J. Cent. South Univ. Technol. (2010) 17: 1196-1200
DOI: 10.1007/s11771-010-0618-x
Influence of copper solvent extractant on microbial community structure of acidophilic microorganisms
CHEN Bo-wei(陈勃伟), LI Wen-juan(李文娟), LIU Xing-yu(刘兴宇),
ZHOU Gui-ying(周桂英), WEN Jian-kang(温建康)
National Engineering Laboratory of Biohydrometallurgy, General Research Institute for Nonferrous Metals,
Beijing 100088, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: The influence of different concentrations of copper solvent extractant ZJ 988 on the growth and activity of acidophilic microorganisms was studied and the microbial community structures were compared by 16S rRNA gene clone library analysis. The total bacteria numbers are reduced when 0.5% (volume fraction) extractant is added. The proportions of Acidithiobacillus ferrooxidans and Acidiphilium organovorum are increased, whereas the proportion of Leptospirillum ferriphilum is reduced. When the concentration of extractant is elevated to 1%, growth of all bacteria is inhibited. Clone library results reveal that the dominant bacteria in the culture solution with/without the extractant are At. ferrooxidans, A. organovorum and L. ferriphilum. The sensitivity order of the three bacteria to the extractant from the most to the least is found to be L. ferriphilum>At. ferrooxidans>A. organovorum.
Key words: extractant; acidophile; 16S rRNA gene; biohydrometallurgy
1 Introduction
For several decades, biohydrometallurgy techniques have been intensively applied on industrial scale for the extraction of metals from low-grade ores (i.e., containing gold, copper, nickel, cobalt or uranium) due to its environmentally friendly aspect and low cost, low investment, and simple equipment [1]. Many heaps or stirred-tank bioleaching plants, most of which were used to treat copper ore, have been built around the world including China [2-3]. Copper produced by biohydrometallurgy accounts for 1/4 of the world copper production [4].
Industrial heap bioleaching-SX-EW is a closed cycle process. The recycled raffinate contains different amounts of organic matter depending on the solubility of the solvent used and mechanical entrainment. Raffinate is used for irrigation, so microorganisms inevitablely get touch with extractant in this cycling process. Phenol and nonylphenol in the extractant are toxic to bioleaching microorganisms, thus influencing bioleaching efficiency [5].
Many researches have been done on the effect of solvent extraction reagent, organic compounds and flotation chemicals on the growth and oxidation ability of bioleaching bacteria [6-8]. Most of the studies focused on the effects on pure cultures, while the influence on mixed cultures was less studied. QIU et al [9] found that the bacteriostasis order of the organic matter was as follows: 260# kerosene+1.5% LIX 984N>260# kerosene>circulated organic phase sampled from factory>octanol + 1.5% LIX 984N>octanol. WATLING et al [10] studied the effects of the copper extractant LIX 984N 20% (volume fraction) in Shellsol 2046 on the growth and activity of Acidithiobacillus ferrooxidans, Sulfobacillus thermosulfidooxidans and Acidiphilium cryptum. They found that copper extraction was reduced to about one third with S. thermosulfidooxidans in the presence of 50 mg/L SX reagent compared with no SX reagent. At 250 mg/L SX reagent, S. thermosulfidooxidans growth and ferrous ion oxidation were significantly inhibited. However, solvent extraction reagent had little impact on At. ferrooxidans at concentrations up to 250 mg/L. A. cryptum grew well at 250 mg/L SX reagent but did not utilise the reagent.
Up to now, according to our knowledge, no papers have been published on the microbial community structure of acidophiles subjected to copper solvent extractant. In this work, the influence of different concentrations of copper solvent extractant ZJ 988 on the growth and activity of acidophilic microorganisms was studied and the microbial community structures were compared by 16S rRNA gene clone library analysis.
