
Transformation behavior of lead fractions during composting of lead-contaminated waste
LIU Jian-xiao(刘剑潇)1, XU Xiang-min(徐祥民)1, 2, HUANG Dan-lian(黄丹莲)2, ZENG Guang-ming(曾光明)1, 2
1. College of Environmental Science and Engineering, Ocean University of China, Qingdao 266003, China;
2. College of Environmental Science and Engineering, Hunan University, Changsha 410082, China
Received 2 June 2008; accepted 3 March 2009
Abstract: The transformation behaviors of Pb fractions during composting of Pb-polluted waste without inoculants and with the inoculants of Phanerochaete chrysosporium were studied. Results show that the active Pb ions with high toxicity and transferability are transformed into the inactive Pb with low toxicity and transferability, confirming that Pb ions can be efficiently immobilized during composting without or with the inoculants. The soluble-exchangeable Pb in composting without inoculants reaches 49.0 mg/kg at day 60, while that with the inoculants is reduced to 0 mg/kg dry mass compost. The higher contents of organic-bound Pb (59.0 mg/kg) and residual Pb (69.2 mg/kg) with low toxicity are found after 60-day composting with inoculants, compared with those without inoculants. The above data indicate the better immobilization effect of Pb and the greater alleviation of Pb hazards in composting with the inoculants of Phanerochaete chrysosporium than without inoculants, which may be due to the more microbial biomass and the higher pH value in composting of Pb-polluted waste with inoculants.
Key words: lead fractions; lead-contaminated waste; composting; transformation behavior
1 Introduction
The production of compost from agricultural wastes and municipal wastes is an important means of recovering organic matter. The organic matters can be degraded, stabilized and disinfected by composting, and the final products can be applied to land as the fertilizer or soil conditioner[1-2]. However, the presence of heavy metal in composts may cause adverse effects on environment, which limits the compost application[3]. Lead (Pb) has been recognized as one of the most hazardous heavy metals. The primary sources of Pb-contamination come from the mining and smelting activities, combustion of leaded gasoline, battery disposal and Pb-bearing products[4]. Such irregular inputs of Pb result in the increase of Pb content in solid waste, which causes the increase of Pb content in compost product. The application of the compost with active Pb poses a serious potential threat to environment, ecosystem and public health because active Pb in the soluble-exchangeable fraction can transfer from the compost into soil, groundwater and plants and even the food chain[5]. Therefore, how to reduce active Pb ions effectively in the composting of solid waste by fungi so as to alleviate the Pb hazards in the final compost product receives much concern[6-8]. Understanding the change of Pb fractions during composting is important for the improvement of composting method and the risk assessment of compost application[9-10]. However, little information is available on the transformation behavior of Pb fractions during composting of Pb-polluted waste by fungal inoculants. Composting of Pb-polluted waste by fungi with the ability of immobilizing Pb as a promising method also needs further studies. Phanerochaete chrysosporium has been used to remove Pb ions in wastewater because it is capable of accumulating metal ions in their cells by intracellular uptake, as many researchers validated, and can also chelate metal ions by the carboxyl, hydroxyl or other active functional groups on cell (including the dead cell) wall surface[11]. Meanwhile, Phanerochaete chrysosporium was proved to be adapted to the complex polluted environment after being inoculated into solid waste[12]. So it is of its own advantage to be applied to the composting of Pb-polluted waste.
In this study, Pb contents and the transformation behavior of Pb fractions during composting without inoculants and with the inoculants of Phanerochaete chrysosporium were investigated. And the probable reasons of the transformation of Pb fractions in the composting process were demonstrated by the correlation analysis of experimental data.
