DOI: 10.11817/j.issn.1672-7207.2015.01.006
氢氧化钠分解氟碳铈矿的热力学分析
张文娟,李江涛,王小波
(中南大学 冶金与环境学院,湖南 长沙,410083)
摘要:结合已有热力学数据,对Ce-F-C-H2O体系、La-F-C-H2O体系和Nd-F-C-H2O体系进行热力学平衡计算,绘制25 ℃下各体系可溶解组分的lg c-pH图。利用热力学平衡图,对氟碳铈矿分解过程进行分析。研究结果表明:在较低的pH下,氟碳铈矿可被酸分解,生成难溶于酸的ReF3和可溶的氯化稀土,故酸分解过程中,氟碳铈矿只能被部分分解;在一定碱度条件下,氟碳铈矿可被碱转化为含氟量很低,易被酸处理的混合氢氧化稀土,并与溶液中的氟化钠及碳酸钠分离。
关键词:氟碳铈矿;氢氧化钠;热力学
中图分类号:TF845.6 文献标志码:A 文章编号:1672-7207(2015)01-0034-07
Thermodynamics analysis on sodium hydroxide decomposition of bastnaesite
ZHANG Wenjuan, LI Jiangtao, WANG Xiaobo
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: The thermodynamic equilibrium of Ce-F-C-H2O system, La-F-C-H2O system and Nd-F-C-H2O system was calculated according to the known thermodynamic data. The lg c-pH diagrams for three systems were established at 25 ℃. Using these diagrams,thermodynamic analysis was carried out to show the decomposition of bastnaesite. The results show that part of bastnaesite can be decomposed by acid at low pH to get insoluble ReF3 and soluble rare earth chloride. Bastnaesite can be converted into hydroxide with little fluorine under the condition of certain alkalinity and separated from sodium fluoride and sodium carbonate in solution.
Key words: bastnaesite; sodium hydroxide; thermodynamic
稀土元素以其特殊性能广泛应用于航空航天、军工、特种钢材、石油化工、功能材料等。随着现代科学技术的发展,稀土已成为21世纪重大战略元素之一。我国稀土资源储量位居世界第一,占世界总储量的55%,其中氟碳铈矿作为储量最大的稀土矿物,占稀土总量的50%以上[1-2]。氟碳铈矿属于碳酸盐类矿物,主要以轻稀土元素铈、镧和钕为主,铈占稀土元素的50%左右[3]。目前,氟碳铈矿主要的处理工艺有氧化焙烧-盐酸浸出、硫酸焙烧法、盐酸-氢氧化钠二步分解法及烧碱分解法(即氢氧化钠-盐酸优溶法)[2-5]。采用焙烧分解氟碳铈矿的过程中,会产生氟化氢气体,且由于物料形态转化次数多使得工艺流程冗长,化工材料消耗大[5]。盐酸-氢氧化钠二步分解法工艺过程简单,但浓盐酸分解时,温度高,时间长,酸雾大。烧碱分解过程不会产生氟化氢气体,其酸溶过程的优溶率达到了传统盐酸-氢氧化钠分解方式的2.5倍[6]。烧碱法分解氟碳铈矿基于如下反应:
ReFCO3+3NaOH = Re(OH)3+NaF+Na2CO3
氟碳铈矿在碱作用下,分解为难溶的氢氧化稀土,伴生金属也生成相应的氢氧化物,与易溶的氟化钠及碳酸钠分离[7]。该法到目前为止,关于其理论方面的研究报道很少,本文作者利用已有的热力学数据绘制25 ℃时Ce-F-C-H2O体系、La-F-C-H2O体系和Nd-F-C-H2O体系的lg c-pH图,并对浸出过程热力学进行分析。
