爆炸喷涂制备铁基非晶合金涂层的摩擦磨损特性及耐腐蚀性能
来源期刊:中国有色金属学报(英文版)2016年第6期
论文作者:吴宏 兰小东 刘咏 李飞 张卫东 陈紫瑾 宰雄飞 曾晗
文章页码:1629 - 1637
关键词:铁基非晶涂层;爆炸喷涂;显微组织;摩擦行为;腐蚀行为
Key words:Fe-based metallic glass coating; detonation gun spraying; microstructure; tribological behavior; corrosion behavior
摘 要:在Q235不锈钢板上利用爆炸喷涂工艺制备名义组分为Fe51.33Cr14.9Mo25.67Y3.4C3.44B1.26(摩尔分数,%)的铁基非晶涂层。采用扫描电镜、X射线衍射仪、维氏显微硬度计、摩擦磨损试验机和电化学测量方法对涂层的组织结构、相组成、硬度、摩擦磨损特性和耐腐蚀性能进行表征。显微组织结构分析结果表明,涂层组织均匀、结构致密、平均孔隙率低于2.1%。干磨条件下的磨损行为显示,在同一磨损条件下,非晶涂层的相对耐磨性为基体材料的5倍。摩擦过程中,磨损形式为粘着磨损和磨粒磨损的综合作用,在非晶涂层中粘着磨损起主导作用。涂层在质量分数为3.5% NaCl 水溶液中存在明显的钝化现象,有较低的钝化电流密度和较宽的钝化区间,呈现出优异的耐腐蚀性能。
Abstract: A metallic glass coating with the composition of Fe51.33Cr14.9Mo25.67Y3.4C3.44B1.26 (mole fraction, %) on the Q235 stainless steel was developed by the detonation gun (D-gun) spraying process. The microstructure and the phase aggregate were analyzed by scanning electron microscopy and X-ray diffractometry, respectively. Microhardness, wear resistance and corrosion behavior were assessed using a Vickers microhardness tester, a ball-on-disk wear testing machine and the electrochemical measurement method, respectively. Microstructural studies show that the coatings possess a densely layered structure with the porosity less than 2.1%. The tribological behavior of the coatings examined under dry conditions shows that their relative wear resistance is five times higher than that of the substrate material. Both adhesive wear and abrasive wear contribute to the friction, but the former is the dominant wear mechanism of the metallic glass coatings. The coatings exhibit low passive current density and extremely wide passive region in 3.5% NaCl solution, thus indicating excellent corrosion resistance.
Trans. Nonferrous Met. Soc. China 26(2016) 1629-1637
Hong WU1, Xiao-dong LAN1, Yong LIU1, Fei LI2, Wei-dong ZHANG1, Zi-jin CHEN1, Xiong-fei ZAI1, Han ZENG1
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. State Key Laboratory of Construction Machinery, Zoomlion Heavy Industry Science and Technology Co., Ltd., Changsha 410013, China
Received 30 July 2015; accepted 9 March 2016
Abstract: A metallic glass coating with the composition of Fe51.33Cr14.9Mo25.67Y3.4C3.44B1.26 (mole fraction, %) on the Q235 stainless steel was developed by the detonation gun (D-gun) spraying process. The microstructure and the phase aggregate were analyzed by scanning electron microscopy and X-ray diffractometry, respectively. Microhardness, wear resistance and corrosion behavior were assessed using a Vickers microhardness tester, a ball-on-disk wear testing machine and the electrochemical measurement method, respectively. Microstructural studies show that the coatings possess a densely layered structure with the porosity less than 2.1%. The tribological behavior of the coatings examined under dry conditions shows that their relative wear resistance is five times higher than that of the substrate material. Both adhesive wear and abrasive wear contribute to the friction, but the former is the dominant wear mechanism of the metallic glass coatings. The coatings exhibit low passive current density and extremely wide passive region in 3.5% NaCl solution, thus indicating excellent corrosion resistance.
Key words: Fe-based metallic glass coating; detonation gun spraying; microstructure; tribological behavior; corrosion behavior
1 Introduction
In the past decades, metallic glasses have attracted significant interest due to their superior mechanical, physical and chemical properties, compared with their crystalline counterparts [1-3]. It is well known that metallic glass alloys exhibit high hardness, high tensile strength and good fracture toughness [4]. The unique properties of bulk glassy alloys originate from the random atomic arrangement of metallic glasses that contrasts with the regular atomic lattice arrangement found in crystalline alloys, which makes bulk glassy alloys extremely attractive for structural and functional uses [5].
