文章编号:1004-0609(2013)06-1605-06
碳热还原法合成TiB2的反应传质机理
马爱琼,蒋明学,武志红
(西安建筑科技大学 材料与矿资学院,西安 710055)
摘 要:在XRD、SEM、能谱分析、TEM、TG-DSC等实验分析的基础上,对以TiO2、B2O3、C为原料,通过碳热还原法合成TiB2粉末的反应传质机理进行了研究,阐明碳热还原法合成TiB2的反应传质机理,建立碳热还原法合成TiB2的反应传质模型。研究表明:在碳热还原TiO2的过程中,由低温到高温,最稳定的还原产物分别是Ti4O7和Ti3O5,尤其当温度超过1300℃以后,Ti3O5为最稳定的还原产物。在碳热还原TiO2与B2O3合成TiB2的反应过程中,DDSC曲线上有几个明显的吸热峰,这分别对应于TiO2→Ti4O7→Ti3O5→TiB2的反应阶段。碳与氧化物颗粒之间是通过CO/CO2气体偶实现质量传递的。在反应体系中,B2O2(g)气相、Ti3O5(s)固相分别是形成TiB2的前驱体。
关键词:TiB2;碳热还原;传质机理;传质模型
中图分类号:TQ037 文献标志码:A
Reactionary mass transfer mechanism of TiB2 synthesized by carbothermal reduction method
MA Ai-qiong, JIANG Ming-xue, WU Zhi-hong
(College of Materials and Mineral Resources, Xi’an University of Architecture and Technology, Xi’an 710055, China)
Abstract: On the basis of experiment analysis such as XRD, SEM, energy spectrum analysis, TEM and TG-DSC, the reactionary mass transfer mechanism of synthesizing TiB2 by carbothermal reducing TiO2 and B2O3 was studied. The reactionary mass transfer mechanism of synthesizing TiB2 by carbothermal reduction method was analyzed, and the reactionary mass transfer model of synthesizing TiB2 by carbothermal reduction method was built. The results show that, during carbothermal reduction of TiO2, the most stable reduction products are Ti4O7 and Ti3O5 from low temperature to high temperature, respectively, when the reduction temperature is over 1 300 ℃, Ti3O5 is the most stable reduction product.There are several endothermic peaks on DDSC curve in the process of synthesizing TiB2 by carbothermal reducing TiO2 and B2O3, which correspond to reaction stage of TiO2→Ti4O7→Ti3O5→TiB2, respectively. The mass transfer from carbon to the oxide particle is realized by the CO/CO2 gas couple. In reactionary system, B2O2(g) and Ti3O5(s) are the precursors of formation TiB2 by carbothermal reduction.
Key words: TiB2; carbothermal reduction; mass transfer mechanism; mass transfer model
