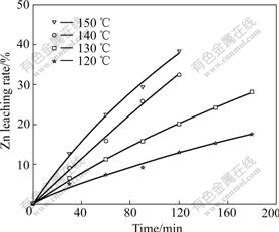
Fig.3 Effect of temperature on Zn leaching rate
Substituting the experimental results into the following kinetic equation yields
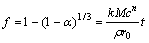 (6)
(6)
where t is the leaching time, min; α is the zinc leaching rate, %; k is a rate constant, mol/(L?min-2); ρ is the density of particle, g/cm3; r0 is the initial radius of particle, cm; c is the concentration of leaching reagent H2SO4, mol/L; M is the relative atomic (molecular) mass of solid reactant.
The calculated results are shown in Fig.4.
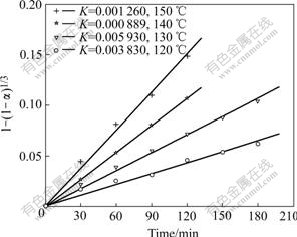
Fig.4 Relationship of f with temperature
Fig.3 and Fig.4 indicate that effect of temperature on Zn leaching rate of high iron sphalerite concentrate is very remarkable. In Eqn.(6), when the concentration and temperature are kept invariable, the value of kMcn/ρr0 is a constant. This constant can be named apparent rate constant k′ and equals the slope of each straight line in Fig.4. By plotting the natural logarithm of the apparent rate constant (-lnk′) against the reciprocal of the reaction temperature (1/T), the Arrhenius line can be obtained, as shown in Fig.5.
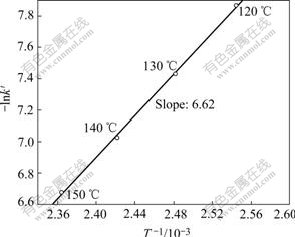
Fig.5 Arrhenius line
From Fig.5 a kinetic equation about the effect of temperature on Zn leaching rate can be derived:
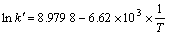 (7)
(7)
Because the slope of the Arrhenius line is equal to the activation energy divided by the gas constant(-E/R), the value of the activation energy of the leaching reaction can be calculated i.e. E=55.04 kJ/mol. This result accords closely with the value (E=51.05 kJ/mol) reported in Ref.[11]. According to the criteria for determining the rate-controlling step[8], it can be concluded that the leaching process of high iron sphalerite concentrate is controlled by surface chemical reactions because the activation energy exceeds the criterion value of 42 kJ/mol.
3.4 Effect of oxygen partial pressure on Zn leaching rate
The vapor tension of dilute sulfuric acid solution under the experiment condition is obtained, and it is 0.3 MPa. So, relationship equation of pressure to oxygen partial pressure is p=p(O2)+0.3. The experimental results under the same conditions are shown in Fig.6.
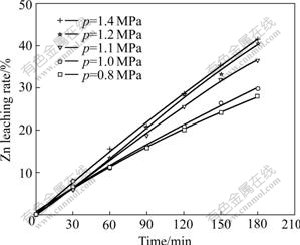
Fig.6 Effect of oxygen partial pressure on Zn leaching rate
When substituting the experimental results into Eqn.(6), Fig.7 can be constructed.
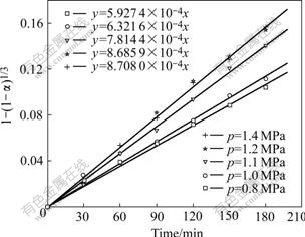
Fig.7 Relationship between f and oxygen partial pressure
By plotting the natural logarithm of the slope of the each line against the natural logarithm of the pressure (lnp) in Fig.7, Fig.8 can be obtained.