However, as a matter of fact, industrial applications of metallic glass alloys have been restricted due to the difficulties encountered in the production of bulk quantities and brittleness of bulk metallic glass alloys [6]. To overcome the limitations and expand the applications of metallic glass alloys, researchers have developed the process to deposit metallic glass coatings on structural or functional components, which can combine excellent mechanical properties of the substrates and metallic glass coatings and significantly improve the properties such as corrosion resistance and wear resistance. Fe-based metallic glass alloys have been selected as a prominent candidate to work as surface coatings that protect the surface of structural materials owing to their excellent wear and corrosion resistance, and relatively low cost [7]. Up to now, there exist various techniques to produce metallic glass coatings, such as high velocity oxygen fuel (HVOF) spraying [8], magnetron sputtering [9], vacuum plasma spraying [10] and laser surface processing [11]. Each of these techniques has advantages and disadvantages over its counterparts. Compared with the plasma spray process and laser clad, the detonation gun (D-gun) spraying technique is an especially suitable method to prepare Fe-based metallic glass coatings due to its ability to produce high-density coatings with larger compressive stress and improved metallurgical bonding to the substrate. The previous works also demonstrated that Fe-based metallic glass coatings prepared by D-gun spraying can increase the density of the coating up to 98% and thus improve the wear, adhesion and mechanical properties moderately [12]. Some attempts have been made on preparation of amorphous alloy coatings by the D-gun spraying process [13-15], and most of the coatings show prominent properties. However, there is a lack of information concerning the wear behavior and related wear mechanism of Fe-based metallic glass coatings.
In the present study, the microstructure characteristics and tribological behavior of Fe-based metallic glass coatings fabricated by the D-gun spaying were systematically investigated in order to understand the wear mechanism, and the comprehensive corrosion behavior of the coatings in NaCl solutions was also examined.
2 Experimental
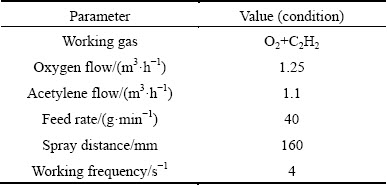
The powders with nominal composition of Fe51.33Cr14.9Mo25.67Y3.4C3.44B1.26 (mole fraction, %) were manufactured using water atomization technique. The water- atomized powders with particle sizes in the range of 50-100 μm were deposited via D-gun spaying onto the Q235 steel sheet substrate with dimensions of 300 mm × 90 mm × 8 mm for their good fluidity. The surface of the substrate was activated by grit-blasting pretreatment after the removal of the oxide layer. Then, the substrate was degreased by acetone and dried in air. The detailed parameters of the spraying are listed in Table 1 and the thickness of the Fe-based metallic glass coatings is between 300 and 500 μm.
Table 1 Parameters employed in D-gun spraying
The as-deposited coatings were cut by wire electrical discharge machining and polished to mirror finish according to the standard procedures. The morphology of the powders and cross-sectional microstructures of as-deposited coatings were characterized by scanning electron microscopy (SEM) combined with EDAX analyzer. X-ray diffraction (XRD) was carried out using a Rigaku D/max-2550VB diffractometer (Cu Kα radiation) with 2θ diffraction angle ranging from 20° to 80°, at a scanning rate of 0.02 (°)/s. Jade software 6.0 was used for analysis of the amorphous phase content in the D-gun deposited metallic glass coatings. Firstly, the XRD pattern of the sample was smoothed in order to remove all kinds of chance fluctuations (noise). Secondly, the strength of amorphous peak was fitted in the whole pattern and then fitted manually until all the diffraction peaks in XRD pattern were fitted. Finally, the crystallinity (Xc) of the sample was calculated according to the Jade formula:
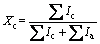 (1)
(1)
where Ic is the total diffraction integral strength for the crystallization part and Ia is the total diffraction integral strength for the amorphous part. Microhardness measurement of the coatings (Vickers scale) was conducted on the central part of the cross-section samples using a Vickers microhardness tester with an applied load of 50 g and indentation time of 10 s. The average of 10 measurements was determined to be the final value of the microhardness. Friction and wear experiments were conducted under dry condition using a ball-on-disk sliding apparatus (CERT UMT-3, USA). Wear loss and friction coefficient were constantly recorded during sliding. The corrosion behavior of the Fe-based metallic glass coatings was investigated by electrochemical measurement on CHI600B. Electrolyte used was 3.5% NaCl solution. Electrochemical measurements were conducted in a three-electrode cell using a platinum counter electrode and a saturated calomel reference electrode. Potentiodynamic polarization curves were measured at a potential sweep rate of 30 mV/min in the solutions open to air at 298 K after immersing the specimens for several minutes,when the open-circuit potentials became almost steady. For comparison, Q235 steel sheet and Fe-based metallic glass coatings sprayed by D-gun spraying were selected to perform the electrochemical measurements under the same conditions.
3 Results and discussion
3.1 Coating characteristics
The typical SEM images of the water-atomized Fe-based alloy powders with diameters of 50-100 μm are presented in Fig. 1. It is shown that most of the as-atomized powders are spherical or near-spherical although some of them have small satellites attached. During the water atomization, different particle sizes have different solidification rates. It is universally accepted that the solidification rate is inversely proportional to the particle size. Thus, the powders with smaller particle size experience a faster solidification rate. Collision could happen between the droplets with different particle sizes which have not completely solidified in the turbulent region. When larger droplet of molten alloys is still in the liquid state or semi-solid state, the smaller one may have already solidified because of the difference of time needed for solidification. Attachment may occur during the collision, leading to small satellites bonded to the large powders. The diameters of the powders range from 50 to 100 μm and most of them exhibit smooth surface, indicating a good fluidity.