2 Experimental
2.1 Materials
The fungus Phanerochaete chrysosporium strain BKM-F-1767 was used. Stock cultures were maintained on malt extract agar slants at 4 ℃. Spore suspensions were prepared in sterile distilled water. The fungal concentration was measured and adjusted to 2.0×106 spores per milliliter. The uncontaminated soil, wheat straw, kitchen waste and bran were blended in the mass ratio of 28?8?13?7, in which the Pb content was about 2.9 mg/kg. The mixture was then blended with Pb(NO3)2 solution for adding Pb2+ 200 mg/kg dry mass to simulate Pb-polluted compost materials. The compost materials were placed for 14 d so that the simulated Pb-contaminated waste became relatively stabile, and no change of Pb fractions was observed. Then the carbon- to-nitrogen ratio (C/N ratio) and the organic matter content of the compost materials were measured, which were 30?1 and 60%, respectively. The water content of compost materials was adjusted to be about 70%.
2.2 Composting method
Two parallel sets of experimental apparatus were prepared and labeled as Reactor A and B, and the glass vessel of 5 L was used as reactor. Experimental apparatus used for this research mainly consisted of a composting reactor as shown in Fig.1. The above simulated wastes were evenly distributed into reactors A and B. Reactor A was with no inoculants, and Reactor B was inoculated with 2% of spore suspensions (mass fraction). The environment temperature was maintained at 30 ℃ by a temperature controller. A blower fan was used for aeration with air flow of 0.1 m3/h by flowmeter during composting. All composting lasted for 60 d.
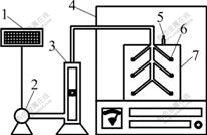
Fig.1 Schematic diagram of experimental apparatus A (B is the same as A)
2.3 Chemical and microbiological analyses
At each sampling day, triplicate samples collected from different depths of each pile were mixed thoroughly for analysis. All the analyses were considered time-zero and performed in triplicate for the mixed samples. The following parameters were measured, such as Pb content in different fractions, pH and microbial biomass carbon, etc.
Because the toxicity of heavy metal was associated with the metal bioavailability in different fractions, the freeze-dried samples were analyzed for Pb content in different fractions according to the sequential extract ion procedure of TESSIER et al[13]. Five fractions of Pb were extracted in turn as follows: the soluble-exchangeable Pb is likely to be affected by changing ionic composition in water as well as sorption-desorption processes; the carbonate-bound Pb is susceptible to change in pH; the Fe-Mn oxides-bound Pb is thermodynamically unstable under anoxic conditions; the organic-bound Pb can be degraded (oxidizing), leading to a release of soluble metals under oxidizing conditions; and the residual Pb is the most stable and the less transferable fraction among five fractions. The transferability and toxicity of the five fractions of Pb were in turn decreased. The concentration of Pb in each extracted solution was determined by an atomic absorption spectrometer (Agilent 3510, USA).
10 g compost sample was placed in a flask and 100 mL water was added in. The suspension was agitated on a mechanical vibrator at 200 r/min for 1 h. The supernatant was centrifuged at 6 000 r/min for 15 min, and then filtered to get the filtrate as the compost extract. pH was determined in the compost extract using a 716 DMS Titrino pH meter (Metrohm Ltd. CH.-9101 Herisau, Switzerland) fitted with a glass electrode[2, 14]. Microbial biomass carbon(Cmic) was analyzed by the fumigation of fresh compost sample with ethanol-free chloroform and extraction with 0.5 mol/L K2SO4, and Cmic was calculated from the organic carbon in K2SO4 extraction measured by American OI 1010 TOC instrument[2, 15].
2.4 Statistical analysis
The averages of three replicates were calculated to present experimental results. Since the microorganisms and pH in compost might be the influencing factors of Pb fractions, analyzing the relationships between Pb fractions and the two factors might benefit the understanding of the transformation behavior of Pb fractions. Linear correlation analysis was used to estimate the relationships between Pb fractions and pH and the relationships between Pb fractions and microbial biomass.