硼化钛(TiB2)是Ti-B二元系中唯一稳定的化合物[1],属六方晶系,其晶体结构参数为:a=0.302 36 nm,c=0.322 04 nm[2]。TiB2的熔点高达3 225 ℃,这使其成为超高温材料的首选,硼化钛具有多方面综合优异性能,在许多领域均有广阔的应用前景。在结构材料方面,由于其较高的强度与硬度,可制造硬质工具材料和刀具、拉丝模、喷砂嘴等,同时可以作为各种复合材料的添加剂,在功能材料方面,由于TiB2具有与纯铁相似的电阻率,从而在功能材料应用上大有可为。利用TiB2的电性能,还可制造PTC材料。利用TiB2陶瓷优良的电性能和在过渡金属及轻金属熔体中具有良好的稳定性,使它成为在金属蒸镀技术领域具有广泛用途的一种新型的多功能材料[3-6]。
通常,主要通过碳热还原钛和硼的氧化物来制备TiB2[7-11]。在TiB2粉末合成过程中,为了得到合适的粉末,对反应机理的充分理解是十分必要的。研究碳热还原TiO2与B2O3合成TiB2的反应传质机理,不仅有助于理解TiB2粉末合成反应的本质、合成反应中所出现的物相,而且对采用碳热还原法制备其他金属陶瓷粉末也有指导意义。
一直以来,关于碳热还原法合成TiB2的反应机理,国内外看法并不统一。根据作者以往的热力学分析,碳热还原生成TiB2的反应温度低于单质钛与单质硼的开始生成温度(约300℃),在TiO2-B2O3-C系统的优势区相图内,单质B与Ti的还原产物之间没有稳定共存的区域,由单质B与Ti生成TiB2的反应机理不能得到热力学证实[12]。因此,到目前为止,对这一反应传质机理尚未完全清楚。基于此,本文作者在前人研究工作的基础上,并结合作者以往热力学分析的结论,对碳热还原法合成硼化钛的反应传质机理进行了研究,建立了碳热还原法合成硼化钛的反应传质模型。
1 实验
将TiO2粉末(金红石型,纯度为99.95%,平均粒径为1.75 μm)、B2O3粉末(纯度为98.8%,平均粒径为2.62 μm)、石墨粉(纯度为99.49%,平均粒径为0.5 μm)按1:2:5.5(摩尔比)放入尼龙球磨罐中以蒸馏水混合,以刚玉为研磨介质,其中料:球:水的质量比为1:5:1.5,湿磨24 h后,取出料浆,在烘箱中进行110 ℃、24 h干燥,过88 μm筛。
将3%无水乙醇和0.2%(质量分数)的糊精加入粉料中,搅拌碾压均匀,用内径为d 38 mm的模具,在20 MPa的压力下压制成d 38 mm×35 mm的圆柱形试样。将压制好的生坯试样在烘箱中进行110 ℃、24 h干燥。
将制备好的试样置于ZT-50-20Y真空碳管炉内,对炉内抽真空至50 Pa以下,并充氩气洗炉2~3次,最后,在保持抽真空的条件下加热至设定的合成温度,反应温度分别设定为900、1 000、1 100、1 200、1 300、1 400和1 450 ℃。升温速度平均为10 ℃/min,在合成温度下保温3 h,当合成反应结束后,以15 ℃/min的降温速率迅速冷却至室温。
采用日本理学公司生产的D/MAX-2400型X射线衍射仪对合成试样进行XRD分析;采用德国耐驰公司生产的STA409PC TG-DSC联用分析仪对原料混合物进行热重-差示扫描量热(TG-DSC)分析;采用美国FEI公司生产的Quanta 200型扫描电子显微镜对试样进行SEM分析;采用日本产JEM-3010型高分辨率透射电子显微镜对试样进行TEM分析;采用英国产INCA ERERGY250能谱仪对试样进行能谱分析。
2 结果与讨论
2.1 合成试样的XRD分析与能谱分析
图1所示为在不同温度下保温3 h后合成产物的XRD谱。由图1可见,随着合成反应温度的升高,TiO2的碳热还原反应开始于1 100 ℃,还原顺序依次为TiO2→Ti4O7→Ti6O11→Ti5O9→Ti3O5→Ti2O3。

图1 不同温度下合成产物的XRD谱
Fig. 1 XRD patterns of synthesized products at different temperatures
其中,Ti4O7在1 100~1 200 ℃之间稳定存在,Ti3O5在1 200 ℃以上稳定存在,而Ti2O3的生成温度较高,当还原反应温度超过1 300 ℃以后才开始生成,随温度升高,Ti2O3生成量的增加并不明显。这表明,在TiO2的还原产物中,由低温到高温,最稳定的还原产物分别是Ti4O7、Ti3O5,尤其当温度超过1 300 ℃以后,Ti3O5为最稳定的还原产物。
