J. Cent. South Univ. Technol. (2011) 18: 406-410
DOI: 10.1007/s11771-011-0711-9
Synthesis and electrochemical performance of TiO2-B as anode material
WANG Xin-yu(王新宇), XIE Ke-yu(谢科予), LI Jie(李劼), LAI Yan-qing(赖延清),
ZHANG Zhi-an(张治安), LIU Ye-xiang(刘业翔)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: TiO2-B was synthesized by solid-state reaction. The structures, surface morphologies and electrochemical performances of TiO2-B were characterized by X-ray diffractometry (XRD), scanning electron microscopy (SEM) and electrochemical measurement, respectively. The effects of calcining temperature, molar ratio of K2O to TiO2 and calcining time on the characteristics of TiO2-B were investigated. The results show that the calcining time exerts a significant influence on the electrochemical performances of TiO2-B. The TiO2-B is obtained with good crystal structure and suitable size by using K2Ti4O9, which is prepared at 950 °C for 24 h under the condition of x(K2O)/x(TiO2)=1:3.5. The TiO2-B delivers all initial discharge capacity of 231.6 mA?h/g. And the rate capacity is 73.2 mA?h/g at 1 675 mA/g, which suggests that TiO2-B is a promising anode material for the lithium ion batteries.
Key words: lithium ion battery; TiO2-B; solid state method; anode material
1 Introduction
Li-ion batteries have been widely applied as power sources for portable electronic devices, especially laptop computers, wireless telephones and cameras, due to their advantages such as high energy density, low self- discharge and no pollution. Recently, Li-ion batteries are considered as the most promising energy storage technology for hybrid, plug-in hybrid, and electric vehicle applications. For the emerging large-scale applications, however, fundamental improvements are needed with regard to safety, cycle life, cost and so on. At present, lithium secondary batteries are composed of a graphite/carbon-related anode, which has some defects for large-scale applications [1-3]: 1) crucial safety concerns exist in connection with carbonaceous anode, which is attributed to its low operating potential; 2) a large irreversible capacity loss related to solid electrolyte interface (SEI) film formation during the initial cycle; 3) the change of volume is about 10% when lithium ion is inserted and extracted, which decreases the cycle stability. There has been much interest in Li4Ti5O12 as an alternative anode to graphite, due to its special characteristics, such as a stable insertion potential at 1.55 V vs Li/Li+, which avoids the reduction reaction of the electrolyte and a very small volume change during the charge/discharge processes, which enables a long and stable cycle life [4-6]. Unfortunately, the charge and discharge capacities of Li4Ti5O12 are limited by theoretical ones (175 mA?h/g and 607 mA?h/cm3), which are unfavorably lower than those of commercial graphite (372 mA?h/g and 855 mA?h/cm3) [7].
Combined with low cost, low toxicity and good chemical and thermal stability, TiO2 has the same advantages as Li4Ti5O12: higher potential (>1.5 V vs Li/Li+) and small volume change during charge/discharge processes (about 3%) [8]. Moreover, it has the capacities of intercalate lithium (335 mA?h/g and 1 246 mA?h/cm3) [7] almost twice those of Li4Ti5O12. Hence, TiO2 is a promising candidate for a highly potential negative electrode in the Li-ion batteries. Among the various polymorphs of TiO2, the structure of TiO2-B (bronze) is more open than other polymorphs, in which inserted Li+ may undergo facile transport. ARMSTRONG et al [9-10] have synthesized TiO2-B nanowire and nanotube via hydrothermal reaction. The specific capacities of TiO2-B nanowires and nanotubes are 305 mA?h/g and 338 mA?h/g, respectively. These nanomaterials also have excellent cycleablity and rate-ability. Moreover, TiO2-B nanoribbons [11] and TiO2-B/C nanoribbons [12] have also been synthesized by hydrothermal reaction. However, hydrothermal synthesis is not suitable for industrial production, because of the high production cost. More recently, a precursor, K2Ti4O9, was prepared by a solid-state reaction, and TiO2-B powder was obtained by ion exchange followed by calcining, aiming at reducing the cost of the material. However, the electro-chemical characteristics of TiO2-B were only focused in different electrolytes [7], and the effects of synthesis conditions on the morphologies and electrochemical performances of TiO2-B were not mentioned.