3 Results and discussion
3.1 Transformation of Pb fractions
Pb contents in five fractions in Reactors A and B changed obviously (Fig.2). The soluble-exchangeable Pb content in both Reactors A and B displayed the highest value on day 6, and then remarkably decreased with the composting time. The soluble-exchangeable Pb in Reactor A was reduced to 49.0 mg/kg till day 60, while that in B even dropped to 0 mg/kg on day 50. The carbonate-bound Pb, organic-bound Pb and residual Pb in both A and B increased obviously after day 6, whereas Fe-Mn oxides-bound Pb was observed to increase after day 18. The higher contents of organic-bound Pb (59.0 mg/kg) and residual Pb (69.2 mg/kg) with the low toxicity and transferability were found in Reactor B after 60-day composting, compared to those in Reactor A. It confirmed that the active Pb ions could be immobilized by composting, and the composting method with inoculants of Phanerochaete chrysosporium showed the better immobilization effects.
Fig.2 also shows the main existence fraction of Pb at the different composting stages. It was observed that Pb mainly existed in the soluble-exchangeable form in Reactors A and B at the beginning of composting stage. The order of abundance of the other four fractions of Pb in both reactors on day 0 was Fe-Mn oxides-bound Pb>organic-bound Pb>carbonate-bound Pb>residual Pb. However, the order of abundance of the five fractions of Pb in Reactor B on day 60 was residual Pb>organic- bound Pb>Fe-Mn oxides-bound Pb>carbonate-bound Pb>soluble-exchangeable Pb, while that in Reactor A was Fe-Mn oxides-bound Pb>soluble-exchangeable Pb>organic-bound Pb>residual Pb>carbonate-bound Pb. The results revealed that the more active form of Pb with high toxicity was transformed into other inactive forms with low toxicity in Reactor B than that in Reactor A, indicating the potential harm of Pb in compost was alleviated by composting with Phanerochaete chrysosporium.
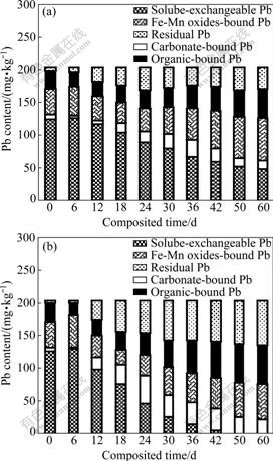
Fig.2 Pb contents in five existence fractions in composting without inoculants (a) and with inoculants of Phanerochaete chrysosporium (b)
The greater reduction of active Pb with high bioavailability and toxicity in the composting with Phanerochaete chrysosporium than that without inoculants might be attributed to the following two reasons: 1) Phanerochaete chrysosporium inoculated into Reactor B could chelate Pb ions by the carboxyl, hydroxyl or other active functional groups on cell wall surface to reduce Pb activity[11]; and 2) Phanerochaete chrysosporium could improve the degradation of organic matter, as reported previously[16], which might promote the humus formation, resulting in the enhancement of the chelation of Pb with humus.
3.2 Relationship between Pb-fractions and changes in pH
After 6 d of composting, pH values in both reactors increased significantly and later tended to be stable (Fig.3). On day 60, pH in Reactors A and B reached 6.93 and 7.45, respectively. Another mechanism that could account for the better immobilization of active Pb in Reactor B is the higher pH compared with Reactor A during the whole composting process. The ammonification of organic nitrogen and the formation and solubilization of the ammonia nitrogen might be responsible for the increasing pH observed in both reactors with composting time. The higher pH in Reactor B compared with Reactor A might be because the inoculants of Phanerochaete chrysosporium promoted the fungal transformation of organic nitrogen to ammonia nitrogen or the volatilization of organic acid [16].