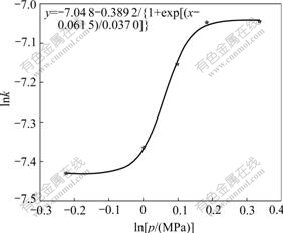
Fig.8 Relationship of lnk′ to lnp
From Fig.8 a kinetic equation about the effect of oxygen partial pressure on Zn leaching rate can be derived by regression analysis:
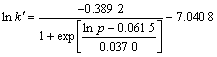 (8)
(8)
3.5 Effect of initial acid concentration on Zn leaching rate
Fig.9 indicates the effect of initial sulfuric acid concentration on Zn leaching rate when other conditions are unchangeable. The experimental results show that the higher the initial sulfuric acid concentration, the lower the Zn leaching rate is.
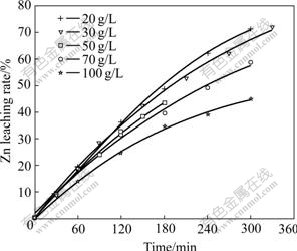
Fig.9 Effect of initial sulfuric acid concentration on Zn leaching rate
When substituting the experimental data into Eqn.(6), Fig.10 can be obtained.
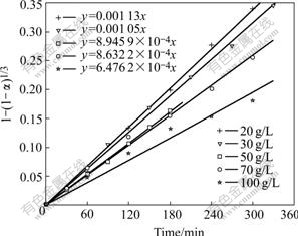
Fig.10 Relationship between f and initial sulfuric acid concentration
By plotting the natural logarithm of the slope of the each line (lnk′) against the natural logarithm of initial sulfuric acid concentration (ln[H2SO4]) in Fig.10, Fig.11 can be drawn.
Fig.11 indicates that lnk′ is a linear function of ln[H2SO4], and the apparent reaction order of -0.318 2 can be found from the slope of the straight line. So, a kinetic equation about the effect of the initial sulfuric acid concentration on Zn leaching rate can be obtained as follows:
lnk′=-7.251 6-0.318 2lnc (9)
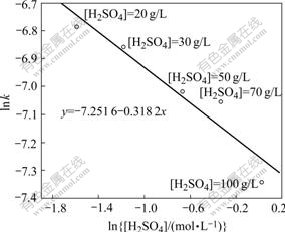
Fig.11 Relationship of lnk′to ln[H2SO4]
In general, Zn leaching rate should increase with a rise in initial sulfuric acid concentration. But the result shown in Fig.11 is contradictory. This abnormality is reported in Ref.[11], but it is not studied further. The general explanation is that if the amount of sulfuric acid added in experiment is over twice the stoichiometric amount, there will be the “abnormality”, i.e. Zn leaching rate decreases with a rise in the initial sulfuric acid concentration. The mechanism proposed for reaction (1) is
ZnS+H2SO4→ZnSO4+H2S (10)
H2S+Fe2(SO4)3→2FeSO4+H2SO4+S0 (11)
2FeSO4+H2SO4+1/2O2→Fe2(SO4)3+H2O (12)
When the amount of sulfuric acid added is over twice the stoichiometric amount, reaction(11) will go to left and more and more H2S will be produced. Because H2S restrains the dissolution of zinc[11-13], the leaching rate of zinc will decrease with an increase in the initial sulfuric acid concentration. In Fig.9, the acid amounts added are all more than ten times the stoichiometric amount, so the experimental results accord with those in the related reports.
3.6 Effect of iron content in concentrate on Zn leaching rate
Leaching experiments are carried out on the samples of km-01, km-02 and dl-01, and the results are shown in Fig.12. The experimental data indicate that within some range of sulfuric acid, the higher iron content in the high iron sphalerite concentrate, the higher the Zn leaching rate is.
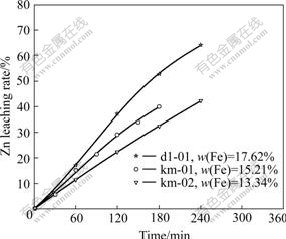
Fig.12 Effect of iron content in concentrate on Zn leaching rate
When substituting the experimental data into Eqn.(6), Fig.13 can be constructed.