1 热力学数据估算
氟碳铈矿的化学分子式为ReFCO3或Re2(CO3)3·ReF3,是稀土碳酸盐和稀土氟化物的复合化合物[8]。由于缺乏ReFCO3的热力学数据,本文通过其在水中的溶解度对溶度积常数Ksp进行估算,各氟碳酸盐在水中的溶解度数据如表1所示。
对于ReFCO3矿物,Ksp与溶解度存在下列关系:
 (1)
(1)
式中:Sm为ReFCO3矿物的溶解度; 为水溶液中总的稀土浓度与Re3+浓度的比值;
为水溶液中总的稀土浓度与Re3+浓度的比值; 为总氟浓度与F-浓度的比值;
为总氟浓度与F-浓度的比值;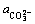 为总碳浓度与
为总碳浓度与 浓度的比值。ReFCO3在水溶液中溶解平衡时,存在
浓度的比值。ReFCO3在水溶液中溶解平衡时,存在

 (2)
(2)
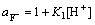 (3)
(3)
 (4)
(4)
其中:“[ ]”为浓度。由于碳酸盐矿物的溶解度受大气中CO2分压(p(CO2))的影响,大气中p(CO2)=1.01× 101.5 Pa,根据CO2在水中的溶解平衡
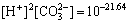 (5)
(5)
由于ReFCO3溶解产生的 很少,因此,
很少,因此, 的初始浓度可看做是
的初始浓度可看做是 ,代入式(5)可得到H+的浓度,结合表1中给出的ReFCO3的溶解度数据及式(1)~(4)可得出1个近似的
,代入式(5)可得到H+的浓度,结合表1中给出的ReFCO3的溶解度数据及式(1)~(4)可得出1个近似的 ,此时游离的
,此时游离的 较准确的浓度为
较准确的浓度为
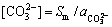 (6)
(6)
代入式(5),可得更精确的H+浓度。按上述方式反复运用逐步逼近法,可得到CeCO3F,LaCO3F和NdCO3F的pKsp分别为17.53,17.97和17.29。
表1 氟碳酸盐在水中的溶解度
Table 1 Solubility of rare earth fluorocarbonate in water

2 溶解组分lg c-pH图的绘制
在缺少NdF3溶度积数据的情况下,查阅文献[9]可知25 ℃时NdF3与CeF3在水中的溶解度分别为4.0×10-6 mol/L和8.2×10-6 mol/L,由于NdF3与CeF3物理化学性质相近,故本文中NdF3溶度积可认为近似等于CeF3的溶度积。溶液中存在的平衡反应式见表2~4。在缺少有关离子活度的情况下,文中计算均以浓度代替活度[10]。由所有平衡关系式可得出,溶液中各游离离子均满足表2~4中所列方程。
根据质量守恒和同时平衡原理可知:溶液中总的稀土、总碳和总氟浓度分别为
[Re]=[Re3+]+[ReOH2+]+ +[Re(OH)3]+
+[Re(OH)3]+ 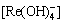 +[ReF2+]+
+[ReF2+]+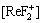 +[ReF3]+
+[ReF3]+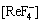 +
+ 
[C]= +
+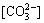 +[
+[ ]+ [H2CO3]
]+ [H2CO3]
[F]=[ReF2+]+ +[ReF3]+
+[ReF3]+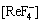 +[F-]+[HF]
+[F-]+[HF]
氟碳铈矿的分解过程中恒有ReFCO3的溶解平衡,故Re3+, 和F-的浓度恒满足如下关系式:
和F-的浓度恒满足如下关系式:

从所有的平衡关系式可以看出:在不同pH范围为内,稳定存在的固体不同。
在pH较低的时候,发生如下反应:
3ReFCO3+6H+ = ReF3+2Re3++CO2+H2O
由于CO2的产生对工业生产无实际指导意义,故此条件下不予以讨论。此时体系中固体只有ReF3,在该pH范围内均满足:
表2 Ce-F-CO3-H2O系平衡反应及平衡常数(25 ℃)
