Influence of thermo hydrogen treatment onhot deformation behavior of Ti600 alloy
来源期刊:中国有色金属学报(英文版)2009年第1期
论文作者:赵敬伟 丁桦 王耀奇 侯红亮
文章页码:65 - 71
Key words:Ti600 alloy; thermo hydrogen treatment; hot deformation; flow stress; activation energy
Abstract: Hot compressive deformation of Ti600 alloy after thermo hydrogen treatment (THT) was carried out within hydrogen content range of 0-0.5%, temperature range of 760-920 ℃ and strain rate range of 0.01-10 s-1. The flow stress of Ti600 alloy after THT was obtained under hot deformation condition, and the influence of hydrogen on work-hardening rate (S*), strain energy density (U*), and deformation activation energy (Q) was analysed. The results show that the flow stress of Ti600 alloy decreases remarkably with the increase of hydrogen when the hydrogen content is less than 0.3%. Both S* and U* decrease with the increase of hydrogen when the hydrogen content is less than 0.3%, and when the hydrogen content is more than 0.3%, S* and U* increase with hydrogen addition. The value of Q decreases with the increase of strain at the same hydrogen content. The addition of small quantity of hydrogen leads to an increase of Q at small strain values, and when the strain reaches 0.6, the value of Q decreases gradually with the increase of hydrogen. When the hydrogen content is within the range of 0.1%-0.3%, the flow stress of Ti600 alloy is decreased when being deformed at the temperature range of 760-920 ℃.

ZHAO Jing-wei(赵敬伟)1, DING Hua(丁 桦)1, WANG Yao-qi(王耀奇)2, HOU Hong-liang(侯红亮)2
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
2. Beijing Aeronautical Manufacturing Technology Research Institute, Beijing 100024, China
Received 30 May 2008; accepted 16 September 2008
Abstract: Hot compressive deformation of Ti600 alloy after thermo hydrogen treatment (THT) was carried out within hydrogen content range of 0-0.5%, temperature range of 760-920 ℃ and strain rate range of 0.01-10 s-1. The flow stress of Ti600 alloy after THT was obtained under hot deformation condition, and the influence of hydrogen on work-hardening rate (S*), strain energy density (U*), and deformation activation energy (Q) was analysed. The results show that the flow stress of Ti600 alloy decreases remarkably with the increase of hydrogen when the hydrogen content is less than 0.3%. Both S* and U* decrease with the increase of hydrogen when the hydrogen content is less than 0.3%, and when the hydrogen content is more than 0.3%, S* and U* increase with hydrogen addition. The value of Q decreases with the increase of strain at the same hydrogen content. The addition of small quantity of hydrogen leads to an increase of Q at small strain values, and when the strain reaches 0.6, the value of Q decreases gradually with the increase of hydrogen. When the hydrogen content is within the range of 0.1%-0.3%, the flow stress of Ti600 alloy is decreased when being deformed at the temperature range of 760-920 ℃.
Key words: Ti600 alloy; thermo hydrogen treatment; hot deformation; flow stress; activation energy
1 Introduction
Hydrogen is an especial element in titanium alloy, and it can be easily added and removed without melting. After hydrogenation of titanium alloy, the phase transition ((α+β)→β) temperature is decreased, which causes the ratio of β to increase. Because β phase, with body-centered cubic (BCC) structure, can be easily deformed at high temperature, the use of hydrogen as a temporary alloying element is always added into titanium alloy during hot deformation processing. In 1970s, scholars[1-5] in former Soviet Union began to investigate the influence of hydrogen on thermo- plasticity of titanium alloy, and it was found that the addition of hydrogen reduced the flow stress and enhanced the plasticity of the α, near α, α+β and intermetallic-base alloys at lower temperatures of hot deformation. When 0.2% (mass fraction) hydrogen was added into the alloy, the peak stress of Ti3Al-base alloy during hot compression deformation was evidently decreased after hydrogenation, the deformation temperature can be decreased by 50 ℃, and the strain rate could be increased by an order of magnitude at 900-1 000 ℃[6]. The work of KOLACHOV[7] had shown that the flow stress after hydrogenation was only 1/3 of that without hydrogen after 0.6% (mass fraction) hydrogen was added into Ti3Al-base CT5 alloy, moreover, there was no crack occurred even though 80% deformation was carried out at 900 ℃. In early work, KERR et al[8] found that the flow stress of Ti-6Al-4V alloy decreased with the increase of hydrogen. When 0.4% (mass fraction) hydrogen was added, the flow stress reached a minimum, and then it increased slightly with the increase of hydrogen. BIRLA et al[9] suggested that the forged flow stress of Ti-6Al-2Sn-4Zr-6Mo alloy with 0.4% (mass fraction) hydrogen decreased by 0.3-0.35 times of that without hydrogen when being deformed at 730 ℃. LI et al[10] found that the yield strength of Ti-60 alloy decreased by 0.7 times of that without hydrogen after 0.3% (mass fraction) hydrogen was added into the alloy when being deformed at 900 ℃. Tensile deformation tests had been carried out by ZHANG and ZHAO[11], and the results revealed that the flow stress was decreased and the plasticity was improved when hydrogen content was less than 0.32% (mass fraction). Recently, an increased interesting on thermo hydrogen treatment (THT), or the use of hydrogen as a temporary element for modifying microstructure and enhancing mechanical properties of titanium alloy, has been emphasized.