In this work, a precursor, K2Ti4O9, was prepared by a solid-state reaction, and TiO2-B powder was obtained by ion exchange followed by calcining. The effects of calcining temperature, molar ratio of K2O to TiO2 and calcining time on the characteristics of the precursor K2Ti4O9 and on the electrochemical characteristics of TiO2-B were systematically investigated.
2 Experimental
2.1 Preparation and characterization of TiO2-B
First, the precursor, K2Ti4O9, was obtained by heating K2CO3 (AR) and TiO2 (anatase, AR) in different molar ratios at 950 °C for 6, 12, 24, 36 and 48 h, respectively. Second, the resulting solid is ground in agate mortar and then hydrolyzed for 72 h in 1 mol/L HCl. After filtering, the H2Ti4O9 powder was heated at 500 °C in air for 2 h.
The X-ray diffraction (XRD) patterns were recorded on a Rigaku D-MAX2500VB with Cu Kα radiation. The morphology of the TiO2-B was characterized by scanning electron microscopy (SEM, JSM-5600LV).
2.2 Electrochemical measurements
The charge–discharge performance and the cycling stability of the TiO2-B electrode were tested with an electrochemical equipment (Wuhan Land Electro- chemical Equipment Company, China). The 2025 coin- type cell consisted of a working electrode (mass ratio of active material to carbon black and to PVDF of 8:1:1), a metal lithium foil counter electrode, and a Celgard 2400 separator. The electrolyte was 1 mol/L LiPF6 in the EC/DMC/DEC (1:1:1 in volume fraction) (Ferro Co.). The cells were assembled in a dry glove box. The charge–discharge cycling test was carried out galvanostatically at different current densities. The cutoff voltages for the charge and discharge processes were 3 and 1 V, respectively. Experiments were carried out at room temperature. Cyclic voltammetry (CV) for TiO2-B/Li half cells was measured using 2025 coin-type cells. The tests were conducted using PARSTAT 2273 electrochemical measurement system (PerkinElmer instrument, USA). CV test was performed with a scan rate of 0.1 mV/s between 1.20 and 2.60 V.
3 Results and discussion
3.1 Effect of different TiO2-to-K2O molar ratios on precursor K2Ti4O9
Fig.1 shows the phase diagram of K2O/TiO2 [13]. From the phase diagram, the precursor K2Ti4O9 whiskers will appear at temperatures above 926 °C, when the molar ratios of K2O to TiO2 is 65%-80% [14]. Moreover, at 710 °C, the K2CO3 will decompose into hydrosoluble non-crystal K2O, which will volatilize at even higher temperatures. And this would lead to the mismatched molar ratio of K2O to TiO2. Therefore, the precursor K2Ti4O9 whiskers were prepared through the solid-state reaction at 950 °C for 24 h with different molar ratios of K2O to TiO2 in the air.
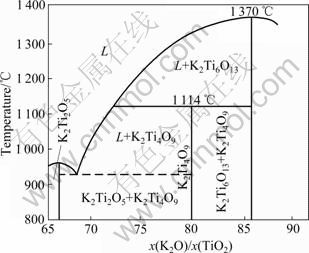
Fig.1 Phase diagram of K2O/TiO2
The X-ray diffraction patterns of the precursor K2Ti4O9 prepared with different molar ratios of K2O to TiO2 in the air are shown in Fig.2. It can be found that single-phase K2Ti4O9 is synthesized with the molar ratios of K2O to TiO2 being 1:3 and 1:3.5, respectively. In addition, the peaks of these samples are all narrow and strong, indicating the high crystallinity of the precursor K2Ti4O9. However, when the molar ratio of K2O to TiO2 is 1:4, the single-phase K2Ti4O9 structure transforms to a mixed phase structure of K2Ti4O9 and K2Ti6O13 (2θ= 11.46°, 13.76° and 28.02°), as shown in Fig.2(c). The reason might be the volatilization of K2O at high temperature, which makes the molar ratio of K2O to TiO2 exceed 1:4. And the mixed phase of K2Ti4O9 and K2Ti6O13 in the product heated at 950 °C can also be explained by the phase diagram of K2O and TiO2.