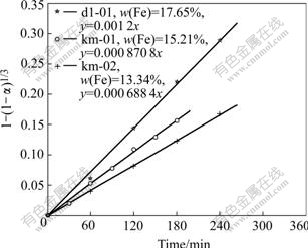
Fig.13 Relationship between f and iron content in concentrate
By plotting the natural logarithm of the slope of each line (lnk′) against the iron content in the concentration in Fig.13, Fig.14 can be drawn.
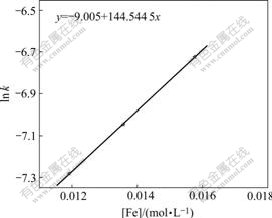
Fig.14 Relationship of lnk′ with [Fe]
From Fig.14 a kinetic equation about the effect of the iron content in the concentrate on Zn leaching rate can be obtained:
lnk′=-9.005+144.544 5[Fe] (13)
3.7 Effect of concentration of Fe2+ added into solution on Zn leaching rate
The Fe2+ is added in the form of FeSO4, and the leaching results are shown in Fig.15.
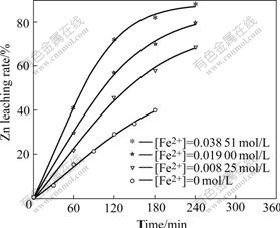
Fig.15 Effect of concentration of Fe2+ added on Zn leaching rate
Substitute the experimental data into Eqn.(6) and Fig.16 can be constructed.
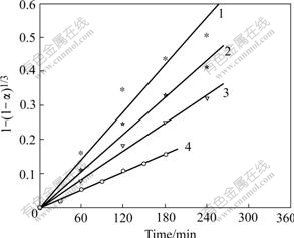
Fig.16 Relationship between f and concentration of Fe2+ added: 1 [Fe2+]=0.038 51 mol/L, y=0.002 33x; 2 [Fe2+]=0.019 00 mol/L, y=0.001 8x; 3 [Fe2+]=0.008 25 mol/L, y=0.001 38x; 4 [Fe2+]=0 mol/L, y=0.000 883 5x
By plotting the slope of each line (k′) in Fig.16 against the concentration of Fe2+ added ([Fe2+]), Fig.17 can be drawn.
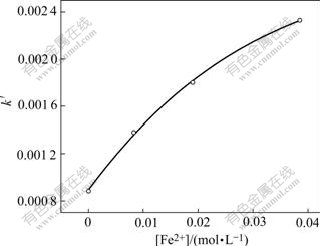
Fig.17 Relationship of k′to [Fe2+]
From this figure, a kinetic equation about the effect of the concentration of Fe2+ added into the solution on Zn leaching rate can be obtained:
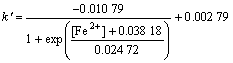 (14)
(14)
4 Development of kinetic mathematical model
The above experimental relationships indicate that Eqns.(7), (9) and (13) are linear, and Eqns.(8) and (14) are S-shaped curve. According to Ref.[18], S-shaped curve y=exp[β0+β1/x] is nonlinear in form, and it can be converted into linear relationship through the medium of change of variables, i.e. lny=β0+β1x1 (x1=1/x). A linear equation can be developed finally by regression analysis, and this is so-called essential linear relationship. By use of SPSS statistical software, a relationship of lnk′ with various factors can be obtained:
lnk′=6.974-6 606.005×(1/T)+0.779×lnp-
0.312×lnc+142.425×[Fe]+27.941×[Fe2+] (15)
The calculated values on the basis of Eqn.(15) and the experimental values are compared and the results are listed in Table 2.
Table 2 Comparison of calculated values with experimental ones for lnk′
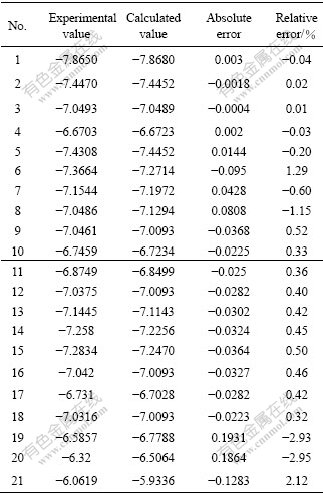
It can be seen that the accuracy of Eqn.(15) is good.