The serving temperature of aircraft engine is as high as 600 ℃, and the materials used for aircraft engine are required to work stably under a long-term load in temperature range of 550-600 ℃. At present, the typical titanium alloys that can meet such application are Ti-1100 alloy (U.S.)[12], IMI834 alloy (U.K.)[13] and BT36 alloy (Russia)[14], and these alloys have been used successfully in different aircraft engines. However, because of the poor plasticity, these alloys cannot be deformed by using conventional processing method. The addition of hydrogen can remarkably decrease the flow stress of these alloys, which makes the deformation process possible at relatively low temperature. Ti600 alloy is a new type of near α high-temperature titanium alloy which can work stably at 600 ℃. Ti600 alloy has been regarded as one of the materials for aircraft engine serviced at the temperature of 600 ℃. The rolling process, thermal deformation behavior and creep theory of Ti600 alloy have currently received much attention[15-17]. However, the influence of hydrogen on the hot deformation behavior of Ti600 alloy has been less investigated extensively. In this work, the hot compression deformation behavior of Ti600 alloy after THT is studied, with emphases on flow stress, work-hardening rate, strain energy density and activation energy of deformation.
2 Experimental
The material used in this investigation is a Ti600 alloy, and the chemical composition is as follows (in mass fraction, %): 6 Al, 2.8 Sn, 4 Zr, 0.5 Mo, 0.4 Si, 0.1 Y (rare-earth element) and balance Ti. Cylindrical specimens, with 8 mm in diameter and 15 mm in length, were machined, and all the specimens were hydrogenated at 750 ℃ for 2 h followed by air-cooling to room temperature. Specimens with various contents of hydrogen in the range of 0-0.5% (mass fraction) were obtained by controlling the hydrogen pressure as
?p?V=?n?RT (1)
where ?p is the pressure change before and after THT, ?n is the amount of hydrogen added into specimen, V is the total volume of hydrogenated system, R is the gas constant, and T is the hydrogenated temperature. The actual hydrogen content in specimen was determined by weighing the specimen before and after THT.
The hot deformation tests were performed on a Gleeble-1500 hot-simulator at deformation temperature from 760 to 920 ℃, strain rate from 0.01 to 10 s-1, and the maximum true strain of 0.69. The deformation process was controlled by computer, and all the tested data were collected automatically.
3 Results and discussion
3.1 Influence of hydrogen on flow stress
Fig.1 shows the σ—ε curves for specimens with various hydrogen contents at ![]() 0.1 s-1 and temperature range of 760-920 ℃. It can be seen that the flow stress of Ti600 alloy decreases with the increase of deformation temperature at a given strain rate. For the specimens without hydrogen (Fig.1(a)), the value of peak stress at 920 ℃ is σp=169.9 MPa, which is far less than that at 760 ℃ (σp=520.3 MPa). Hydrogenation causes a decrease of the flow stress of specimens with 0.1%-0.5% H at given temperature and strain rate. After 0.1% hydrogen is added (Fig.1(b)), specimens exhibit work hardening at first, then soften gradually with the increase of strain in the deformation temperature range of 760-840 ℃. When being deformed at 880-920 ℃, the flow stress changes unconspicuously with the increase of strain, and the value of flow stress at 880 ℃ shows a little difference from that at 920 ℃. The specimens with 0.3% H have a greater drop in flow stress than that with 0.1% H in temperature range of 760-840 ℃ (Fig.1(c)). The flow stress of specimens with 0.3% H deformed at 880-920 ℃ shows a less drop in flow stress than that with 0.1% H; moreover, the flow stress changes little with the increase of strain. Fig.1(d) shows the σ—ε curves for specimens with 0.5% H. It can be seen that the curves show a similar characteristic to that with 0.3% H, and there is unconspicuous difference between the curves for specimens with 0.3% and 0.5% H.