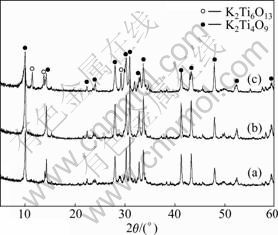
Fig.2 XRD patterns of samples prepared under different molar ratios of K2O to TiO2: (a) 1:3; (b) 1:3.5; (c) 1:4
3.2 Effect of calcining time on precursor K2Ti4O9
The shape of TiO2-B is determined in the first calcination process by solid-state reaction [7]. So, the effects of calcining time on the structures and the surface morphologies of TiO2-B are further investigated. The XRD patterns of TiO2-B prepared for different times from K2Ti4O9 are shown in Fig.3. The diffraction peaks of all the samples could be indexed to TiO2-B (JCPDS 46-1237), which belongs to space group C2/m with monoclinic unit cell. Furthermore, the integrated intensity ratios of the (110) to (002) peaks (I(110)/I(002)) for samples prepared at 950 °C for 6, 12, 24, 36 and 48 h, are listed in Table 1. With increasing the heat-treatment time, the value of I(110)/I(002) increases. This means that the layer structure of TiO2-B is well developed with increasing the heat-treatment time.
Fig.4 shows the SEM images of TiO2-B prepared for different times from K2Ti4O9. All the powders have needle-like structure with different diameters and lengths. When the calcining time is 6 h, the diameter and length of TiO2-B are about 300 nm and about 2.5 μm, respectively. It is found that the diameter and length of TiO2-B increase rapidly with increasing the calcining time. When the calcining time is 48 h, the diameter of TiO2-B is about 1 μm and the length of TiO2-B is up to 10 μm.
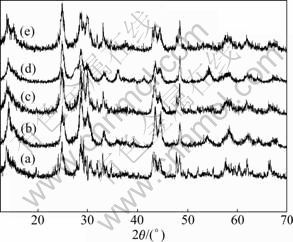
Fig.3 XRD patterns of TiO2-B prepared for different times from K2Ti4O9: (a) 6 h; (b) 12 h; (c) 24 h; (d) 36 h; (e) 48 h
Table 1 Ratio of I(110) to I(002) of TiO2-B prepared for different times from K2Ti4O9


Fig.4 SEM images of TiO2-B prepared for different times from K2Ti4O9: (a) 6 h; (b) 12 h; (c) 24 h; (d) 36 h; (e) 48 h
3.3 Electrochemical characteristics of TiO2-B
Cyclic voltammetry is an effective analytical method for understanding the mechanism of Li+ inserting and extracting of TiO2-B. Fig.5 shows the cyclic voltammogram of TiO2-B (24 h). A pair of redox peaks are observed on the CV curve at 1.42 V and 1.70 V vs Li/Li+, for cathodic and anodic scans, respectively, which demonstrates the typical behavior of Li insertion/ extraction processes in TiO2-B electrode. The redox peaks of TiO2-B (1.70 V/1.42 V) are lower than those of other polymorphs, with 1.95 V/1.75 V [15], 1.90 V/ 1.75 V [16] and 2.02 V/1.7 V [17] for anatase, rutile and brookite, respectively. This means that the cells, incorporating a TiO2-B anode, would have higher voltage than that based on other polymorphs of TiO2.
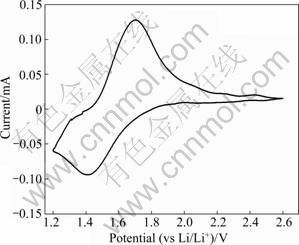
Fig.5 Cyclic voltammogram of TiO2-B (24 h)
The first three charge-discharge curves of these TiO2-B/Li cells, between 1 and 3 V at a constant current of 33.5 mA/g, are shown in Fig.6. The first charge and discharge capacity are 194 mA?h/g and 231.6 mA?h/g, respectively, with the coulombic efficiency of 83.8%. In the following two cycles, the discharge capacities are 194.6 mA?h/g and 192.2 mA?h/g, respectively, exhibiting high coulombic efficiency above 94%. The reason for the irreversible capacity is still unclear [18]. The existence of an exceptionally high irreversible capacity could be attributed to the surface reactions, such as solvent decomposition. Another possible reason for the large irreversible capacity in the first cycle might be the surface defect of TiO2-B, such as oxide vacancy, which can trap Li+. Further work is necessary to explore the origins of the low coulombic efficiency of the first cycle. Moreover, there is no obviously flat potential upon charging or discharging, which reveals that TiO2-B has fast transport channels for Li+ insertion and extraction, without two-phase reactions in electrode materials during the lithium inserting and extracting ion process [19].