Table 2 Equilibrium reactions and constants for Ce-F-CO3-H2O system (25 ℃)

表3 La-F-CO3-H2O系平衡反应及平衡常数(25 ℃)
Table 3 Equilibrium reactions and constants for Ce-F-CO3-H2O system (25 ℃)
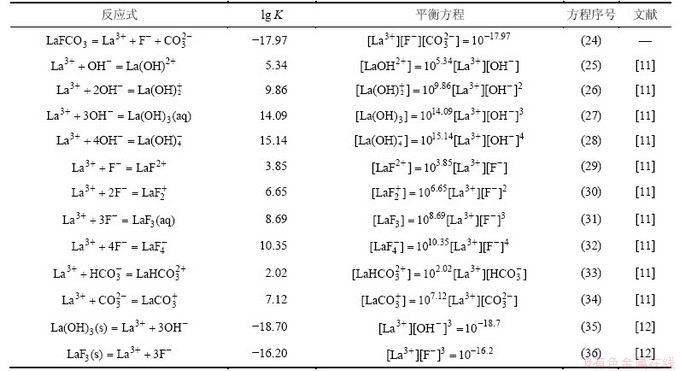
表4 Nd-F-CO3-H2O系平衡反应及平衡常数(25 ℃)
Table 4 Equilibrium reactions and constants for Ce-F-CO3-H2O system (25 ℃)

 2[C]+[F]=3[Re] (50)
2[C]+[F]=3[Re] (50)
根据平衡关系式,当体系中只有ReF3时,Ce-F-C-H2O体系中,溶液中各游离组分满足方程(7)~(17),(19)~(22)和(50);La-F-C-H2O体系中,溶液中各游离组分满足方程(24)~(34),(20)~(22),(36)和(50);Nd-F-C-H2O体系中,溶液中各游离组分满足方程(37)~(47),(20)~(22)和(49)~(50)。
在pH较高的时候,发生如下反应:
ReFCO3+3NaOH = Re(OH)3+NaF+Na2CO3
此时体系中固体只有Re(OH)3,在该pH范围内均满足:
 [C] = [F] (51)
[C] = [F] (51)
当体系中只有Re(OH)3时,Ce-F-C-H2O体系中,溶液中各游离组分满足方程(7)~(18),(20)~(22)和(51);La-F-C-H2O体系中,溶液中各游离组分满足方程(24)~(35),(20)~(22)和(51);Nd-F-C-H2O体系中,溶液中各游离组分满足方程(37)~(49),(20)~(22)和(51)。
当体系中固体只有ReFCO3时,可得
[C] = [F] = [Re] (52)
此时,Ce-F-C-H2O体系中,溶液中各游离组分满足方程(7)~(17),(20)~(22)和(52);La-F-C-H2O体系中,溶液中各游离组分满足方程(24)~(34),(20)~(22)和(52);Nd-F-C-H2O体系中,溶液中各游离组分满足方程(37)~(47),(20)~(22)和(52)。
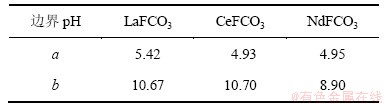
根据以上计算和平衡关系,可求得生成ReF3和Re(OH)3的边界pH分别为a和b,如表5所示;并绘制出25 ℃ 时Ce-F-C-H2O体系、La-F-C-H2O体系和Nd-F-C-H2O体系溶液组分的lg c-pH图,见图1~3。
时Ce-F-C-H2O体系、La-F-C-H2O体系和Nd-F-C-H2O体系溶液组分的lg c-pH图,见图1~3。
表5 ReFCO3分解的边界pH
Table 5 Boundary pH of ReFCO3 decomposition
3 结果讨论
由于铈组元素性质相近,各体系中离子的存在形式相同,故3个体系的lg c-pH图只存在细微差别。由图1~3可以看出:3个体系中各离子浓度变化趋势一致,在a<pH<b范围内,ReFCO3稳定存在,pH低于或高于这个范围,氟碳铈矿开始分解。
在pH<a时, 会结合H+生成H2CO3,进而分解产生CO2,部分Re3+结合F-生成稳定的ReF3沉淀,该过程实际上是氟碳铈矿的酸分解。在酸分解过程中,氟碳铈矿经分解得到可溶的氯化稀土和难溶的ReF3。这点与其他研究人员的实验结果相一致。文献[15]报道:直接采用浓盐酸浸出氟碳铈矿,稀土浸取率不足15%,且反应后所得固体需经过碱分解和酸溶处理。本文作者认为,这可能是因为反应生成的ReF3附着在未反应的固体颗粒表面,而阻碍了浸出剂的扩散和反应的继续进行,故直接采用盐酸浸出氟碳铈矿存在着稀土分解率低等严重问题。
会结合H+生成H2CO3,进而分解产生CO2,部分Re3+结合F-生成稳定的ReF3沉淀,该过程实际上是氟碳铈矿的酸分解。在酸分解过程中,氟碳铈矿经分解得到可溶的氯化稀土和难溶的ReF3。这点与其他研究人员的实验结果相一致。文献[15]报道:直接采用浓盐酸浸出氟碳铈矿,稀土浸取率不足15%,且反应后所得固体需经过碱分解和酸溶处理。本文作者认为,这可能是因为反应生成的ReF3附着在未反应的固体颗粒表面,而阻碍了浸出剂的扩散和反应的继续进行,故直接采用盐酸浸出氟碳铈矿存在着稀土分解率低等严重问题。