0.1 s-1 and temperature range of 760-920 ℃. It can be seen that the flow stress of Ti600 alloy decreases with the increase of deformation temperature at a given strain rate. For the specimens without hydrogen (Fig.1(a)), the value of peak stress at 920 ℃ is σp=169.9 MPa, which is far less than that at 760 ℃ (σp=520.3 MPa). Hydrogenation causes a decrease of the flow stress of specimens with 0.1%-0.5% H at given temperature and strain rate. After 0.1% hydrogen is added (Fig.1(b)), specimens exhibit work hardening at first, then soften gradually with the increase of strain in the deformation temperature range of 760-840 ℃. When being deformed at 880-920 ℃, the flow stress changes unconspicuously with the increase of strain, and the value of flow stress at 880 ℃ shows a little difference from that at 920 ℃. The specimens with 0.3% H have a greater drop in flow stress than that with 0.1% H in temperature range of 760-840 ℃ (Fig.1(c)). The flow stress of specimens with 0.3% H deformed at 880-920 ℃ shows a less drop in flow stress than that with 0.1% H; moreover, the flow stress changes little with the increase of strain. Fig.1(d) shows the σ—ε curves for specimens with 0.5% H. It can be seen that the curves show a similar characteristic to that with 0.3% H, and there is unconspicuous difference between the curves for specimens with 0.3% and 0.5% H.
Fig.2 shows the influence of hydrogen on the flow stress of specimens deformed at 800 and 880 ℃. It can be seen that the values of peak flow stress without hydrogen are 485.5 MPa and 283.8 MPa when being deformed at 800 and 880 ℃, respectively. After 0.3% hydrogen is added, the values of peak flow stress drop to 221.1 MPa and 96.9 MPa, respectively. The peak flow stress decreases approximately by 54%-66% when being deformed in temperature range of 800-880 ℃. When the hydrogen content is more than 0.3%, the flow stress increases appreciably with the increase of hydrogen content. As shown in Fig.2(a), the flow stress of the specimen with 0.5% H is higher than that with 0.3% H at 800 ℃. When the deformation temperature increases to 880 ℃, as shown in Fig.2(b), the flow stress of specimen with 0.5% H is larger than that with 0.3% H and 0.1% H. Therefore, the hydrogen content within proper range can decrease the flow stress of Ti600 alloy remarkably.
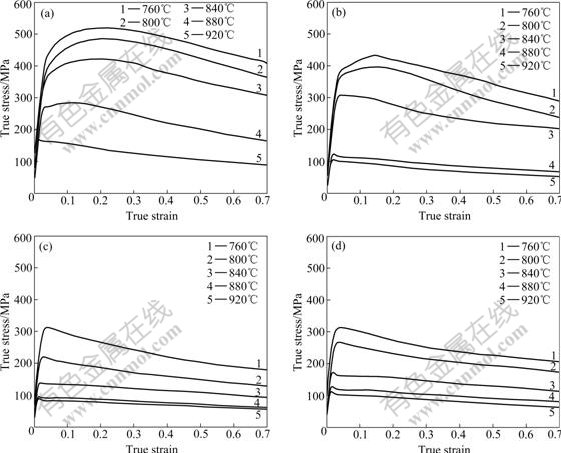
Fig.1 Flow curves for specimens with various hydrogen contents (![]() 0.1 s-1): (a) 0% H; (b) 0.1% H; (c) 0.3% H; (d) 0.5% H
0.1 s-1): (a) 0% H; (b) 0.1% H; (c) 0.3% H; (d) 0.5% H
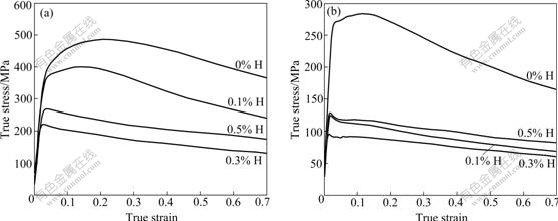
Fig.2 Influence of hydrogen on flow stress at different temperatures (![]() 0.1 s-1): (a) 800 ℃; (b) 880 ℃
0.1 s-1): (a) 800 ℃; (b) 880 ℃
The dependences of flow stress and peak stress on hydrogen content are exhibited in Fig.3. Fig.3(a) shows the relationship between hydrogen content and flow stress at given strain and strain rate. It can be seen that, with the decrease of temperature, the flow stress increases gradually. For a given deformed temperature, the flow stress drops fast at first, then decreases to a minimum when the hydrogen content is 0.3%, and finally increases slightly with the increase of hydrogen content. Fig.3(b) shows that the flow stress increases with the increase of strain rate at a given deformation temperature. Similarly, the flow stress decreases to a minimum with the increase of hydrogen content, then increases slightly when the hydrogen content is more than 0.3%. The relationship between hydrogen content and peak stress is presented in Fig.3(c), which shows a similar characteristic of flow stress change with hydrogen content as shown in Fig.3(a) and Fig.3(b). Summarily, the same conclusion as Fig.2 can be obtained, i.e., when the hydrogen content is within a certain range, the flow stress can be decreased effectively.