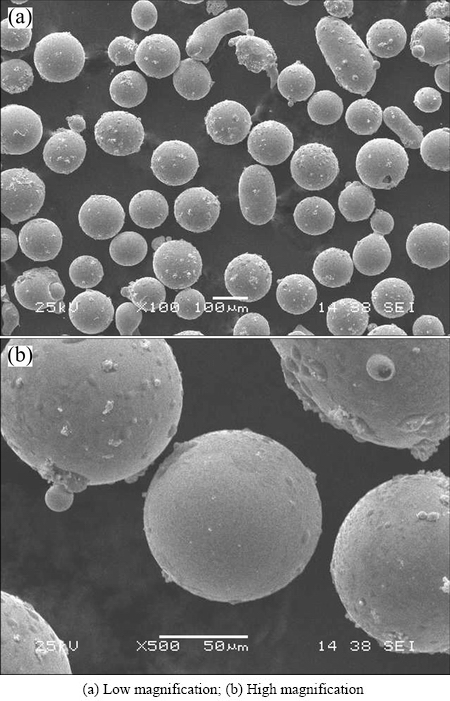
Fig. 1 SEM images of water-atomized Fe-based alloy powders
The XRD measurement was conducted to confirm the structure of the atomized powders and as-deposited coatings, as shown in Fig. 2. A typical broad halo peak can be seen from the XRD pattern of the Fe-based powders (Fig. 2(a)), indicating the existence of the amorphous phase. The rest of the sharp peaks mean that the powders also contain some crystalline phases. It is notable that only a single broad halo peak appears in the as-deposited coating (Fig. 2(b)) and no obvious sharp peaks appear, implying its high content of the amorphous phase. The high content of the amorphous phase of the as-deposited coating is mainly attributed to the high cooling rate of the D-gun process and proper spraying parameters selected in the present study [16]. The content of the amorphous phase in the coating is about 85.54% (volume fraction) calculated by MDI Jade. The origin of the high glass forming ability (GFA) is that the element composition chosen obeys Inoue’s three empirical rules for the achievement of high GFA [17]. Besides, quite few oxides can be detected in the coating. This may be ascribed to good oxidation resistance of the Cr-containing alloys [18].
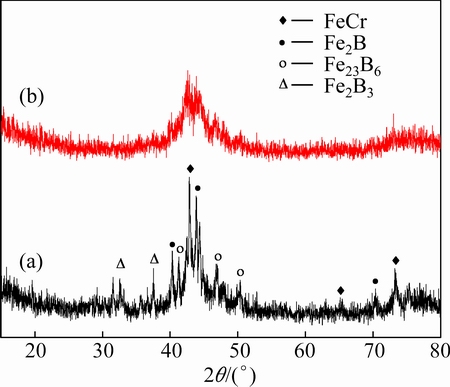
Fig. 2 XRD patterns of Fe-based powders (a) and coating (b) deposited by D-gun spraying
Figure 3 reveals the typical SEM image from the cross-sections of the coating. It can be seen that the coating is stacked by sheet-shaped particles with good deformation ability, and the coating shows a dense layered structure corresponding to the typical characteristic of thermally sprayed deposits. No obvious flaws are found in the coating. The results of the EDS analysis in different regions of the Fe-based metallic glass coating show that the major part of the coating exists as grey amorphous phase (shown in Fig. 3(a)) which basically remains the nominal composition of the atomized powder (shown in Fig. 3(b)), and the composition of the grey regions is quite homogeneous. The dark grey regions are rich in boron (shown in Fig. 3(d)) that exists in the form of boride with high hardness. The boride serves as the wear-resistant skeleton which can significantly improve the wear resistance of the Fe-based metallic glass coatings during dry sliding. The lamellar phase which is discretely distributed at the interface of the grey amorphous matrix is oxide (shown in Fig. 3(c)). As the D-gun spraying process is conducted without protective atmosphere, oxidation may occur during the solidification of thermal spraying molten droplets. The oxide not only suppresses the formation of amorphous phase and affects the combination of lamellar-type particles, but also significantly changes the force and heat transfer of the coating during wear. Thus, stress concentration occurs, which will lead to the emergence of fatigue crack in the early stage. Therefore, to control the content of oxide is one of the major methods to improve the properties of metallic glass coatings. Some pores appearing as very dark regions can be observed from the image (Fig. 3(a)).
The large pores located between flattened droplets are mainly caused by the loose packed layer structure or gas porosity phenomenon [19], while the small ones within the flattened particles originate from the shrinkage porosity [20]. Despite the presence of these defects, the coatings exhibit a low porosity lower than 2.1% calculated by image analysis software, which is a typical character of the D-gun sprayed coatings [21].