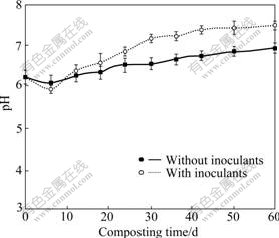
Fig.3 Changes of pH in composting without inoculants and with inoculants of Phanerochaete chrysosporium
The soluble-exchangeable Pb content decreased as the pH increased (Fig.2 and Fig.3). The pH is known to affect the ionic form and chemical mobility, so a high pH value can decrease the solubility of metals in the medium [17]. The correlation analysis indicated that the pH was significantly correlated with the soluble-exchangeable Pb and the organic-bound Pb (Fig.4). No obvious relationship was found between pH value and another three fractions of Pb. The decreasing contents of soluble-exchangeable Pb in both reactors during composting might be due to the increase of pH value. The high pH value might facilitate the cationic heavy metal retention to sample surfaces via adsorption, organic complexation, and/or precipitation[18]. The change in pH was also proved to be important to the stability of metal precipitates because it could directly affect the solubility of metal hydroxides, as well as metal carbonates and phosphates. As a result, the higher pH values were helpful to the immobilization of metal ions [19].
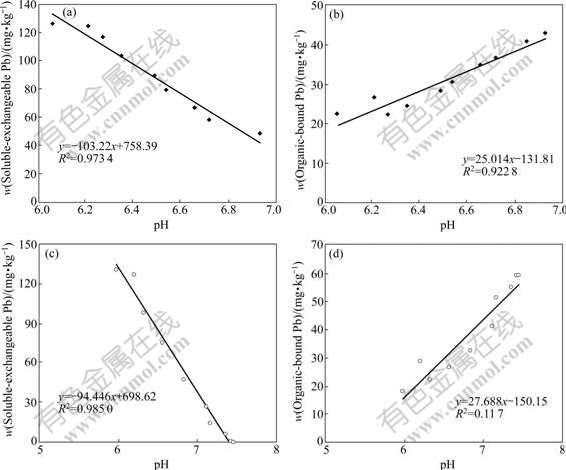
Fig.4 Relationship between content of Pb-fractions and changes in pH: (a) Soluble-exchangeable Pb and pH in compost without inoculants; (b) Organic-bound Pb and pH in compost without inoculants; (c) Soluble-exchangeable Pb and pH in compost with inoculants of Phanerochaete chrysosporium; (d) Organic-bound Pb and pH in compost with inoculants of Phanerochaete chrysosporium
3.3 Relationship between Pb-fractions and changes in microbial biomass
The changes in the content of microorganism might affect the immobilization of active Pb ions because some of microorganisms could adsorb and accumulate Pb on their cell wall and chelate Pb with the fungal metabolic products. So, Cmic was analyzed in this study. Cmic in both reactors increased rapidly after 3 d of composting (Fig.5). Cmic kept much higher in Reactor B than in Reactor A after 6-day composting, and Cmic in Reactor B reached the peak value (2 952.3 mg/kg dry compost sample) on day 27, while that in Reactor A was only 2 309.6 mg/kg. The results indicated that the inoculants of Phanerochaete chrysosporium increased the total content of microorganism, as a result, the organic matter degradation and Pb-fractions transformation might be promoted. The inoculated Phanerochaete chrysosporium could accumulate metal on cell wall and deposite metal intracellularly[11], and the toxicity of metal was reduced so as to provide a condition favorable to the growth of the indigenous microorganisms in composting. This might be responsible for the higher Cmic in Reactor B than Reactor A.

Fig.5 Microbial biomass in composting without inoculants and with inoculants of Phanerochaete chrysosporium
By analyzing microbial biomass and Pb-fractions, it was found that the increasing microorganisms during composting might accelerate the transformation and redistribution of Pb fractions (Fig.2 and Fig.5). The relationship between the Pb-fractions transformation and microbial biomass was estimated by linear correlation analysis, as shown in Fig.6. Cmic values in Reactors A and B maintained significantly negative correlations with the content of soluble-exchangeable Pb. The content of residual Pb was correlated with the microbial biomass positively in Reactor B, while in Reactor A there was no remarkable correlation between them, indicating that the inoculants of Phanerochaete chrysosporium might contribute to the transformation of other fractions of Pb to the residual Pb. However, the mechanisms of accelerating the residual Pb formation by Phanerochaete chrysosporium are not clear and need further studies. The content of soluble-exchangeable Pb significantly decreased with the increasing microbial biomass, which might be mainly ascribed to the immobilization of Pb ions by fungal biosorption and complexation[7, 20]. The sorption of metal to polysaccharides, proteins, or other molecules on the cell wall surface of microorganism probably plays the most important role. In addition, fungi could evolve active defense mechanism to alleviate the toxicity of metals, which is usually based on the immobilization of heavy metals using extracellular chelating compounds[11].