Fig.3 Dependence of flow stress (a, b) and peak stress (c) on hydrogen content: (a) ε=0.69, ![]() 0.1 s-1; (b) ε=0.69, θ=760 ℃; (c)
0.1 s-1; (b) ε=0.69, θ=760 ℃; (c) ![]() 0.1 s-1
0.1 s-1
Although the influence of hydrogenation on the mechanism of high temperature plasticity of titanium has currently received much attention, an accordant conclusion has not been acquired by now. At present, it has been generally suggested that the decrease of flow stress is related to the increase of β phase. It is known that hydrogen alloying stabilizes the ductile high-temperature BCC β phase in titanium, so the β→(α+β) phase transition temperature is decreased, and the temperature interval of the two-phase (α+β) range is increased with the addition of hydrogen. Because there are more slip systems in β phase than that in α phase, a hydrogen-induced increase of β phase will certainly lead to a decrease of flow stress, and also, an increase of the high temperature plasticity. When the hydrogen content is within a proper range, the flow stress decreases continuously with the increase of hydrogen. The eutectoid reaction βH→α+δ will happen at higher hydrogen contents, and then a new phase δ is formed. Because the specific volume of δ is different from that of β matrix, stress fields are generated when δ precipitates from β matrix, and then distortion of lattices is caused, leading to large number of dislocations around δ. It has been observed[18-19] that the hydrogen-induced softening of titanium is associated with the interaction of dislocations with hydrogen. When being deformed at high temperature, hydrogen in solution in titanium lowers down the dislocation interactions with various obstacles, and dislocation mobility is enhanced, which leads to the improvement of high temperature plasticity[20]. In addition, the hydrogen in solution, being highly mobile and occupying interstitial sites near dislocations, prevents other solutes from segregating to the mobile dislocations[21]. Moreover, as the diffusion ability of alloying elements is increased, dynamic recovery and recrystallization are facilitated, lowering the flow stress in this way[22]. Additionally, because the content of β phase increases after hydrogenation, the concentration of the alloying element dissolved in β phase reduces, and the solution strength is weakened, which will cause the flow stress to decrease to a certain extent.
Although the flow stress decrease in Ti600 alloy, when the hydrogen content is increased, is caused by an increase in the volume fraction of β phase, the flow stress increases at higher hydrogen content because of the coarsening of β phase at high temperature. In addition, the solubility of hydrogen in β phase is far more than that in α phase, and large number of hydrogen atoms will enter the interstitial sites and function as solution strengthening elements at higher hydrogen content. Additional reason causing the flow stress to increase could be due to a short range ordering that occurs at relatively high hydrogen content. Although dislocation glide is expected to destroy such ordering, it may be restored continuously during deformation processing because of the high mobility of hydrogen.