XRD分析表明,TiB2的生成温度开始于1 300 ℃左右,随着反应温度的升高,TiB2的生成量逐渐增加;当反应温度达到1 450 ℃时,TiB2的生成已达100%。这一点可以由图2所示对1 450 ℃反应后所得试样的能谱分析结果得到验证。由图2可见,此时试样中存在的元素为Ti和B元素,根据它们的摩尔分数可知产物为TiB2。
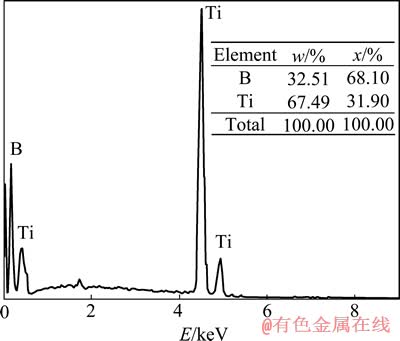
图2 1 450 ℃反应后试样的能谱分析结果
Fig. 2 Energy spectrum analysis results of synthesized product at 1 450 ℃
2.2 反应试样的热重-差示扫描量热(TG-DSC)分析
为了对碳热还原TiO2、B2O3合成TiB2的反应过程进行系统研究,对金红石、石墨和氧化硼(摩尔比为1:2:5.5)的混合物在标准状况下进行热重-差示扫描量热(TG-DSC)分析,分析结果如图3所示。图3中dW/dt为微商热重,W为质量损失率,t为时间;dH/dt为热流率,H为热流。
由图3可看出,在反应发生的几个阶段均有质量损失,这对于DTG曲线而言尤为明显。在低温时(100 ℃左右)的微小质量损失是由于温度升高,吸附水的挥发所致,此时的质量损失率为0.55%,在DDSC曲线上表现为吸热。626 ℃左右,在DTG曲线上有一个较小的质量损失峰,对应于DDSC曲线,为一个明显的吸热峰,这与在此温度下,混合物中的非晶态B2O3转变为熔融液态吸热有关。在高温下,DDSC曲线上出现了几个吸热峰,这分别对应于TiO2被碳逐级还原形成Ti4O7、 Ti3O5、 Ti2O3和 TiB2。由TG曲线可见,在从室温到1 400 ℃的温度范围内,质量损失为17.12%,低于理论质量损失,这与加热到最高温度以后,没有保温一段时间有关。由DDSC曲线可见,对应于1 000 ℃以上的4个吸热峰,在DSC曲线上表现为连续的吸热,根据热力学计算结果,这些峰值所对应的温度,与表1所列的反应开始温度一致,并且反应的焓变△H与DSC分析结果吻合,表中所列的反应在相应温度下的焓变△H分别为7.46、70.06、139.31 和285.33 kJ/mol,因此,形成TiB2的反应属吸热反应。由此可见,TG-DSC分析与热力学计算结果完全吻合,由前面的XRD分析结果,这些物相组成已得到相应的实验验证,这进一步证实了Ti3O5是形成TiB2的先驱产物,而并非TiO或单质Ti。由热力学计算结果可知,由Ti3O5、Ti2O3形成TiO和单质Ti的温度分别高达1 752和2 139 K,在此温度下,TiB2的合成反应早已进行完全。
2.3 合成产物的显微形貌分析
采用扫描电镜、透射电镜对不同温度下合成产物的显微形貌进行了分析,其结果如图4所示。由图4可见,当反应温度为1 100 ℃时(见图4(a)),合成TiB2的反应并没有开始,从图中仍可见到片状的石墨颗粒,这表明,在此条件下,TiO2的还原反应刚刚开始;当温度升高到1 450 ℃时(见图4(b)),合成TiB2的反应已经完成,产物为基本发育完整的六方短柱状TiB2晶粒,可以清晰地看到在TiB2晶粒中存在大大小小的孔洞,这表明在合成TiB2的反应中,存在气相传质机理,反应中有CO等气体释放。

图3 金红石-石墨-氧化硼混合物的TG-DSC曲线
Fig. 3 TG-DSC curves for mixture of rutile-graphite-boride
表1 热力学计算标准状况下Ti4O7、Ti3O5、Ti2O3和TiB2的形成温度
Table 1 Forming temperatures of Ti4O7, Ti3O5, Ti2O3 and TiB2 from thermal calculation


图4 合成产物的SEM像
Fig. 4 SEM image of synthesized products
图5所示为1 450 ℃合成条件下TiB2的TEM像。由图5(a)所示,TiB2晶粒发育较完整,呈规则的六方柱状。由图5(b)所示,在TiB2晶粒中有许多小而开放的孔洞结构,显而易见,这些孔洞是由于反应过程中有诸如CO和CO2等气体释放而留下的。由此可见,SEM、TEM分析的结果与XRD、能谱分析和热力学分析结果完全吻合。

图5 1 450 ℃合成产物的TEM像
Fig. 5 TEM images of product synthesized at 1 450 ℃