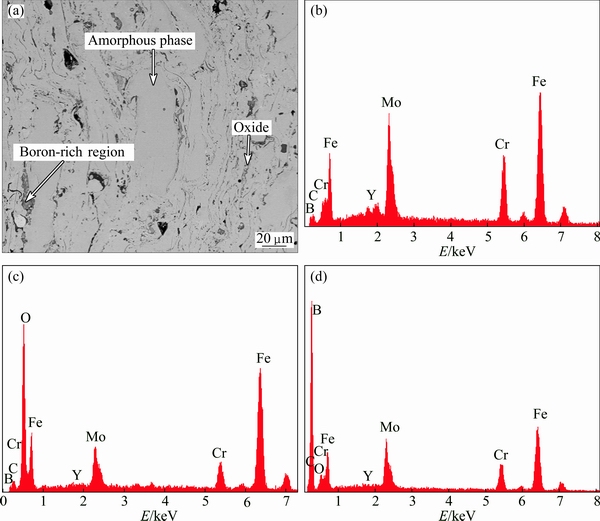
Fig. 3 Cross-sectional SEM image of as-deposited Fe-based alloy coating (a) and EDS patterns for amorphous phase (b), oxide (c) and boron-rich region (d)
3.2 Tribological behavior
Hardness is one of the key factors to assess the tribological behavior of the coating [22]. In general, the extremely dense random packed atomic configurations of the amorphous structure can effectively resist the plastic deformation caused by applied load, thus leading to high hardness performance of the metallic glass coatings [23]. In addition, the dispersion strengthening of hard phase precipitated from amorphous matrix also contributes to the high hardness of as-deposited coatings. The results show that the average value of microhardness is about HV 1095.6, which is higher than not only that of the Q235 stainless steel, but also that of most of the crystal metallic alloys. This indicates that Fe-based metallic glass coating deposited by D-gun spraying is a typically hard coating. It is expected that the wear resistance is generally proportional to the hardness [24].
The wear resistance under dry sliding of the Fe-based metallic glass coatings was evaluated by calculating the wear loss after wear test. The results are listed in Table 2, and the data of Q235 steel substrate are included for comparison. It is apparently that the as-deposited metallic glass coatings exhibit much lower wear loss. The relative excellent wear resistance of metallic glass coatings, which is inversely proportional to wear loss [23], can achieve approximately 5 times higher than that of the substrate material. Therefore, the D-gun spraying technique is a promising way to produce excellent wear-resistant metallic glass coatings.
Table 2 Wear loss of Fe-based metallic glass alloy coating deposited by D-gun spraying

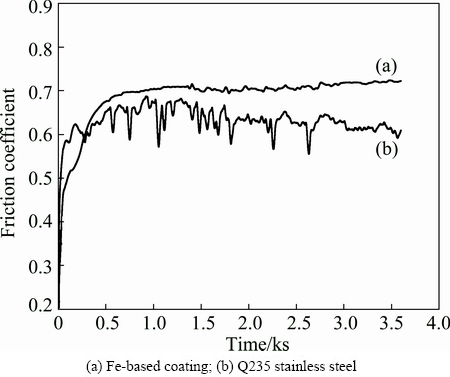
Fig. 4 Relationship between friction coefficient and time at load of 20 N
Figure 4 presents the friction coefficients as a function of time for the Fe-based metallic glass coating and Q235 substrate at room temperature. The curves show that the wear process can be divided into running-in and steady state regimes. In the running-in regime, the tiny concave convex surface gets spalled and polished by the matching material. Since the coating surface tends to match the counterface, the friction coefficient increases with time (Fig. 4). The reason is that the friction coefficient reflects the interaction of surface topography, the contact mode of friction, load and sliding speed. The contact mode between the friction pairs in the sliding experiment is ball-to-disk. The Fe-based metallic glass coating is a kind of material with high hardness and high strength. When the hard asperity with different heights and slopes indents into the surface of the counterface, the hard abrasive particles dug into the sliding sample surface and plow out material to form grooves. Thus, the contact way transfers from ball-to-disk to disk-to-disk, and the roughness of the surface increases. Friction gets serious when the role of plowing gets enhanced, which makes the increase of friction coefficient. The real contact area increases with the coating getting polished gradually. The contact face between friction pairs gets smoother and smoother, and then enters steady-state regimes. The friction coefficient of the coating is approximately 0.7, whereas that of the substrate fluctuates at 0.6.
The friction coefficient curve of the Q235 steel substrate shows large fluctuation in the steady state regime. This may be attributed to the low hardness of the substrate (approximately HV 200). Bearing cyclic loading stress, the surface is easy to fatigue and spalls to create new contact surface. The decrease of friction results in the decrease of friction coefficient. Furthermore, the substrate material gets soft and then adhesion happens between the materials of friction pairs under the effect of friction heat, leading to the increase of friction coefficient. Thus, the friction coefficient fluctuates slightly around a certain value in the steady state regimes. Compared with the substrate, the Fe-based metallic glass coating shows a quite stable friction coefficient in the steady state regimes. This means that the metallic glass coating can keep a slow and stable wear loss even under longer friction time, showing pretty good wear resistance. The good wear resistance and stable tribological behavior of Fe-based metallic glass coating make it an ideal candidate to work as effective protecting materials in the field of surface engineering.
Figure 5 shows the worn surfaces of the Q235 stainless steel substrate and the Fe-based metallic glass coating, respectively. The worn surface of the substrate is distinguished by long continuous parallel grooves with different widths and depths after sliding, as shown in Fig. 5(a). The morphology of the worn surface is consistent with the typical abrasive wear. During the wear test, the hard abrasive particles dug into the sliding sample surface and plow out material to form furrows. The morphology of wide and deep furrows supports the great wear loss of the substrate material. Therefore, abrasive wear is the main wear mechanism.