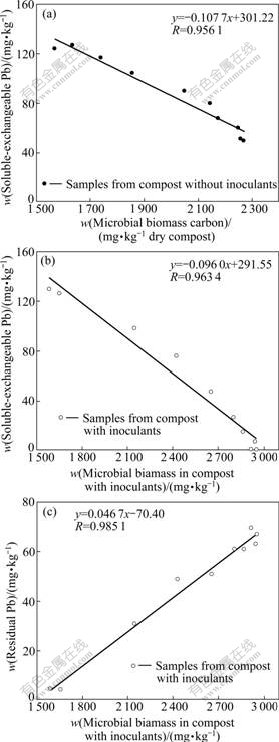
Fig.6 Relationship between content of Pb-fractions and changes in microbial biomass: (a) Soluble-exchangeable Pb and microbial biomass in compost without inoculants; (b) Soluble- exchangeable Pb and microbial biomass in compost with inoculants of Phanerochaete chrysosporium; (c) Residual Pb and microbial biomass in compost with inoculants of Phanerochaete chrysosporium
4 Conclusions
1) It was proved that the composting methods without inoculants and with inoculants of Phanerochaete chrysosporium could effectively transform Pb fractions, reduce active Pb and alleviate the potential harm of Pb-contaminated waste. The transformation behavior of Pb fractions might result from the fact that the Pb ions could be accumulated by fungal mycelium and chelated by the humus formed in the composting.
2) The content of soluble-exchangeable Pb was positively correlated with pH value and microbial biomass, indicating that increasing pH and microbial biomass were important to the immobilization of Pb during composting.
3) The better immobilization effect of active Pb was found in the composting method with inoculants of Phanerochaete chrysosporium compared with that without inoculants, which might be due to the more microbial biomass and higher pH value in composting of Pb-polluted waste with inoculants. It was also observed that the inoculants might be responsible for the increase of the content of residual Pb content during composting.
References
[1] HU Tian-jue, ZENG Guang-ming, HUANG Dan-lian, YU Hong-yan, JIANG Xiao-yun, DAI Fang, HUANG Guo-he. Use of potassium dihydrogen phosphate and sawdust as adsorbents of ammoniacal nitrogen in aerobic composting process [J]. Journal of Hazardous Materials, 2007, 141(3): 736-744.
[2] JIANG Xiao-yun, ZENG Guang-ming, HUANG Dan-lian, CHEN yang, LIU Fang, HUANG Guo-he, LI Jian-bing. Remediation of pentachlorophenol-contaminated soil by composting with immobilized Phanerochaete chrysosporium [J]. World Journal of Microbiology and Biotechnology, 2006, 22(9): 909-913.
[3] HSEU Z Y. Evaluating heavy metal contents in nine composts using four digestion methods [J]. Bioresource Technology, 2004, 95(1): 53-59.
[4] TSEZOS M. Biosorption of metals: The experience accumulated and the outlook for technology development [J]. Hydrometallurgy, 2001, 59(2/3): 241-243.
[5] WARMAN P R, TERMEER W C. Evaluation of sewage sludge, septic waste and sludge compost applications to corn and forage: Ca, Mg, S, Fe, Mn, Cu, Zn and B content of crops and soils [J]. Bioresource Technology, 2005, 96(9): 1029-1038.
[6] WONG J W C, FANG M. Effects of lime addition on sewage sludge composting process [J]. Water Research, 2000, 34(15): 3691-3698.
[7] ZENG Guang-ming, HUANG Dan-lian, HUANG Guo-he, HU Tian-jue, JIANG Xiao-yun, FENG Chong-ling, CHEN Yao-ning, TANG Lin, LIU Hong-liang. Composting of lead-contaminated waste with inocula of white-rot fungus [J]. Bioresource Technology, 2007, 98(2): 320-326.