图1 25 ℃时La-F-CO3-H2O体系可溶解组分的lg c-pH图
Fig. 1 lg c-pH diagram for La-F-CO3-H2O system at 25 ℃
在a<pH<b时,即ReFCO3的稳定区内, 结合H+主要以
结合H+主要以 形式存在,随着pH升高,
形式存在,随着pH升高, 浓度升高。由于OH-浓度的增加,Re3+与OH-的结合能力也逐渐增强,
浓度升高。由于OH-浓度的增加,Re3+与OH-的结合能力也逐渐增强, 浓度不断增加,使得游离Re3+的浓度受到抑制而不断下降。由于
浓度不断增加,使得游离Re3+的浓度受到抑制而不断下降。由于 ,在该范围内,F-浓度变化很小。
,在该范围内,F-浓度变化很小。
当pH>b时,随着pH升高,OH-浓度增加,Re(OH)3的浓度达到饱和,使得游离的Re3+浓度急剧下降。由于氟碳铈矿的溶解平衡,溶液中总碳浓度和总氟浓度逐渐增大,不断析出Re(OH)3固体,分解过程为碱分解。对于萤石含量较高的高钙氟碳铈矿,直接用NaOH分解,不仅增加了碱用量,且反应生成的Ca(OH)2在后续工序中随稀土同时进入浸出液中,影响到了产品质量[16],因此,该法不适宜直接处理含钙高的氟碳铈矿。
由图1~3可以看出:在碱分解氟碳铈矿的过程中,NdFCO3,LaFCO3 和CeFCO3几乎同时被分解。此时,ReFCO3不断转变为氢氧化物,与可溶性的 和 F-分离,Re(OH)3易被盐酸分解,从而可得到混合氯化稀土。由热力学分析可以看出:碱度越高,越有利于氟碳铈矿的分解。如文献[17]中在温度为473~623 K的条件下,测定了氢氧化钠浓度为1~5 mol/L范围内,氟碳铈矿浸出率与氢氧化钠浓度的关系。结果表明:在所有分解温度下,回收率随氢氧化钠浓度上升而增加,这一点与本文分析结果相吻合。
和 F-分离,Re(OH)3易被盐酸分解,从而可得到混合氯化稀土。由热力学分析可以看出:碱度越高,越有利于氟碳铈矿的分解。如文献[17]中在温度为473~623 K的条件下,测定了氢氧化钠浓度为1~5 mol/L范围内,氟碳铈矿浸出率与氢氧化钠浓度的关系。结果表明:在所有分解温度下,回收率随氢氧化钠浓度上升而增加,这一点与本文分析结果相吻合。
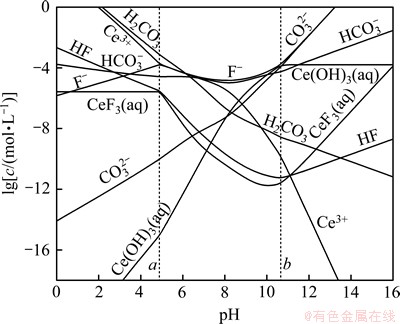
图2 25 ℃时Ce-F-CO3-H2O体系可溶解组分的lg c-pH图
Fig. 2 lg c-pH diagram for Ce-F-CO3-H2O system at 25 ℃

图3 25 ℃时Nd-F-CO3-H2O体系可溶解组分的lg c-pH图
Fig. 3 lg c-pH diagram for Nd-F-CO3-H2O system at 25 ℃
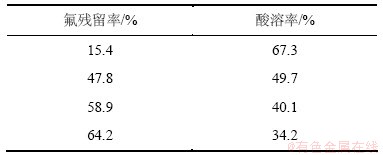
ReF3比ReFCO3更稳定,更难被酸溶解。因此,碱分解产物的含氟量直接影响了它在酸中的溶解率及氟碳铈矿的分解率。这与赵仕林等[6]的实验结果相吻合,如表6所示。氟碳铈矿在碱分解过程中,由于结构中的F-被碱溶出,使得碱分解产物的酸溶性大为提高。张其春等[18]用140 ℃的浓氢氧化钠溶液转化氟碳铈矿,矿碱比采用 1:1.1,反应时间为4 h,经过滤,氢氧化稀土与可溶性的氟化钠和碳酸钠分离,得到的碱转化产物中F-含量为0.13%,酸溶率可达90%以上。
由于氟碳铈矿与NaOH溶液的反应属于非均相反应,反应生成的氢氧化稀土沉淀会附着在未反应的固体颗粒表面,且随着反应的进行Re(OH)3固体层越来越厚,阻碍了NaOH向矿物颗粒表面的扩散[19]。为此,文献[20]报道了在工频电场下用烧碱分解稀土精矿的工艺研究,通过电场为分解反应提供热源,加快了反应的进行,减少了扩散对反应的影响。虞宝煜[20]通过实验研究认为,工频电场的作用是一种交流电直接通过物料而引起的内加热。这实质上是在高浓度碱液中,通过提高反应温度而加速了扩散的结果。
表6 氟残留率对氟碳铈矿分解率的影响
Table 6 Effects of residual rate of fluorine on decomposition of bastnasite.