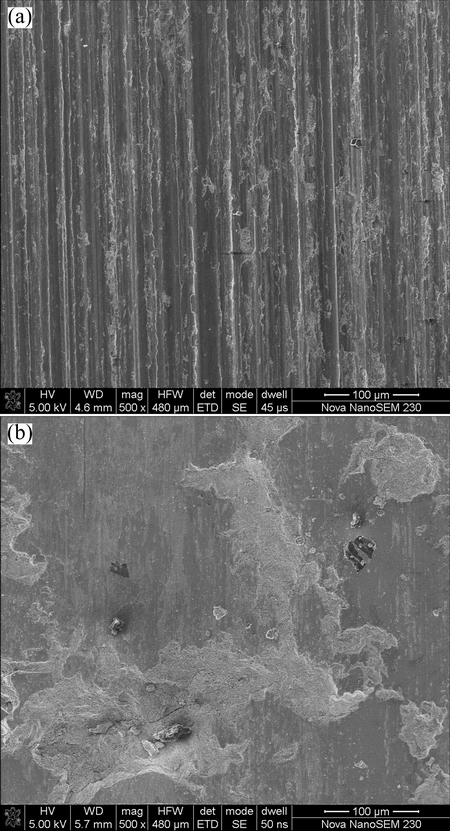
Fig. 5 Morphologies of worn surface of Q235 stainless steel (a) and Fe-based metallic glass coating (b)
Figure 5(b) shows the worn surface of the Fe-based metallic glass coating. It is seen that the coating exhibits a typical morphology of brittle fracture and splat detachment, and some slight scratches can be observed along the sliding direction. Since the Fe-based metallic glass coatings possess poor ductility and toughness, and the splats tend to suffer detachment from the coating under high Hertz load and repeated shear stress [25]. The wear mechanism of metallic glass coatings deposited by detonation gun spraying has been seldom reported [26,27]. The worn morphology of Fe-based metallic glass coating deposited by D-gun spraying process was examined in this paper. The main reason for the damage of coating is that there are few defects existing in the coating which is well bonded. When the temperature rises to right below Tg (glass transition temperature) during the friction, viscoplastic deformation will happen and the coating will get adhered to the friction pair, leading to the occurrence of debris. The particles and flake-like debris are similar to those in the Fe41Co7Cr15Mo14C15B6Y2 BMG observed by HUANG et al [28] and YOON et al [29]. During the wear process, the abrasive particles such as hard amorphous phases and hard phase particles dug into the sliding sample surface, and deformation of coating takes place inside of coating splats initially. The severity of wear for conventional materials can usually be described by means of the dimensionless parameter, K, in Archard’s equation [30]:
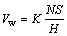 (2)
(2)
where Vw is the total volume of material removed by wear, N is the normal load, S is the total relative sliding distance, H is the material hardness, and K is the wear coefficient. Typical values of K are in the range of 10-2-10-4 for severe wear and 10-4-10-6 for mild wear [31]. Wear coefficient K of the metallic glass coating is calculated to be 2.3×10-5, thus implying the dominant mild adhesive wear.
Figure 6 shows the friction coefficient as a function of time for the Fe-based metallic glass coating under different loads, i.e., 5, 20 and 35 N. It can be seen that there is an obvious fluctuation for the friction coefficient of the Fe-based metallic glass coating under the load of 5 N. The friction coefficient becomes steady when the load is 20 N. The average value of friction coefficient decreases as the load increases from 5 to 20 N. The friction coefficient is the most steady when the load increases to 35 N. However, the average value increases slightly compared with that under the load of 20 N.
Generally, the surface contact area of the Fe-based metallic glass coating is nonlinear to the load. When the load is small (5 N), the contact pressure between the coating and the counterface will be small as well. As a result, the grinding effect of the friction pair on the hard phase particles will impose on the unsmooth surface of the coating. Due to the roughness of friction surface, the average friction coefficient is larger. When the load increases to 20 N, properly prolonged wear time is beneficial to improving the surface roughness of friction pair and coating since Fe-based metallic glass coating is a kind of material that has high hardness and high strength, resulting in the decrease of the friction coefficient. At the load of 35 N, the friction coefficient increases slightly because the hard phase particles in the Fe-based metallic glass coating are easy to leave the surface and the surface becomes unsmooth, both surface roughness and friction coefficient increase.
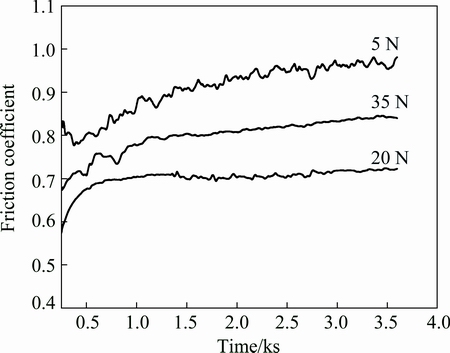
Fig. 6 Friction coefficient of Fe-based coating with time at different loads
It is apparent that the wear loss of metallic glass coatings nonlinearly varies with the load (shown in Fig. 7). The effect of load on the tribological behavior is related to the deformation and the contact area. The increase of load makes the contact area enlarge significantly during sliding. The larger the effective contact area is, the more the abrasive particles are involved in the contact surface. Thus, it appears severer wear. Thus, increasing the load can increase the wear degree and wear loss of the coating.