From Eqns.(6) and (15), a mathematical model for zinc leaching rate in the process of pressure leaching high iron sphalerite concentrate can be developed:
 (16)
(16)
where α is the Zn leaching rate, %; T is the temperature, K; p is the total pressure, MPa; c is the initial acid concentration, mol/L; [Fe] is the iron content in the concentrate, mol/L; t is the leaching time, min; r0 is the initial radius of particle, cm.
The comparison of the calculated Zn leaching rate (based on Eqn.(16)) with the experimental value is shown in Figs.18 and 19.
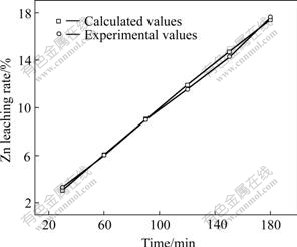
Fig.18 Demonstration of Eqn.(16) (Particle size is 147-154 μm)
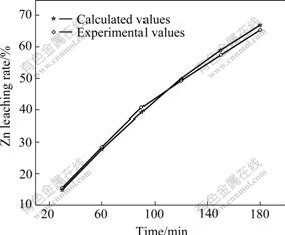
Fig.19 Demonstration of Eqn.(16) (Particle size is 30-50 μm)
Fig.18 shows that the experimental values accord closely with the calculated values (the relative error <2%) under the condition that the single size classification is 147-154 μm.
And Fig.19 shows that the experimental values deviate somewhat from the calculated values under the condition that the non-single size classification is 30-50 μm. But the deviation is small (the relative error <2.5%), so the model can be still used to guide the practice of production.
5 Conclusions
1) In the process of pressure leaching high iron sphalerite concentrate, when the agitation rate is greater than 600 r/min, its influence on Zn leaching rate is not substantial, which suggests that diffusion is not the control step of the leaching reaction under this condition.
2) The leaching process is controlled by the surface chemical reaction and follows the surface chemical reaction control kinetic law of “ shrinking of unreacted core”. The activation energy for pressure leaching high iron sphalerite concentrate is 55.04 kJ/mol.
3) The apparent reaction order for the initial sulfuric acid concentration is -0.318 2. When the amount of sulfuric acid in the leaching solution is over twice the stoichiometric amount, Zn leaching rate decreases gradually with a rise in the acid concentration because the intermediate product H2S restrains the dissolution of zinc.
4) Fe3+ in the leaching solution facilitates electron transfer and catalyzes the leaching reaction, which can be seen from the effects of Fe content in the concentrate and Fe2+ concentration in leaching solution on Zn leaching rate.
5) Within the condition range of this experiment, the kinetic equations for the effects of temperature, oxygen partial pressure, initial acid concentration, Fe content in the concentrate and Fe2+ concentration in leaching solution on Zn leaching rate are derived respectively. By combinating the effects of various factors and by use of SPSS statistical software, a mathematical model for the effects of multiple factors on Zn leaching rate is obtained, and a kinetic mathematical model for pressure leaching high iron sphalerite concentrate is finally developed:
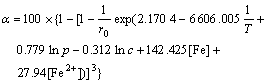
The experimental results demonstrate that the Zn leaching rate calculated on the basis of the kinetic mathematical model accords closely with the experimental data.