3 碳热还原法合成TiB2的反应传质机理探讨
WEIMER等[13-14]、BERGER等[15-17]经过长期的实验研究认为,通常的碳热还原反应可以描述为由两种固态反应物(如C和TiO2颗粒)反应生成新的固相产物(如Ti4O7、Ti3O5等)和气相(CO),在此条件下,其中的一种固态反应物就是反应的前驱体或者认为新的固相产物由两种气相中间产物形成。
由图4(b)和5(b)可知,合成的TiB2颗粒中含有许多孔洞。图6所示为作者以往热力学分析中所绘制的TiO2-B2O3-C系统的优势区相图[12]。由图6可见,图中实线部分区域为B-C-O系统中各物相的稳定存在区域,虚线区域为Ti-C-O系统中各物相的稳定存在区域。图中Ti-C-O系统和B-C-O系统有部分重叠区域,其中B2O2-Ti3O5-C重叠面积最大,这表明,反应过程中的质量传递是通过CO/CO2气体偶来实现的。根据前面的XRD分析和TG-DSC分析,并结合此处的优势区相图分析可见,在反应体系中,B2O2、Ti3O5分别是形成TiB2的前驱体。根据这一结论和实验研究结果可知,碳热还原法合成TiB2的反应传质过程分为3个阶段,这3个阶段的反应传质模型如图7所示。
由图7可见,反应第一阶段所涉及到的反应为
4TiO2(s)+C(s)=Ti4O7(s)+CO(g) (1)
随着反应温度升高,固态C颗粒与TiO2颗粒扩散速率加快,反应活性增强,二者在接触边界发生反应,反应属固相反应过程。XRD分析证实,这一阶段的反应开始于1 100 ℃左右,与热力学计算的反应开始温度吻合。

图6 TiO2-B2O3-C系统的优势区相图[12]
Fig. 6 Diagram of stability relations of phases in TiO2- B2O3-C system[12]

图7 碳热还原法合成TiB2的反应传质模型
Fig. 7 Reaction mass transfer model of TiB2 synthesized by carbothermal reduction
反应第二阶段所涉及的反应为
3Ti4O7(s)+CO(g)=4Ti3O5(s)+CO2(g) (2)
C(s)+CO2(g)=2CO(g) (3)
随着Ti4O7的生成,在反应过程中伴随有CO气体释放并充当还原剂进一步还原Ti4O7,此时的反应属于气-固相反应,由于CO气相扩散速率高于固态C的,反应将不局限于物料直接接触的界面,而可能是沿整个反应物颗粒的自由表面同时进行。而通过CO2气体平衡来实现CO的还原能力很可能是这一阶段的还原机理,通过CO/CO2气体偶来实现氧化物颗粒与碳颗粒之间的质量传递。因此,和第一阶段反应相比,这一阶段,由于气态分子扩散迅速,反应速率较快,此阶段的反应由化学反应速率所控制。实验证实,这一阶段的反应温度开始于1 200 ℃左右。
合成反应第三阶段所涉及的反应为
B2O3(l)+C(s)=B2O2(g)+CO(g) (4)
Ti3O5(s)+3B2O2(g)+11C(s)=3TiB2(s)+11CO(g) (5)
在这一阶段由于涉及B2O3被还原及产物相TiB2的生成,通过热力学分析,以上3个反应,应是在还原剂C的参与下完成的,且以上3个反应中,由于B2O3被C还原生成B2O2(g)气相的开始反应温度较高,而B2O2(g)气相是合成TiB2的过程中B的来源。因此,和反应第二阶段相比,这一阶段的反应速率有所下降,由B2O3(l)液相通过固态C的扩散应是整个反应的控制步骤。
4 结论
1) XRD分析和能谱分析证明,在碳热还原TiO2的过程中,由低温到高温,最稳定的还原产物分别是Ti4O7和Ti3O5,尤其当温度超过1 300 ℃以后,Ti3O5为最稳定的还原产物。
2) TG-DSC分析证明,在碳热还原TiO2与B2O3合成TiB2的反应过程中,DDSC曲线上有几个明显的吸热峰,其分别对应于以下反应过程TiO2→Ti4O7→ Ti3O5→ TiB2。
3) 描述了碳热还原TiO2与B2O3合成TiB2的反应传质机理,在反应体系中,B2O2(g)和Ti3O5(s)分别是形成TiB2的前驱体。
REFERENCES
[1] 陈肈友. ZrB2与TiB2质耐火材料[J]. 耐火材料, 2000, 34(4): 224-229.
CHEN Zhao-you. ZrB2 and TiB2 refractories[J]. Refractory, 2000, 34(4): 224-229.