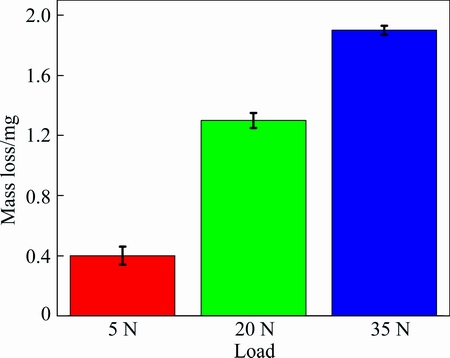
Fig. 7 Wear loss of Fe-based metallic glass coating at different loads
Figure 8 shows the worn surface morphologies of the Fe-based metallic glass coating under dry condition at different loads. All of them exhibit spalling pits. The wear increases distinctly with the increase of load, which is mainly manifested by the increase of pitting area and depth of pits.

Fig. 8 Frictional wear surface morphologies of Fe-based metallic glass alloy coating at different loads
Considering the sliding conducted at the load of 20 N analyzed before, it can be concluded that under different loads, adhesive wear and abrasive wear both contribute to the friction together, but adhesive wear serves as the dominant wear mechanism of the metallic glass coatings.
3.3 Corrosion behavior
To examine the influence of Cl- on the corrosion behavior of Fe-based metallic glass coatings, the potentiodynamic polarization curves of metallic glass coatings and Q235 stainless steel were measured in 3.5% NaCl solution, as shown in Fig. 9. Self-corrosion potential φcorr and corrosion current density can be calculated according to Tafel extrapolation method [32]. As shown in Fig. 9, the Fe-based metallic glass coating shows obvious passivation phenomenon in 3.5% NaCl solution, which means good corrosion resistance. The self-corrosion potential φcorr of the metallic glass coating at point A is about -415 mV and the part of anodic polarization curve is to the right of point A. The current density of the anodic polarization curve of Fe-based metallic glass coatings increases with the increase of polarization potential up to a maximum value near point B. After current density gets across at point B, the growth speed of surface passive film on anode surpasses its dissolution rate, and thus the coating enters a steady passivation stage, manifested as a dense passivation layer covered on the surface of Fe-based metallic glass coating. The passive potential range is from 15 to 510 mV. When it passes point B, the rate of anodic process nearly does not depend on potential value and it is mainly determined by the chemical dissolution rate of the passive film on the coating surface. When polarization potential surpasses point C, the current density of the metallic glass coating increases gradually and at this time the compact passive film gets destroyed correspondingly. This implies that transpassive phenomenon appears, and the characterization represented is that anode chemical dissolution rate of the metallic glass coating increases, and anodic dissolution rate increases. In other words, the passive film on the coating surface cannot resist the erosion of Cl- completely, which shows that the dense passive film formed on the surface of metallic glass coating cannot resist the continuous erosion of Cl-. The reason for this phenomenon is that Cl- belongs to the kind of elements with aggressive erosion ability and the elements in Fe-based metallic glass coating do not belong to the kind of elements with high resistance for pitting. The defects in the coating are also contributed to the erosion process. Nevertheless, the self-corrosion potential φcorr of the Q235 stainless steel is about 98 mV, which is a bit higher than that of Fe-based metallic glass coating. The current density of Q235 stainless steel is much higher than that of Fe-based metallic glass coating when the same polarization potential is given. No steady passive potential range can be observed on the anodic polarization curve. These mean that corrosion resistance of Q235 stainless steel is poor. The high resistance to pitting corrosion of the metallic glass coating is considered to be primarily determined by the formation of a homogeneous single-phase structure without grain boundaries which are sensitive to Cl- absorption [33].
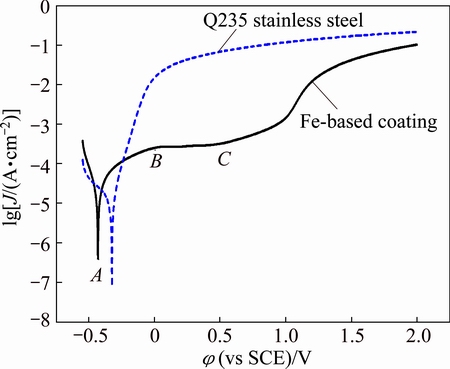
Fig. 9 Potentiodynamic polarization curves of Q235 stainless steel and Fe-based metallic glass alloy coating in 3.5% NaCl solution
4 Conclusions
1) The coating deposited by D-gun spraying is stacked by sheet-shaped particles with good deformation. The coating has a dense layered structure typical of thermally sprayed deposits, which remains the nominal chemical composition of the atomized powders. The XRD pattern shows typical broad halo peak, which indicates that there exists amorphous phase. The content of amorphous phase in the coating is about 85.54% (volume fraction). The porosity calculated by image analysis software is less than 2.1%.