2 Materials and methods
2.1 Bacteria culture and extractant
The acidophilic microorganisms were sampled from the bioleaching plant of Zijinshan Copper Mine, China and maintained on sterilized 9K medium, which was composed of the following mineral salts: (NH4)2SO4 3.0 g/L, K2HPO4 0.5 g/L, MgSO4·7H2O 0.5 g/L, KCl 0.1 g/L, Ca (NO3)2 0.01 g/L, FeSO4·7H2O 45 g/L. The pH of the medium was adjusted to 1.8 using 0.5 mol/L H2SO4. The copper extractant used in this work was ZJ 988 which was made by Zijin Mining Group Co., Ltd., China. The reagent is mainly composed of 2-hydroxy-5- nonylacetophenone oxime and 5-nonylsalicyaldehyde oxime. Both the aldoxime and the ketoxime have inhibitory effects on bacteria growth and oxidation activity.
2.2 Influence of copper solvent extractant on bacteria growth and activity
In order to investigate the influence of copper solvent extraction on acidophilic microorganisms growth and activity, triplicate 250 mL conical flasks containing 180 mL 9K medium were inoculated with 20 mL the above acidophilic microorganisms (1×107 mL-1) without the extractant and with the addition of 0.5% (volume fraction) or 1% extractant. The pH of the solution was adjusted to 1.8 by adding 0.5 mol/L H2SO4. The flasks were cultivated in a shaking incubator (160 r/min) at 33 ℃. The variations of pH, oxidation/reduction potential, and concentrations of Cu2+, Fe2+ and Fe3+ in solution were measured every 24 h. Cell concentrations were estimated using a blood cell counting chamber.
2.3 Clone library construction
When cell concentrations reached stationary phase, a modified nucleic acids extraction method was used [11] to extract DNA from samples of inoculating liquid and culture solution. Y, YC and YD were named as clone library of inoculating liquid, culture solution without SX and culture solution with 0.5% SX, respectively. 16S rRNA genes were amplified with universal primers 27F and 1492R [12]. The amplified 16S rRNA genes were gel purified and inserted into pGEM-T easy vectors (Promega, USA) and transformed into Escherichia coli DH5α. At least 50 white clones were randomly selected from the library and sequenced by Sangon Corp. (Shanghai, China).
2.4 Phylogenetic analysis
Chimeric sequences were detected by the chimera function at the RDP site (http://rdp.cme.msu.edu/), and all chimera sequences were eliminated. Sequences were analyzed using BLAST at the NCBI database (http://ncbi. nlm.nih.gov/BLAST). Alignments of 16S rRNA gene sequences were performed with the CLUSTAL_X program, version 1.64b [13]. A phylip-generated distance matrix was used as the input file to distance-based OTU and richness (DOTUR) [14], which assigns sequences to operational taxonomic units (OTUs) for every possible distance. Rarefaction analysis and the Chao1 non-parametric diversity estimator were applied to the clone library to estimate whether the library had been sufficiently sequenced to extrapolate the total sequence diversity. Other diversity indices were calculated by using SPADE [15]. A neighbour-joining phylogenetic tree was constructed based on evolutionary distances that were calculated with the Kimura two-parameter model using MEGA4 [16].
3 Results and discussion
3.1 Influence of copper solvent extractant on bacteria growth and oxidation activity
The effect of copper solvent extractant on mixed culture of acidophiles from solution is shown in Fig.1. It can be seen that the extractant has a strong inhibition effect on bacteria growth. Bacteria can reach the steady growth state in 6 d without extractant. When 0.5% extractant is added, the time in which bacteria attainted the stationary phase is postponed for 4 d and the total number of bacteria reduces. When the concentration of extractant is elevated to 1%, growth of all bacteria is inhibited.
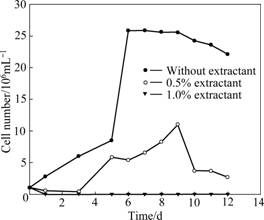
Fig.1 Influence of copper solvent extractant on mixed cultures of acidophiles from solution
Fig.2 shows the impact of copper solvent extractant on bacteria oxidation activity. The oxidation/reduction potential (VOR) is mainly determined by the concentration ratio of Fe3+ to Fe2+ in the solution, so the oxidation/ reduction potential was used as an indication of bacteria oxidation activity. It can be seen that pH value in all experiments goes upwards and then reduces. In the first
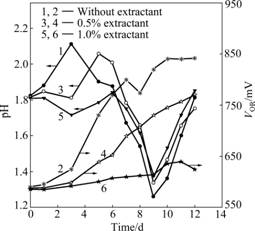
Fig.2 Influence of copper solvent extractant on mixed acidophiles oxidation activity
period, the main reaction is the oxidation of Fe2+, which is an acid consuming reaction, causing the raise of pH. With the increase of Fe3+, the hydrolysis of ferric iron to ferric species such as jarosite and Fe(OH)3 becomes dominant, so the pH begins to decrease. The two reactions are as follows:
4Fe2++O2+4H+→4Fe3++2H2O (1)
Fe3++3H2O→Fe(OH)3+3H+ (2)
When 0.5% extractant is added, the redox potential goes up slowly and the value of redox potential is lower than that without extractant. When the concentration of extractant is elevated to 1%, the redox potential does not obviously increase. This indicates no bacteria growth and the oxidation activity of all bacteria are completely inhibited.