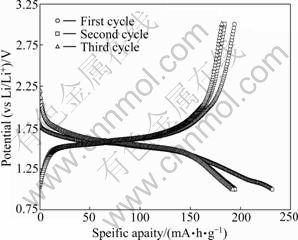
Fig.6 First three charge-discharge curves of TiO2-B (24 h)
Fig.7 shows the cycle performance of TiO2-B electrode, obtained at various calcining times, as a function of cycle number at different current densities. All the samples have good cycleability. For the TiO2-B obtained at calcining time of 24 h, the capacity retention is about 82.3% for the second discharge capacity after 50 cycles. Combined with XRD and SEM, it is shown that both the crystallinity and morphology exert a significant influence on the rate performances of TiO2-B. The sample calcined for 6 h, with the least particle size, has low discharge capacity, due to the low crystallinity. While the sample calcined for 48 h, with the highest crystallinity, also has low discharge capacity, due to the large particle size. Therefore, the sample calcined for 24 h, with the moderate crystallinity and particle size, exhibits the highest discharge capacity and rate performances. The first diacharge capacity is 231.6 mA?h/g, and the rate capacity is 73.2 mA?h/g at 1 675 mA/g.
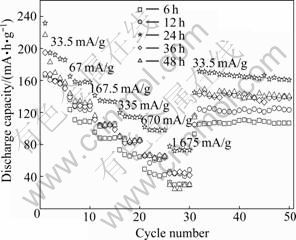
Fig.7 Cycle performance of TiO2-B at different current densities
4 Conclusions
1) Pure TiO2-B can be synthesized by ion exchange followed by calcining precursor K2Ti4O9, which is prepared by a solid-state reaction with controlled calcining temperature, molar ratio of K2O to TiO2 and calcining time. The calcining time exerts a significant influence on the electrochemical performances of TiO2-B. With increasing the calcining time, the crystallinity is developed, which is helpful to improving the discharge capacity. However, the particle size is also increased, which is harmful to the rate performance.
2) TiO2-B, obtained from the precursor K2Ti4O9 prepared at 950 °C for 24 h, has the moderate crystallinity and particle size, and exhibits the highest discharge capacity and rate performance. The first diacharge capacity is 231.6 mA?h/g, and the rate capacity is 73.2 mA?h/g at 1 675 mA/g.
References
[1] AURBACH D, ZINIGRAD E, COHEN Y, TELLER H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions [J]. Solid State Ionics, 2002, 148(3/4): 405-416.
[2] DELL R M. Batteries fifty years of materials development [J]. Solid State Ionics, 2000, 134(1): 139-158.
[3] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries [J]. Nature, 2001, 414: 359-367.
[4] WANG Da, XU Hua-yun, GU Man, CHEN Chun-hua. Li2CuTi3O8- Li4Ti5O12 double spinel anode material with improved rate performance for Li-ion batteries [J]. Electrochemistry Communications, 2009, 11(1): 50-53.
[5] JU S H, KANG Y C. Effects of preparation conditions on the electrochemical and morphological characteristics of Li4Ti5O12 powders prepared by spray pyrolysis [J]. Journal of Power Sources, 2009, 189(1): 185-190.
[6] SHU Jie. Li-Ti-O compounds and carbon-coated Li-Ti-O compounds as anode materials for lithium ion batteries [J]. Electrochimica Acta, 2009, 54(10): 2869-2876.
[7] INABA M, OBA Y, NIINA, F, MUROTA Y, OGINO Y, TASAKA A, HIROTA K. TiO2(B) as a promising high potential negative electrode for large-size lithium-ion batteries [J]. Journal of Power Sources, 2009, 189(1): 580-584.