3.2 Influence of hydrogen on work-hardening rate
Usually, the curves of dτ/dε and dσ/dε versus true stress are used to study the work-hardening rate for single crystal and polycrystal metals, respectively. Here, as shown in Fig.4, S*=dσ/dε is employed to analyze the influence of hydrogen on work-hardening rate of Ti600 alloy. Two stages of work-hardening rate are observed with the changing of true stress, i.e., the first stage with positive S* and the second stage with negative S*. For the specimen without hydrogen, at first stage, S* drops rapidly at first, then decreases more slowly to zero. When being deformed at 840 and 880 ℃, the stresses attained at the end of the first stage of S* are about 421.7 MPa and 283.8 MPa, which correspond to the strains of 0.21 and 0.12, respectively. The value of strain attained at the end of the first stage of S* decreases with the increase of temperature. At the second stage, S* decreases to a minimum at first, then increases slightly with the decrease of true stress. Hydrogenation leads to a more rapid decrease in the values of S* through all the first stage. At the second stage, S* drops to a minimum rapidly, and then increases slightly to zero with the decrease of stress. When hydrogen content is less than 0.3%, the S*—σ curves move to lower stress values gradually with the increase of hydrogen content, i.e., specimens containing more hydrogen have lower work- hardening rate at the same stress or strain levels. However, as the hydrogen content reaches 0.5%, compared with the specimens with 0.3% H, the S*—σ curves move to higher stress values. As shown in Fig.4, when the specimen is deformed at 880 ℃, the work- hardening rate with 0.5% H is higher than that with both 0.3% and 0.1% H, which is only higher than that with 0.3% H at 840 ℃, contrastively. Additionally, the S*—σ curves tend to move to lower stress values, i.e., the work-hardening rate decreases at the same stress value with the increase of temperature at a given hydrogen content.

Fig.4 Influence of hydrogen on work-hardening rate (![]() 0.1 s-1): (a) θ=840 ℃; (b) θ=880 ℃
0.1 s-1): (a) θ=840 ℃; (b) θ=880 ℃
3.3 Influence of hydrogen on strain energy density
The influence of hydrogen on strain energy density at various temperatures is illustrated in Fig.5. The strain energy density, i.e., strain energy per unit volume, is defined as U*=∫σdε (MJ/m3). It can be seen that U* increases linearly with the increase of strain. Hydrogenation in Ti600 alloy leads to decrease in the strain energy density, and the value of U* decreases gradually with the increase of hydrogen content when the hydrogen content is less than 0.3%. Differently, as the hydrogen content reaches 0.5%, the value of U* increases. When the specimen is deformed at 760 ℃ (Fig.5(a)), the strain energy density with 0.5% H is only higher than that with 0.3% H. However, as it is presented in Fig.5(b), the strain energy density of the specimen with 0.5% H is higher than that with both 0.3% and 0.1% H when being deformed at 880 ℃.

Fig.5 Influence of hydrogen on strain energy density at different temperature (![]() 1 s-1): (a) θ=760 ℃; (b) θ=880 ℃
1 s-1): (a) θ=760 ℃; (b) θ=880 ℃
3.4 Influence of hydrogen on activation energy
Arrhenius equations are often employed to study the influence of temperature and strain rate on flow stress. There are three Arrhenius-type equations that have been widely used to describe the relationships between stress and temperature as well as strain rate[23-24]:
![]() (2)
(2)
![]() (3)
(3)
![]() (4)
(4)
where n, n1 and n2 are constants closely related to the strain rate, A, A1, A2 and α are material constants, Q is the activation energy of deformation, R is the gas constant, T is the absolute temperature, σ is flow stress, and ![]() is strain rate. The parameters n1 and n2 are obtained by the slopes of lnσ—ln
is strain rate. The parameters n1 and n2 are obtained by the slopes of lnσ—ln![]() and σ—ln
and σ—ln![]() plots at a given temperature, and the value of α is calculated by α=n2/n1. The mean values of α at different hydrogen content levels are tabulated in Table 1.
plots at a given temperature, and the value of α is calculated by α=n2/n1. The mean values of α at different hydrogen content levels are tabulated in Table 1.
Table 1 Mean values of α at different hydrogen contents

Because the exponential and power Arrhenius-type equations are just extreme cases of hyperbolic-sine Arrhenius-type equation, Eqs.(2) and (3) are not considered. The hyperbolic-sine Arrhenius-type equation (Eq.(4)) is focused here to investigate the high temperature deformation behavior of Ti600 alloy.