2) Compared with the Q235 substrate material, the Fe-based metallic glass coating has quite stable friction coefficient and can keep a slow and steady wear loss. The coatings can achieve about 5 times higher wear resistance than the substrate material.
3) With increasing normal load, the wear loss of Fe-based metallic glass coating increases, and the average friction coefficient firstly decreases and then increases. Both adhesive wear and abrasive wear contribute to the friction, but adhesive wear serves as the dominant wear mechanism of the metallic glass coatings.
4) The corrosion resistance of the metallic glass coatings in the corrosive environments containing Cl- to induce pitting, such as 3.5% NaCl solutions, is far superior to that of Q235 stainless steel materials.
References
[1] INOUE A, TAKEUCHI A. Recent progress in bulk glassy alloys [J]. Materials Transactions, 2002, 43: 1892-1906.
[2] INOUE A. Stabilization of metallic supercooled liquid and bulk amorphous alloys [J]. Acta Materialia, 2000, 48: 279-306.
[3] SCHUH C A, HUFNAGEL T C, RAMAMURTY U. Mechanical behavior of amorphous alloys [J]. Acta Materialia, 2007, 55: 4067-4109.
[4] ZHU S L, WANG X M, QIN F X, INOUE A. A new Ti-based bulk glassy alloy with potential for biomedical application [J]. Materials Science and Engineering A, 2007, 459: 233-237.
[5] TAKEUCHI A, INOUE A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element [J]. Materials Transactions, 2005, 46: 2817-2829.
[6] LIU Dong-yan, GAO Wei, LI Zheng-wei, ZHANG Hai-feng, HU Zhuang-qi. Electro-spark deposition of Fe-based amorphous alloy coatings [J]. Materials Letters, 2007, 61: 165-167.
[7] SHEN B L, INOUE A, CHANG C. Superhigh strength and good soft-magnetic properties of (Fe, Co)-B-Si-Nb bulk glassy alloys with high glass-forming ability [J]. Applied Physics Letters, 2004, 85: 4911-4913.
[8] KIM H J, LIM K M, SEONG B G, PARK C G. Amorphous phase formation of Zr-based alloy coating by HVOF spraying process [J]. Journal of Materials Science, 2001, 36: 49-54.
[9] SHARMA P, ZHANG W, AMIYA K, KIMURA H, INOUE A. Nanoscale patterning of Zr-Al-Cu-Ni metallic glass thin films deposited by magnetron sputtering [J]. Journal of Nanoscience and Nanotechnology, 2005, 5: 416-420.
[10] JAYARAJ J, SORDELET D J, KIM D H, KIM Y C, FLEURY E. Corrosion behaviour of Ni-Zr-Ti-Si-Sn amorphous plasma spray coating [J]. Corrosion Science, 2006, 48: 950-964.
[11] BASU A, SAMANT A N, HARIMKAR S P, MAJUMDAR J D, MANNA I, DAHOTRE N B. Laser surface coating of Fe-Cr-Mo- Y-B-C bulk metallic glass composition on AISI 4140 steel [J]. Surface and Coatings Technology, 2008, 202: 2623-2631.
[12] MURTHY J K N, BYSAKH S, GOPINATH K, VENKATARAMAN B. Microstructure dependent erosion in Cr3C2v-20(NiCr) coating deposited by a detonation gun [J]. Surface and Coatings Technology, 2007, 202: 1-12.
[13] KAUR M, SINGH H, PRAKASH S. Surface engineering analysis of detonation-gun sprayed Cr3C2-NiCr coating under high-temperature oxidation and oxidation–erosion environments [J]. Surface and Coatings Technology, 2011, 206: 530-541.
[14] KAMAL S, JAYAGANTHAN R, PRAKASH S, KUMAR S. Hot corrosion behavior of detonation gun sprayed Cr3C2-NiCr coatings on Ni and Fe-based superalloys in Na2SO4-60%V2O5 environment at 900 °C [J]. Journal of Alloys and Compounds, 2008, 463: 358-372.
[15] RAJASEKARAN B, GANESH SUNDARA RAMAN S, JOSHI S V, SUNDARARAJAN G. Effect of detonation gun sprayed Cu-Ni-In coating on plain fatigue and fretting fatigue behaviour of Al-Mg-Si alloy [J]. Surface and Coatings Technology, 2006, 201: 1548-1558.
[16] ZHOU Z, WANG L, WANG F, ZHANG H, LIU Y, XU S. Formation and corrosion behavior of Fe-based amorphous metallic coatings by HVOF thermal spraying [J]. Surface and Coatings Technology, 2009, 204: 563-570.
[17] SURYANARAYANA C, INOUE A. Bulk metallic glasses [M]. New York: CRC Press, 2010.
[18] SHTANSKY D V, KIRYUKHANTSEV-KORNEEV P V, SHEVEYKO A N, KUTYREV A E, LEVASHOV E A. Hard tribological Ti-Cr-B-N coatings with enhanced thermal stability, corrosion- and oxidation-resistance [J]. Surface and Coatings Technology, 2007, 202: 861-865.