The growth of microbe needs proper surface tension. Extractants are usually surfactant, so the existence of extractant will change the surface tension of the medium, which is commonly 45-65 mN/m. It is serious that the osmosis of cell membrane will alter. This will cause the damage of cell membrane and inhibit the activity of bacteria [5].
3.2 Influence of copper solvent extractant on microbial community structure
For the three clone libraries constructed, a total of 169 sequences were grouped into eight OTUs with a distance level of 3%. The eight OTUs fell into three phylogenetic divisions: Proteobacteria, Nitrospira and Actinobacteria (Fig.3). Proteobacteria are predominant in the clone libraries (59.17% of clones), and Nitrospira are represented in relatively high amount (38.46% of clones). The gene sequences of only four clones are aligned in a clade with Actinobacteria, representing 2.37% of the clones. Of all the identified sequences, four genera are represented. Among these genera, genus Leptospirillum, Acidithiobacillus and Acidiphilum account for a high proportion; each presents 65, 52, 48 clones, respectively. The main species in the three genera are Leptospirillum ferriphilum, Acidithiobacillus ferrooxidans and Acidiphilum organovorum. The collection curve, which plots number of clones sequenced versus the number of unique OTUs (Fig.4), reaches an asymptote, indicating that the clones tested in the experiment are sufficient to detect the level of microbial communities’ diversity. This is consistent with the high estimated sample coverage of each clone library computed by the SPADE (Table 1).
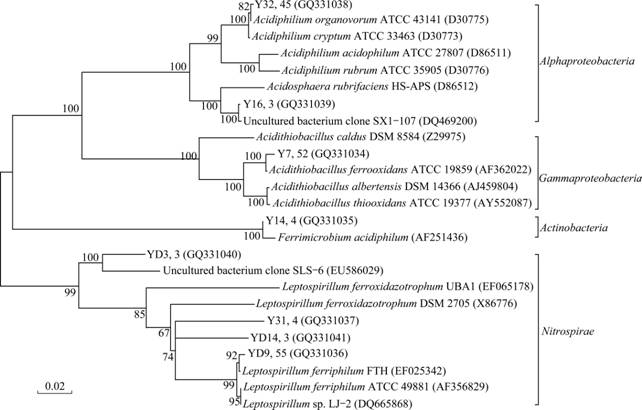
Fig.3 Unrooted phylogenetic tree based on comparative analysis of 16S rRNA gene sequence data from eight OTUs and their close relatives (Cloned 16S rRNA sequences from three clone libraries are arranged as: OTU name, clone numbers (Accession number); Bootstrap value=1 000)
Table 1 Estimated diversity indices for bacterial communities in three clone libraries at distance level of 0.03
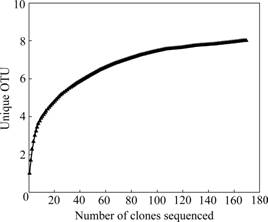
Fig.4 Collection curves of clones and unique OTU
In order to understand the composition differences of the microbial populations in the three samples, the percentage of clones within the library as a surrogate of relative abundance was used for comparison. Fig.5 shows the distribution of the major bacterial assemblages. The 16S rRNA gene inventory reveals that the major bacterial population in the three samples contains three species: Leptospirillum ferriphilum, Acidithiobacillus ferrooxidans and Acidiphilum organovorum. The percentages of L. ferriphilum and At. ferrooxidans do not have great changes without the addition of extractant, while the proportion of A. organovorum is increased from 9.09% to 27.78%. With the addition of 0.5% extractant, the percentage of L. ferriphilum is decreased from 52.73% to 20.00%, while the proportion of At. ferrooxidans is equivalent with that of no extractant and A. organovorum was increased from 9.09% to 41.76%. From the changes of the proportion of the major three 16S rRNA gene in the three sample, the sensitivity order of the three bacteria to the extractant from the most to the least can be concluded as L. ferriphilum>At. ferrooxidans>A. organovorum.