References
[1] WANG Ji-kun, ZHOU Ting-xi, WU Jin-mei. Study on high iron containing sphalerite concentrate by acid leaching under pressure [J]. Nonferrous Metals (Extractive Metallurgy), 2004(1): 5-8. (in Chinese)
[2] WANG Ji-kun, ZHOU Ting-xi. The technology and industrialization of zinc concentrates oxygen pressure leaching [M]. Beijing: Metallurgical Industry Press, 2005: 45-128. (in Chinese)
[3] DENG Ri-zhang, ZHAO Tian-cong, ZHONG Zhu-qian, MEI Guang-gui. Kinetic study on oxidative dissolution of sphalerite in aqueous hydrochloric acid solutions [J]. J Cent South Inst Min Metall, 1992, 23(1): 36-42. (in Chinese)
[4] WU Xi-jun, WANG Yang-sheng. Mathematical modeling of pressure leaching of zinc concentrates [J]. Heavy Metal Nonferrous Metallurgy, 1997(3): 26-32. (in Chinese)
[5] ZENG Qi-an, SUN De-kun, WANG Hui-fang. The technology of zinc concentrates pressure leaching of HBMS [J]. Heavy Metal Nonferrous Metallurgy, 1996(5): 30-33. (in Chinese)
[6] ZHANG Wu-chun, HUANG Zhi-lin, WANG Wan-lu. The experiment of sphalerite concentrates oxygen pressure leaching [J]. Yunnan Metallurgy, 1990(6): 39-42. (in Chinese)
[7] XU Zhi-feng, WANG Hai-bei, QIU Ding-fan. Electrochemical behavior of sphalerite in oxidic-acidic leaching process [J]. Nonferrous Metals, 2005, 57(4): 59-63. (in Chinese)
[8] YANG Xian-wan, QIU Ding-fan. Hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 1998: 154-215; 255-263. (in Chinese)
[9] CHEN Zhi-he, HE Xing-min. The technology of zinc concentrates oxygen pressure leaching [J]. Hunan Nonferrous Metals, 2002, 18(1): 26-28. (in Chinese)
[10] CHEN Jia-yong. Handbook of hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2005: 320-328. (in Chinese)
[11] JAN R J, HEPWORTH M T, FOX V G. A Kinetic study on the pressure leaching of sphalerite [J]. Metallurgical Transactions B, 1976, 7B: 353-361.
[12] TORMA A E. Kinetic evaluation of pressure leaching of a zinc-calcine by SO2 and sphalerite concentrate by oxygen [J]. Metall, 1985, 39(9): 824-828.
[13] HARVEY T J, TAI YEN W, PATERSON J G. A kinetic investigation into the pressure oxidation of sphalerite from acomplex concentrate [J]. Minerals Engineering, 1993, 6: 949-967.
[14] LOCHMANN J, PEDLIK M. Kinetic anomalies of dissolution of sphalerite in ferric sulfate solution [J]. Hydrometallurgy, 1995, 37: 89-96.
[15] SHNEERSON Y M, VIGDORCHIK E M, ZHMARIN E E, LAPIN A Y, SHPAER V M. Mathematical modeling of pressure leaching of sulphide zinc concentrate [C]//COLLINS M J, PAPANGELAKIS V G. Pressure. Hydrometallurgy 2004. Banff, Alberta, Canada: The Canadian Institute of Mining, Metallurgy and Petroleum, 2004: 983-997.
[16] PENG Jin-hui, LIU Chun-peng. Kinetics on leaching sphalerite with FeCl3 solution in microwave field [J]. The Chinese Journal of Nonferrous Metals, 1992, 2(1): 46-49. (in Chinese)
[17] WANG Shao-fen, FANG Zheng. Mechanism of influence of ferric iron on electrogenerative leaching of sulfide minerals with FeCl3 [J]. Trans Nonferrous Met Soc China, 2006, 16(2): 473-476.
[18] XUE Wei. SPSS Statistical Analytical Method and Applications [M]. Beijing: Publishing House of Electronics Industry, 2004: 233-301. (in Chinese)
Foundation item: Project(2002GG01) supported by Yunnan Metallurgical General Company, China
Corresponding author: XIE Ke-qiang; Tel: +86-871-5182554; E-mail: xkqzhh@sina.com
(Edited by YANG Bing)