[2] MROZ C. Annual material review: Titanium diboride[J]. Am Ceram Soc Bull, 1994, 73(6): 136-137.
[3] 向 新, 秦 岩. TiB2及其复合材料研究进展[J]. 陶瓷学报, 1999, 20(2): 112-117.
XIANG Xin, QIN Yan. Development of research on TiB2 and its composites[J]. Journal of Ceramics, 1999, 20(2): 112-117.
[4] WANG Yu-jin, PENG Hua-xin, YE Feng, ZHOU Yu. Effect of TiB2 content on microstructure and mechanical properties of in-situ fabricated TiB2/B4C composites[J]. Transaction Nonferrous Metals Society of China, 2011, 21(s2): s369-s373.
[5] CAO Guo-jian, XU Hong-yu, ZHENG Zhen-zhu, GENG Lin, NAKA M. Grain size effect on cyclic oxidation of (TiB2+TiC)/Ni3Al composites[J]. Transaction Nonferrous Metals Society of China, 2012, 22(7): 1588-1593.
[6] KRISHNARAO R V, SUBRAHMANYAM J. Studies on the formation of TiB2 through carbothermal and B2O3 reduction of TiO2[J]. Mater Sci Eng A, 2003, 362: 145-151.
[7] 王化章, 汤 啸, 杨建红. 氯化物熔体中电化学合成硼化钛[J]. 中国有色金属学报, 1997, 7(2): 34-38.
WANG Hua-zhang, TANG Xiao, YANG Jian-hong. Electrochemical synthesis TiB2 in chloride molten salts[J]. The Chinese Journal of Nonferrous Metals, 1997, 7(2): 34-38.
[8] 张廷安, 杨 欢, 牛丽萍. 镁热还原自蔓延高温合成硼化钛微粉的动力学[J]. 中国有色金属学报, 2001, 11(4): 567-570.
ZHANG Ting-an, YANG Huan, LIU Li-ping. Kinetics of preparation of titanium boride by SHS[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 567-570.
[9] KANG SHIN H, KIM DEUG J. Synthesis of nano-titanium diboride powders by carbothermal reduction[J]. J Eur Ceram Soc, 2007, 27(2): 715-718.
[10] BRUCE N BECKLOFF, JACK W, LACKEY. Process-structure- reflectance correlations for TiB2 films prepared by chemical vapor deposition[J]. J Am Ceram Soc, 1999, 82(3): 503-512.
[11] DARREN A H, MARC A M. Consolidation of combustion- synthesized titanium diboride-based materials[J]. J Am Ceram Soc, 1995, 78(2): 375-381.
[12] 马爱琼, 蒋明学. 硼化钛合成反应机理的优势区相图叠加分析[J]. 中国有色金属学报, 2011, 21(6): 1409-1414.
MA Ai-qiong, JIANG Ming-xue. Predominance area phase diagram analysis about synthetic reaction mechanism of TiB2[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(6): 1409-1414.
[13] WEIMER A, NILSEN K J, COCHRAN G A. Kinetics of carbothermal reduction synthesis of beta silicon carbides[J]. Aiche J, 1993, 39: 493-503.
[14] WEIMER A W, WILLIAM G M, RAYMOND P R. Kinetics of carbothermal reduction synthesis of boron carbide[J]. J Am Ceram Soc, 1992, 75(9): 2509-2514.
[15] BERGER L M, GRUNER W, LANGHOLF E. On the mechanism of carbothermal reduction processes of TiO2 and ZrO2[J]. Int J Refract Met & Hard Mater, 1999, 17: 235-243.
[16] BERGER L M, LANGHOLF E. Mass spectrometric investigations on the carbothermal reduction of titanium dioxide[J]. J Mater Sci Lett, 1999, 18: 1409-1412.
[17] BERGER L M, GRUNER W. Investigation of the effect of a nitrogen-containing atmosphere on the carbothermal reduction of titanium dioxide[J]. Int J Refract Met & Hard Mater, 2002, 20: 235-251.
(编辑 李艳红)
基金项目:陕西省重点学科建设专项资金资助项目
收稿日期:2012-05-30;修订日期:2013-03-08
通信作者:马爱琼,副教授,博士;电话:029-82205798;E-mail: maaiqiong@xauat.edu.cn