Taking the natural logarithm on both sides of Eq.(4), it becomes
![]() (5)
(5)
For a given temperature, the slope of ln[sinh(ασ)]—ln![]() plot gives the parameter n by
plot gives the parameter n by
![]() (6)
(6)
Rearranging Eq.(5) and differentiating with respect to 1/T gives
![]() (7)
(7)
Based on Eqs.(6) and (7), the activation energy of deformation Q can be acquired at given temperature and strain rate values by
![]() (8)
(8)
Fig.6 shows the influence of hydrogen on activation energy of deformation. As shown in Fig.6(a), the values of Q decrease with the increase of strain. When the strain is less than 0.6, the value of Q of the specimen with 0.1% H is higher than that without hydrogen. The Q value of the specimen with 0.1% H is 670.73 kJ/mol, and it is lower than that without hydrogen (674.26 kJ/mol) at strain of 0.6. The Q value of the specimen with 0.5% H is lower than that with 0.3% H, which, including that with both 0.3% and 0.5% H, is far lower than that without hydrogen. Additionally, for a given strain, the specimen with relatively high hydrogen content (up to 0.5% H) has a great drop in activation energy of formation. When the strain is less than 0.6 (Fig. 6(b)), the values of Q increase, go through a maximum (corresponding to the hydrogen content of 0.1%), and then decrease with the increase of hydrogen. The values of Q decrease rapidly with the increase of hydrogen content within hydrogen range of 0.1%-0.3%. When the hydrogen content is more than 0.3%, the values of Q decrease slowly with hydrogen addition. And, when the strain reaches 0.6, the value of Q decreases gradually with the increase of hydrogen content. So, it is concluded that the addition of small quantity of hydrogen leads to an increase of Q at small train values, and when the strain reaches a certain value (ε=0.6, in this work), Q decreases gradually with the increase of hydrogen content.

Fig.6 Influence of hydrogen on activation energy of deformation: (a) Q versus strain curves at different hydrogen contents; (b) Q versus hydrogen content at different strain
4 Conclusions
1) The flow stress of Ti600 alloy decreases gradually with the increase of hydrogen content when the hydrogen content is less than 0.3%. Contrastively, when the hydrogen content is more than 0.3%, the flow stress increases with hydrogen addition.
2) The addition of hydrogen decreases the values of S* and U*. When the hydrogen content is less than 0.3%, the specimen with relatively high hydrogen content has a great drop in both S* and U*. Differently, the values of S* and U* increase with the increase of hydrogen content when the hydrogen content is more than 0.3%.
3) The activation energy of formation of Ti600 alloy decreases gradually with the increase of strain at a given hydrogen content level. The addition of small quantity of hydrogen leads to an increase of Q at small strain values, and when the strain reaches 0.6, the value of Q decreases gradually with the increase of hydrogen content.
4) The addition of hydrogen (0.1%-0.3%H) decreases the flow stress of Ti600 alloy when it is deformed in the temperature range of 760-920 ℃.
References
[1] SENKOV O N, FROES F H. Thermohydrogen processing of titanium alloys [J]. International Journal of Hydrogen Energy, 1999, 24(6): 565-576.
[2] LIN Ying-ying, PAN Hong-si, LI Miao-quan. Hydrogen treatment and its effect on superplasticity of titanium alloys [J]. Materials Engineering, 2005, 5: 60-64. (in Chinese)
[3] TAL-GUTELMACHER E, ELIEZER D, BOELLINGHAUS T. Investigation of hydrogen-deformation interactions in β-21S titanium alloy using thermal desorption spectroscopy [J]. Journal of Alloys and Compounds, 2007, 440(1/2): 204-209.
[4] SHAN D B, ZONG Y Y, L? Y, GUO B. The effect of hydrogen on the strengthening and softening of Ti-6Al-4V alloy [J]. Scripta Materialia, 2008, 58(6): 449-452.
[5] LI Miao-quan, ZHANG Wei-fu. Effect of hydrogenation content on high temperature deformation behavior of Ti-6Al-4V alloy in isothermal compression [J]. International Journal of Hydrogen Energy, 2008, 33(11): 2714-2720.
[6] ZHANG Yong, ZHANG Shao-qing. Hydrogen effects on high temperature deformation characteristics of a cast Ti-14Al-19Nb-3V- 2Mo alloy [J]. Scripta Materialia, 1997, 37(9): 1315-1321.
[7] KOLACHOV B A, LIVANOV L A, NOSOV V K. Influence of hydrogen on hot deformability of titanium alloys with different phase compositions [C]// Titanium and Titanium Alloys: Scientific and Technological Aspects (Proceedings of the 3rd International Conference on Titanium), USA, New York: Plenum Press, 1982: 1833-1842.