[8] HUANG Dan-lian, ZENG Guang-ming, JIANG Xiao-yun, FENG Chong-ling, YU Hong-yan, HUANG Guo-he, LIU Hong-liang. Bioremediation of Pb-contaminated soil by incubating with Phanerochaete chrysosporium and straw [J]. Journal of Hazardous Materials, 2006, 134(1/3): 268-276.
[9] HUANG Dan-lian, ZENG Guang-ming, FENG Chong-ling, HU Shuang, JIANG Xiao-yun, TANG Lin, SU Feng-feng, ZHANG Yu, ZENG Wei, LIU Hong-liang. Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity [J]. Environmental Science and Technology, 2008, 42(13): 4946-4951.
[10] ZORPAS A A, ARAPOGLOU D, PANAGIOTIS K. Waste paper and clinoptilolite as a bulking material with dewatered anaerobically stabilized primary sewage sludge (DASPSS) for compost production [J]. Waste Manage, 2003, 23(1): 27-35.
[11] BALDRIAN P. Interactions of heavy metals with white-rot fungi [J]. Enzyme and Microbial Technology, 2003, 32(1): 78-91.
[12] PALMANS P, MARES G, POPPE J, HOFTE M. Biodegradation of xenobiotics by heavy metal resistant higher fungi [J]. Med Fac Landbouww Univ Gent, 1995, 60(2): 2563-2566.
[13] TESSIER A, CAMPBELL P G C, BISSON M. Sequential extraction procedure for the speciation of particulate trace metals [J]. Analytical Chemistry, 1979, 51(7): 844-851.
[14] ZENG Guang-ming, YU Hong-yan, HUANG Hong-li, HUANG Dan-lian, CHEN Yao-ning, HUANG Guo-he, LI Jian-bing. Laccase activities of a soil fungus Penicillium simplicissimum in relation to lignin degradation [J]. World Journal of Microbiology and Biotechnology, 2006, 22(4): 317-324.
[15] ANDERSON T H, JOERGENSEN R G. Relationship between SIR and FE estimates of microbial biomass C in deciduous forest soils at different pH [J]. Soil Biology and Biochemistry, 1997, 29(6): 1033-1042.
[16] HUANG Dan-lian, ZENG Guang-ming, HU Tian-jue, HUANG Guo-he. Preliminary study on the application of Phanerochaete chrysosporium in composting of lignin waste [C]// Proceedings of EnerEnv’2003 Conference. Beijing and New York: Science Press, 2003: 907-912.
[17] XING F X, GHOLAMHOSSIN S. Effect of pH on chemical forms and plant availability of cadmium, zinc and lead in polluted soils [J]. Water Air and Soil Pollution, 1989, 45(3): 265-273.
[18] STEWART M A, JARDINE P M, BARNETT M O, MEHLHOM T L, HYDER L K, MCKAY L D. Influence of soil geochemical and physical properties on the sorption and bioaccessibility of chromium (III) [J]. Journal of Environmental Quality, 2003, 32(1): 129-137.
[19] APPEL C, MA L. Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils [J]. Journal of Environmental Quality, 2002, 31(2): 581-589.
[20] AYHAN D. Heavy metal bioaccumulation by mushrooms from artificially fortified soils [J]. Food Chemistry, 2001, 74(8): 293-301.
Foundation item: Project(50808073) supported by the National Natural Science Foundation of China; Project(2005CB724203) supported by the National Basic Research Program of China; Project(2007) supported by the Program for Changjiang Scholars and Innovative Research Team in University, China; Project(2007185) supported by the Environmental Protection Technology Research Program of Hunan Province, China
Corresponding author: ZENG Guang-ming; Tel: +86-731-88822754; E-mail: zgming@hnu.cn
DOI: 10.1016/S1003-6326(08)60453-7
(Edited by YANG Hua)