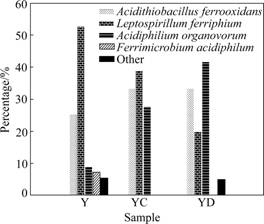
Fig.5 Distribution of abundance of 16S rRNA gene in three samples
No papers have been published on the influence of the extractant on L. ferriphilum, but according to the research of MAZUELOS et al [6], a 15% mix of LIX 64 in kerosene decreased the iron-oxidation rate of L. ferrooxidans in mixed cultures with At. ferrooxidans at concentrations up to 60 mg/L. L. ferriphilum is in the same genus with L. ferrooxidans and it has similar growth conditions [17]. So it can be concluded that the autotroph L. ferriphilum can be inhibited by extractants. At. ferrooxidans usually grows at pH 1.3-4.5, with an optimum of pH 2.5, and at temperatures ranging from 10 to 37 ℃, with an optimum of 30-35 ℃ [18]. It has been used as model microorganisms in the study of bioleaching processes, largely because it is widely distributed in acid mine drainage and bioleaching environments [19]. Previous studies [10] suggested that At. ferrooxidans can grow well in solutions containing LIX 984N at concentrations up to 250 mg/L. It is interesting that strains of At. ferrooxidans show different ferrous iron oxidation rates under organic compounds [20]. This may give an explanation for the higher proportion of At. ferrooxidans over L. ferriphilum with the addition of 0.5% extractant. Another result shows that L. ferriphilum had a strong resistance to kerosene over At. ferrooxidans, while At. ferrooxidans had a strong tolerance of extractant over L. ferriphilum (data not shown). A. organovorum [21], a heterotrophic bacterium, was isolated from a culture of At. ferrooxidans which had been grown autotrophically on FeSO4-basal salts medium for several years. This is why the bacteria were found to be co-cultured with At. ferrooxidans. A. organovorum can utilize reduced forms of sulfur or iron as energy sources. So, in bioleaching environments the heterophile can increase concentrations of soluble ferrous iron and elimilate the inhibitory effect of organic substances [22]. WATLING et al [10] found that Acidiphilum cryptum which was closely related to A. organovorum could not use SX LIX 984N. This suggests that A. organovorum may not utilize extractant either. This can be confirmed by the reduction of cell numbers with the addition of extractant. But due to the tolerance of extractant, A. organovorum became predominat in the medium with extractant. In conclusion, the sensitivity order of the three bacteria to the extractant summarized previously is consistent with the current research results.
4 Conclusions
(1) Copper solvent extractant ZJ 988 is differentially toxic to acidophiles.
(2) 0.5% (volume fraction) extractant can cause the total bacteria number reduced, whereas 1% extractant inhibits all the acidophiles.
(3) At. ferrooxidans, A. organovorum and L. ferriphilum are predominant in the culture solution with/without the extractant.
(4) The sensitivity order of the three bacteria to the extractant from the most to the least is found to be L. ferriphilum>At. ferrooxidans>A. organovorum.
References
[1] BRIERLEY J A, BRIERLEY C L. Present and future commercial applications of biohydrometallurgy [J]. Hydrometallurgy, 2001, 59(2/3): 233-239.
[2] TRIBUTSCH H. Direct versus indirect bioleaching [J]. Hydrometallurgy, 2001, 59(2/3): 177-185.
[3] RUAN Ren-man, WEN Jian-kang, CHEN Jing-he. Bacterial heap-leaching: Practice in Zijinshan copper mine [J]. Hydrometallurgy, 2006, 83(1/4): 77-82.
[4] RAWLINGS D E, DEW D, CHRIS D. Biomineralization of metal-containing ores and concentrates [J]. Trends in Biotechnology, 2003, 21(1): 38-44.