4 结论
1) 利用已有热力学数据,绘制了25 ℃下Ce-F-C-H2O系、La-F-C-H2O系和Nd-F-C-H2O系可溶解组分的lg c-pH图。
2) 利用热力学平衡图,分析了氟碳铈矿分解的热力学条件,在高于或低于某个pH范围时,氟碳铈矿即可被碱或酸分解,并得出在维持一定碱度条件下,氟碳铈矿可很好地转化为氢氧化稀土。
3) 热力学分析表明,直接采用酸分解氟碳铈矿,可得到难溶的ReF3和可溶的氯化稀土;相比之下,在碱分解过程中,由于结构中F-和 被碱溶出,使得氟碳铈矿很好地被转化为易溶于酸的氢氧化物。
被碱溶出,使得氟碳铈矿很好地被转化为易溶于酸的氢氧化物。
参考文献:
[1] 韩小英, 李平, 王勇, 等. 中国稀土发展概况[J]. 中国有色金属, 2010(2): 34-38.
HAN Xiaoying, LI Ping, WANG Yong, et al. Development situation of China’s rare earth[J]. China Nonferrous Metals, 2010(2): 34-38.
[2] 魏莹莹, 付红扬, 李勇. 氟在氟碳铈矿湿法冶炼工艺的影响与作用分析[J]. 辽宁化工, 2012, 41(6): 632-634.
WEI Yingying, FU Hongyang, LI Yong. Analysis of impact and effect of fluorine in the hydrometallurgical process of bastnaesite[J]. Liaoning Chemical Industry, 2012, 41(6): 632-634.
[3] WANG Liangshi, WANG Chunmei, YU Ying, et al. Recovery of fluorine from bastnasite as synthetic cryolite by-product[J]. Journal of Hazardous Materials, 2012, 209/210: 77-83.
[4] 杨庆山, 杨涛. 氟碳铈矿的冶炼新工艺研究[J]. 稀有金属与硬质合金, 2014, 42(1): 1-4.
YANG Qingshan, YANG Tao. Study on new smelting process of bastnasite[J]. Rare Metals and Cemented Carbides, 2014, 42(1): 1-4.
[5] HUANG Yukun, ZHANG Tingan, DOU Zhihe, et al. Study on leaching rare earths from bastnaesite treated by calcification transition[J]. Journal of Rare Earths, 2014, 32(11): 1043-1047.
[6] 赵仕林, 张新申. 氟碳铈矿与NaOH反应的动力学分析及其应用研究[J]. 四川大学学报, 2002, 39(5): 929-932.
ZHAO Shilin, ZHANG Xinshen. Kinetics analysis of the reaction NaOH and bastnasite and study of its application[J]. Journal of Sichuan University, 2002, 39(5): 929-932.
[7] 吴文远. 稀土冶金学[M]. 北京: 化学工业出版社, 2005: 47.
WU Wenyuan. Metallurgy of rare earths[M]. Beijing: Chemical Industry Press, 2005: 47.