[8] KERR W R, GURNEY F J, MARTORELL I A. Pilot plant forging of hydrogenated Ti-6Al-4V. AFWAL-TR-80-4026 [R]. Air Force Wright Aeronautical Labs., Wright-Patterson AFB, OH. 1980.
[9] BIRLA N C, DEPIERRE V. Dehydriding of Ti-6Al-2Sn-4Zr-6Mo hydride powder [J]. Powder Metallurgy, 1975, 18: 15-31.
[10] LI Fang, CHEN Ye-xin, WAN Xiao-jing, WANG Qing-jiang, LIU Yu-yin. Effect of hydrogen on the microstructure and high temperature mechanical properties of Ti-60 alloy [J]. Acta Metallurgica Sinica, 2006, 42(2): 143-146. (in Chinese)
[11] ZHANG Shao-qing, ZHAO Lin-ruo. Effect of hydrogen on the superplasticity and microstructure of Ti-6Al-4V alloy [J]. Journal of Alloys and Compounds, 1995, 218: 233-236.
[12] WEINEM D, KUMPFERT J, PETERS M, KAYSSER W A. Processing window of the near-α-titanium alloy TIMETAL-1100 to produce a fine-grained β-structure [J]. Materials Science and Engineering A, 1996, 206(1): 55-62.
[13] EVANS R W, HULL R J, WILSHIRE B. The effects of alpha-case formation on the creep fracture properties of the high-temperature titanium alloy IMI834 [J]. Journal of Materials Processing Technology, 1996, 56(1/4): 492-501.
[14] TETYUKHIN V, LEVIN I, ILYENKO V. Heatresistant titanium alloys with enhanced, heat resistance, thermal stability [C]// BLENKINSOP P H, EVANS W J, FLOWER H M, ed. Titanium’95: Science and Technology. UK, Cambridge: The University Press, 1996, 2430.
[15] HONG Quan, QI Yun-lian, ZHAO Yong-qing, YANG Guan-jun. Effect of rolling process on microstructure and properties of Ti600 alloy plates [J]. Rare Metal Materials and Engineering, 2005, 34(8): 1334-1337. (in Chinese)
[16] QI Yun-lian. Behavior and processing map of high temperature titanium alloy Ti600 [D]. Xi’an: Northwestern Polytechnical University, 2007. (in Chinese)
[17] HONG Quan, QI Yun-lian, GUO Ping, ZENG Li-ying, ZHAO Yong-qing. Study on the microstruction characteristic and creep properties of Ti600 alloy [J]. Rare Metals Letters, 2007, 26(9): 19-22. (in Chinese)
[18] SHIH D S, ROBERTSON I M, BIRNBAUM H K. Hydrogen embrittlement of α titanium: In situ TEM studies [J]. Acta Metallurgica, 1998, 36(1): 111-124.
[19] BIRNBAUM H K, SOFRONIS P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen-related fracture [J]. Materials Science and Engineering A, 1994, 176(1/2): 191-202.
[20] SENKOV O N, DUBOIS M, JONAS J J. Elastic moduli of titanium hydrogen alloys in the temperature range 20 ℃ to 1 100 ℃ [J]. Metallurgica & Materials Transaction A, 1996, 27(12): 3963-3970.
[21] SENKOV O N, JONAS J J. Dynamic strain aging and hydrogen induced softening in alpha titanium [J]. Metallurgica & Materials Transaction A, 1996, 27(7): 1877-1887.
[22] COLLINSON D C, HODGSON P D, PARKER B A. Modelling of metal rolling processing [M]. London: The Institute of Metals, 1993: 283-295.
[23] WANJARA P, JAHAZI M, MONAJATI H, YUE S, IMMARIGEON J P. Hot working behavior of near-α alloy IMI834 [J]. Materials Science and Engineering A, 2005, 396(1/2): 50-60.
[24] WEI Yan-guang, XIONG Bai-qing, ZHANG Yong-an, LIU Hong-wei, ZHU Bao-hong, WANG Feng. Research on flow stress of spray formed 70Si30Al alloy under hot compression deformation [J]. Rare Metals, 2006, 25(6): 665-670.
Foundation item: Project supported by the Major Basic Research Program of National Security of China
Corresponding author: ZHAO Jing-wei; Tel: +86-24-83687746; E-mail: jwzhaocn@gmail.com
DOI: 10.1016/S1003-6326(08)60230-7
(Edited by YANG Bing)