[5] TORMA A E, ITZKOVITCH I J. Influence of organic solvents on chalcopyrite oxidation ability of Thiobacillus ferrooxidans [J]. Applied and Environmental Microbiology, 1976, 32(1): 102-107.
[6] MAZUELOS A, IGLESIAS N, CARRANZA F. Inhibition of bioleaching processes by organics from solvent extraction [J]. Process Biochemistry, 1999, 35(5): 425-431.
[7] OKIBE N, JOHNSON D B. Toxicity of flotation reagents to moderately thermophilic bioleaching microorganisms [J]. Biotechnology Letters, 2002, 24(23): 2011-2016.
[8] ASTON J E, APEL W A, LEE B D, PEYTON B M. Toxicity of select organic acids to the slightly thermophilic acidophile Acidithiobacillus caldus [J]. Environmental Toxicology and Chemistry, 2009, 28(2): 279-286.
[9] QIU Guan-zhou, LIU Xiao-rong, HU Yue-hua. The effects of organic phases in solvent extraction on bioleaching bacteria [J]. Journal of Central South University of Technology: Natural Science, 2001, 32(3): 243-246. (in Chinese)
[10] WATLING H R, PERROT F A, SHIERS D W, GROSHEVA A, RICHARDS T N. Impact of the copper solvent extraction reagent LIX 984N on the growth and activity of selected acidophiles [J]. Hydrometallurgy, 2009, 95(3/4): 302-307.
[11] OVED T, SHAVIV A, GOLDRATH T, MANDELBAUM R T, MINZ D. Influence of effluent irrigation on community composition and function of ammonia-oxidizing bacteria in soil [J]. Applied and Environmental Microbiology, 2001, 67(8): 3426-3433.
[12] WEISBURG W G, BARNS S M, PELLETIER D A, LANE D J. 16S ribosomal DNA amplification for phylogenetic study [J]. Journal of Bacteriology, 1991, 173(2): 697-703.
[13] THOMPSON J D, GIBSON T J, PLEWNIAK F. The ClustalX windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools [J]. Nucleic Acids Research, 1997, 24: 4876-4882.
[14] SCHLOSS P D, HANDELSMAN J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness [J]. Applied and Environmental Microbiology, 2005, 71(3): 1501-1506.
[15] CHAO A, SHEN T J. Program SPADE (Species Prediction and Diversity Estimation) [EB/OL]. [2009-02-13]. http://chao.stat.nthu. edu.tw.
[16] TAMURA K, DUDLEY J, NEI M, KUMAR S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0 [J]. Molecular Biology and Evolution, 2007, 24: 1596-1599.
[17] CORAM N J, RAWLINGS D E. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40 ℃ [J]. Applied and Environmental Microbiology, 2002, 68(2): 838-845.
[18] RAWLINGS D E. Biomining: Theory, microbes and industrial processes [M]. New York: Springer, 1997: 229-245.
[19] FOWLER T A, HOLMES P R, CRUNDWELL F K. Mechanism of pyrite dissolution in the presence of Thiobacillus ferrooxidans [J]. Applied and Environmental Microbiology, 1999, 65(7): 2987-2993.
[20] FRATTINI C J, LEDUC L G, FERRONI G D. Strain variability and the effects of organic compounds on the growth of the chemolithotrophic bacterium Thiobacillus ferrooxidans [J]. Antonie van Leeuwenhoek, 2000, 77(1): 57-64.
[21] LOBOS J H, CHISOLM T E, BOPP L H, HOLMES D S. Acidiphilium organovorum sp. nov., an acidophilic heterotroph culture isolated from a Thiobacillus ferrooxidans [J]. International Journal of Systematic Bacteriology, 1986, 36(2): 139-144.
[22] COUPLAND K, JOHNSON D B. Evidence that the potential for dissimilatory ferric iron reduction is wide spread among acidophilic heterotrophic bacteria [J]. FEMS Microbiology Letters, 2008, 279: 30-35.
(Edited by YANG You-ping)
Foundation item: Project(50904011) supported by the National Natural Science Foundation of China
Received date: 2009-12-23; Accepted date: 2010-03-15
Corresponding author: WEN Jian-kang, Professor; Tel: +86-10-82241313; E-mail: kang3412@126.com