[8] WAGEMAKER M, KEARLEY G J, WELL A A, MUTKA H, MULDER F M. Multiple Li positions inside oxygen octahedral in lithiated TiO2 anatase [J]. Journal of the American Chemical Society, 2003, 125(3): 840-848.
[9] ARMSTRONG A R, ARMSTRONG G, CANALES J, GARCIA R, BRUCE P. Lithium-ion intercalation into TiO2-B nanowires [J]. Advanced Materials, 2005, 17(7): 862-865.
[10] ARMSTRONG A R, ARMSTRONG G, CANALES J, BRUCE P. TiO2(B) nanotubes as negative electrodes for rechargeable lithium batteries [J]. Electrochemical and Solid-State Letters, 2006, 9(3): A139-A143.
[11] LI Quan-jun, ZHANG Jing-wei, LIU Bing-bing, LI Ming, Liu Ran, LI Xiang-lin, MA Hong-lei, YU Shi-dan, WANG Lin, ZOU Yong-gang, Li Ze-peng, ZOU Bo, CUI Tian, ZOU Guang-tian. Synthesis of high-density nanocavities inside TiO2-B nanoribbons and their enhanced electrochemical lithium storage properties [J]. Inorganic Chemistry, 2008, 47(21): 9870-9873.
[12] LI Quan-jun, ZHANG Jing-wei, LIU Bing-bing, LI Ming, YU Shi-dan, WANG Lin, Li Ze-peng, LIU De-di, HOU Yuan-yuan, ZOU Yong-gang, ZOU Bo, CUI Tian, ZOU Guang-tian. Synthesis and electrochemical properties of TiO2-B@C core-shell nanoribbons [J]. Crystal Growth & Design, 2008, 8(6): 1812-1814.
[13] CHEN Jin-min, WANG Qi-lin, HUANG Zhi-liang, XU Tao, GAO Shu-min. The research of K2Ti4O9 whisker prepared by a sintering method [J]. Journal of Wuhan Institute of Technology, 2007, 29(2): 54-56.
[14] BAO Ning-zhong, FENG Xin, LU Xiao-hua, YANG Zhu-hong. Study on the formation and growth of potassium titanate whiskers [J]. Journal of Materials Science, 2002, 37(14): 3035-3043.
[15] PROCHAZKA J, KAVAN L, ZUKALOVA M, FRANK O, KALBAC M ,ZUKAL A, KLEMENTOVA M, CARBONE D, GRAETZEL M. Novel synthesis of the TiO2(B) multilayer templated films [J]. Chemistry of Materials, 2009, 21(8): 1457-1464.
[16] WANG Dong-hai, CHOI D W, YANG Zhen-guo, VISWANATHAN V V, NIE Z, WANG Chong-min, SONG Yu-jiang, ZHANG Ji-guang, LIU Jun. Synthesis and li-ion insertion properties of highly crystalline mesoporous rutile TiO2 [J]. Chemistry of Materials, 2008, 20(10): 3435-3442.
[17] LEE D H, PARK J G, CHOI K J, CHOI H J, KIM D W. Preparation of brookite-type TiO2/carbon nanocomposite electrodes for application to Li ion batteries [J]. European Journal of Inorganic Chemistry, 2008, 6: 878-882.
[18] TSAI M C, CHANG J C, SHEU H S, CHIU H T, LEE C Y. Lithium ion intercalation performance of porous laminal titanium dioxides synthesized by sol-gel process [J]. Chemistry of Materials, 2009, 21(3): 499-505.
[19] AN Li-ping, LI Guo-ran, HU Tao, GAO Xue-ping, SHEN Pan-wen. Electrochemical lithium storage of TiO2-B nanotubes before and after supporting of transition metal oxides [J]. Chinese Journal of Inorganic Chemistry, 2008, 24(6): 931-936. (in Chinese)
(Edited by YANG Bing)
Foundation item: Project(2007BAE12B01) supported by the National Key Technology R&D Program of China
Received date: 2010-04-09; Accepted date: 2010-10-14
Corresponding author: ZHANG Zhi-an, PhD; Tel: +86-731-88830649; E-mail: zza75@163.com