[8] Kanazawa Y, Kamitani M. Rare earth minerals and resources in the word[J]. Journal of Alloys and Compounds, 2006, 408-412: 1339-1343
[9] 杨燕生, 李源英. 稀土物理化学常数[M]. 北京: 冶金工业出版社, 1978: 331.
YANG Yansheng, LI Yuanying. Physicochemical constant of rare earth[M]. Beijing: Metallurgical Industry Press, 1978: 331.
[10] 张刚, 赵中伟, 李江涛, 等. 氢氧化钠分解钼酸铅矿的热力学分析[J]. 中南大学学报(自然科学版), 2008, 39(5): 902-906.
ZHANG Gang, ZHAO Zhongwei, LI Jiangtao, et al. Thermodynamics analysis on sodium hydroxide decomposition of wulfenite[J]. Journal of Central South University (Science and Technology), 2008, 39(5): 902-906.
[11] Haas J R, Shock E L, Sassani D C. Rare earth element in hydrothermal systems: Estimates of standard partial molal thermodynamic properties of aqueous complexes of the rare earth elements at high pressures and temperatures[J]. Geochim Cosmochim Acta, 1995, 59(21): 4329-4350
[12] Dean J A. Lange’s handbook of chemistry[M]. New York: Mc Graw-Hill Press, 1999: 802-807.
[13] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出社, 1998: 225.
WANG Dianzuo, HU Yuehua. Solution chemistry of flotation[M]. Changsha: Hunan Science and Technology Press, 1988: 225
[14] Poitrasson F, Oelkers E, Schott J, et al. Experimental determination of synthetic NdPO4 monazite end-member solubility in water from 21 °C to 300 °C: Implications for rare earth element mobility in crustal fluids[J]. Geochimica et Cosmochimica Acta, 2004, 68(10): 2208-2218
[15] 李良才, 葛星坊, 李林森. 攀西稀土矿湿法冶炼技术现状与进展[J]. 稀土, 1999, 20(4): 51-55.
LI Liangcai, GE Xingfang, LI Linsen. Development of hydrometallurgical processes for Panxi Bastnaesite[J]. Chinese Rare Earths, 1999, 20(4): 51-55.
[16] XU Yanhui, LIU Haijiao, MENG Zhijun. Decomposition of bastnasite and monazite mixed rare earth minerals calcined by alkali liquid[J]. Journal of Rare Earths, 2012, 30(2): 155-158.
[17] 丰田祥一, 涂峰. 水热法提取氟碳铈矿中的稀土元素[J]. 湿法冶金, 1993, 12(3): 52-57.
Toyota S, TU Feng. Extraction rare earth from bastnasite by hydrothermal method[J]. Hydrometallurgy (China), 1993, 12(3): 52-57
[18] 张其春, 邱克辉. 碱法转化氟碳铈矿制备混合稀土及其机理研究[J]. 矿冶工程, 2000, 20(3): 47-50.
ZHANG Qichun, QIU Kehui. Preparation of rare earth by alkali in inversion of bastnasite and its mechanism[J]. Mining and Metallurgical Engineering, 2000, 20(3): 47-50.
[19] 韩学印, 李良才, 常叔, 等. 浓NaOH溶液分解稀土矿物的研究[J]. 稀土, 1985, 6(3): 39-43.
HAN Xueyin, LI Liangcai, CHANG Shu, et al. Study of the technology for the decomposition of rare earth ore by concentrated NaOH solution[J]. Chinese Rare Earth, 1985, 6(3): 39-43.
[20] 虞宝煜. 工频电场下烧碱法分解稀土精矿机理的研究[J]. 中国稀土学报, 1993, 11(3): 218-221.
YU Baoyu. Study on mechanism for the decomposition of rare earth ore by alkali in power frequency electric field[J]. Journal of the Chinese Rare Earth Society, 1993, 11(3): 218-221.
(编辑 杨幼平)
收稿日期:2014-03-10;修回日期:2014-05-12
基金项目(Foundation item):国家科技支撑项目(2012BAB10B04);国家青年科学基金资助项目(51304246) (Project(2012BAB10B04) supported by the National Science and Technology of China; Project(51304246) supported by the National Science Foundation for Young Scientists of China)
通信作者:李江涛,讲师,从事钨钼的冶金工艺研究;E-mail: jiangtaolee@hotmail.com