[19] ZHOU Z, WANG L, HE D Y, WANG F C, LIU Y B. Microstructure and electrochemical behavior of Fe-based amorphous metallic coatings fabricated by atmospheric plasma spraying [J]. Journal of Thermal Spray Technology, 2011, 20: 344-350.
[20] SOBOLEV V V, GUILEMANY J M. Investigation of coating porosity formation during high velocity oxy-fuel (HVOF) spraying [J]. Materials Letters, 1994, 18: 304-308.
[21] ZHOU Z, WANG L, WANG F, LIU Y. Formation and corrosion behavior of Fe-based amorphous metallic coatings prepared by detonation gun spraying [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(S3): s634-s638.
[22] SHAHA K P, PEI Y T, MARTINEZ-MARTINEZ D, de HOSSON J T M. Influence of hardness and roughness on the tribological performance of TiC/a-C nanocomposite coatings [J]. Surface and Coatings Technology, 2010, 205: 2624-2632.
[23] WU H, BAKER I, LIU Y, WU X C, MUNROEC P R, ZHANG J G. Tribological studies of a Zr-based bulk metallic glass [J]. Intermetallics, 2013, 35: 25-32.
[24] YOON S, KIM J, BAE G, KIM B, LEE C. Formation of coating and tribological behavior of kinetic sprayed Fe-based bulk metallic glass [J]. Journal of Alloys and Compounds, 2011, 509: 347-353.
[25] LI C J, OHMORI A. Relationships between the microstructure and properties of thermally sprayed deposits [J]. Journal of Thermal Spray Technology, 2002, 11: 365-374.
[26] JIN H W, PARK C G, KIM M C. Friction-induced amorphous phase formation observed in Fe-Cr-B-Ni-Mo alloy thermal spray coatings [J]. Scripta Materialia, 1999, 41: 589-595.
[27] JIN H W, RHYIM Y M, HONG S G, PARK C G. Microstructural evolution of the rapidly quenched Fe-Cr-B alloy thermal spray coatings [J]. Materials Science and Engineering A, 2001, 304: 1069-1074.
[28] HUANG D, LI R, HUANG L, JI V, ZHANG T. Fretting wear behavior of bulk amorphous steel [J]. Intermetallics, 2011, 19: 1385-1389.
[29] YOON S, KIM J, KIM B D, LEE C. Tribological behavior of B4C reinforced Fe-base bulk metallic glass composite coating [J]. Surface and Coatings Technology, 2010, 205: 1962-1968.
[30] ARCHARD J F. Contact and rubbing of flat surfaces [J]. Journal of Applied Physics, 1953, 24: 981-988.
[31] GREER A L, RUTHERFORD K L, HUTCHINGS I M. Wear resistance of amorphous alloys and related materials [J]. International Materials Reviews, 2002, 47: 87-112.
[32] MCCAFFERTY E. Validation of corrosion rates measured by the Tafel extrapolation method [J]. Corrosion Science, 2005, 47: 3202-3215.
[33] PANG S J, ZHANG T, ASAMI K, INOUE A. Synthesis of Fe-Cr-Mo-C-B-P bulk metallic glasses with high corrosion resistance [J]. Acta Materialia, 2002, 50: 489-497.
吴 宏1,兰小东1,刘 咏1,李 飞2,张卫东1,陈紫瑾1,宰雄飞1,曾 晗1
1. 中南大学 粉末冶金国家重点实验室,长沙 410083;
2. 中联重科股份有限公司 建设机械关键技术国家重点实验室,长沙 410013
摘 要:在Q235不锈钢板上利用爆炸喷涂工艺制备名义组分为Fe51.33Cr14.9Mo25.67Y3.4C3.44B1.26(摩尔分数,%)的铁基非晶涂层。采用扫描电镜、X射线衍射仪、维氏显微硬度计、摩擦磨损试验机和电化学测量方法对涂层的组织结构、相组成、硬度、摩擦磨损特性和耐腐蚀性能进行表征。显微组织结构分析结果表明,涂层组织均匀、结构致密、平均孔隙率低于2.1%。干磨条件下的磨损行为显示,在同一磨损条件下,非晶涂层的相对耐磨性为基体材料的5倍。摩擦过程中,磨损形式为粘着磨损和磨粒磨损的综合作用,在非晶涂层中粘着磨损起主导作用。涂层在质量分数为3.5% NaCl 水溶液中存在明显的钝化现象,有较低的钝化电流密度和较宽的钝化区间,呈现出优异的耐腐蚀性能。
关键词:铁基非晶涂层;爆炸喷涂;显微组织;摩擦行为;腐蚀行为
(Edited by Wei-ping CHEN)
Foundation item: Project (51301205) supported by the National Natural Science Foundation of China; Project (20130162120001) supported by the Doctoral Program of Higher Education of China; Project (K1502003-11) supported by the Changsha Municipal Major Science and Technology Program, China; Project (K1406012-11) supported by the Changsha Municipal Science and Technology Plan, China; Project (2016CX003) supported by the Innovation-driven Plan in Central South University, China
Corresponding author: Yong LIU; Tel: +86-731-88830406; E-mail: yonliu@csu.edu.cn, yonliu11@aliyun.com
DOI: 10.1016/S1003-6326(16)64271-1

