Influence of aluminium and lead on activation of magnesium as anode
来源期刊:中国有色金属学报(英文版)2010年第8期
论文作者:王乃光 王日初 彭超群 冯艳 张翔宇
文章页码:1403 - 1411
Key words:Mg alloy anode; AP65 Mg alloy; alloying; activation mechanism; electrochemistry
Abstract: Mg-6%Al, Mg-5%Pb and Mg-6%Al-5%Pb (mass fraction) alloys were prepared by induction melting with the protection of argon atmosphere. Their electrochemical activations in different electrolyte solutions were investigated by galvanostatic test. The microstructures of these alloys and their corroded surfaces were studied by scanning electron microscopy, X-ray diffractometry and emission spectrum analysis. The results show that the activation of magnesium is not prominent when only aluminum or lead exists in the magnesium matrix, but the coexistence of the two elements can increase the activation. The activation mechanism of Mg-Al-Pb alloy is dissolving-reprecipitating and there is a synergistic effect between aluminium and lead: the precipitated lead oxides on the surface of the alloy can facilitate the precipitation of Al(OH)3, which can peel the Mg(OH)2 film in the form of 2Mg(OH)2·Al(OH)3 and activate the magnesium matrix.

WANG Nai-guang(王乃光)1, WANG Ri-chu(王日初)1, PENG Chao-qun(彭超群)1,
FENG Yan(冯 艳)1, ZHANG Xiang-yu(张翔宇)2
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Changsha Research Institute of Mining and Metallurgy, Changsha 410012, China
Received 9 July 2009; accepted 18 November 2009
Abstract: Mg-6%Al, Mg-5%Pb and Mg-6%Al-5%Pb (mass fraction) alloys were prepared by induction melting with the protection of argon atmosphere. Their electrochemical activations in different electrolyte solutions were investigated by galvanostatic test. The microstructures of these alloys and their corroded surfaces were studied by scanning electron microscopy, X-ray diffractometry and emission spectrum analysis. The results show that the activation of magnesium is not prominent when only aluminum or lead exists in the magnesium matrix, but the coexistence of the two elements can increase the activation. The activation mechanism of Mg-Al-Pb alloy is dissolving-reprecipitating and there is a synergistic effect between aluminium and lead: the precipitated lead oxides on the surface of the alloy can facilitate the precipitation of Al(OH)3, which can peel the Mg(OH)2 film in the form of 2Mg(OH)2·Al(OH)3 and activate the magnesium matrix.
Key words: Mg alloy anode; AP65 Mg alloy; alloying; activation mechanism; electrochemistry
1 Introduction
Magnesium alloys have been developed as anode materials used in seawater battery system. These alloys have rapid activation, high cell voltage, wide voltage range, high power density capability, relatively light mass in unactivated state and long unactivated storage life[1-4], which make them used widely in sonobuoys, beacons, emergency equipment, balloon batteries, life jackets and cathodic protection[5-8].
Electrochemical activation is a very important performance for magnesium anode materials used in seawater battery. High activation always leads to negative stable potential, short incubation period for activation process and high power density capability. AP65 is one of these magnesium anode materials used in seawater battery with the nominal compositions of 6% Al and 5% Pb (mass fraction). It is reported that aluminium, among other activator elements, added to magnesium matrix improves surface activation and affects the corrosion resistance in NaCl solution[9-12]. It has also been demonstrated that magnesium activation and corrosion resistance will be increased with lead in the magnesium matrix even though lead is not environment friendly[13]. UDHAYAN and BHATT[14] studied the electrochemical behavior of AP65 in various concentrations of magnesium perchlorate solutions and found that its electrode/electrolyte interfacial process is determined by an activation-controlled reaction. But so far the activation mechanism of AP65 in NaCl aqueous solution is not clearly understood. The aim of this work is to study the activation mechanism in the activation process of magnesium with the coexistence of aluminium and lead. The electrochemical behavior of the magnesium alloys was investigated in the presence of Al3+ and Pb2+ ions in NaCl aqueous solutions and also with metallic Al and Pb in the magnesium matrix.
2 Experimental
Mg-6%Al, Mg-5%Pb and Mg-6%Al-5%Pb (mass fraction) alloys were prepared from ingots of pure magnesium (99.99%), pure aluminium (99.99%) and pure lead (99.99%) by induction melting at 750 °C with the protection of argon atmosphere. The molten metals were poured into stainless steel molds and cooled down to room temperature in argon atmosphere. All these magnesium alloy ingots were homogenized at 400 °C for 24 h and water quenched.
The specimens used for electrochemical measurements were encapsulated in epoxy resin with an exposed surface of 10 mm×10 mm. The surface was mechanically ground with 400 grit SiC paper. The auxiliary electrode was Pt sheet with the size of 20 mm×20 mm. Potentials were measured against a saturated hydrogen electrode (SHE). Galvanostatic tests were performed with a Potentiostat-Galvanostat (IM6ex) at a current density of 180 mA/cm2 for 1 000 s. The electrolyte solutions were 3.5% NaCl solution, 3.5% NaCl+0.5 mol/L AlCl3 solution and 3.5% NaCl+PbCl2 saturated solution as Al3+ and Pb2+ ions could be obtained from the ionization of AlCl3 and PbCl2 in water, respectively, which did not bring other negative ions but only Cl- ions. The volume of the electrolyte solution was 80 mL. The test temperatures were 25 °C and 80 °C. As the PbCl2 was insoluble in water at room temperature, the temperature of 80 °C was chosen in order to obtain the PbCl2 saturated aqueous solution. At least, three specimens were used for each galvanostatic test.
The chemical compositions of the specimens and the electrolyte solutions after galvanostatic tests were obtained by atomic absorption spectrometry analysis. The microstructures and the corroded surface of each specimen were examined by scanning electron microscopy (SEM). The phases of the corroded products were investigated by X-ray diffractometry (XRD) and emission spectrum analysis (ESA).
3 Results and discussion
3.1 Electrochemical activations of magnesium anodes
Fig.1 shows the galvanostatic potential-time curves of Mg-6%Al, Mg-5%Pb and Mg-6%Al-5%Pb alloys in 3.5% NaCl solution at 25 °C. The potential-time response obtained at 180 mA/cm2 demonstrates that a very active behavior is attained. According to Fig.1, the curve of Mg-5%Pb alloy polarizes severely and its mean potential is -1.154 V that is the most positive among three specimens, indicating that the electrochemical activation of Mg-5%Pb alloy is the worst and the depolarization of lead to magnesium is weak. The electrochemical activation of Mg-6%Al alloy is better than that of Mg-5%Pb alloy. The stable potential of Mg-6%Al alloy is -1.325 V and its depolarization is very good. This indicates that aluminium has better depolarization effect than lead. Mg-6%Al-5%Pb alloy has the best electrochemical activation as well as the most negative stable potential(-1.400 V). So, it can be concluded from Fig.1 that the magnesium activation produced by aluminium is stronger than that produced by lead but the effect of activation is not prominent when only aluminium or lead exists in the magnesium matrix. The coexistence of the two elements in the magnesium

Fig.1 Galvanostatic curves of Mg-6%Al, Mg-5%Pb and Mg-6%Al-5%Pb alloys at current density of 180 mA/cm2 in 3.5% NaCl solution at 25 °C
matrix can increase this effect.
Fig.2 shows the galvanostatic polarization curves of Mg-5%Pb alloys in 3.5% NaCl solution and 3.5% NaCl +0.5 mol/L AlCl3 solution at 25 °C. As Al3+ ions are added into the NaCl solution, the electrochemical activation and the depolarization effect of Mg-5%Pb alloy increase rapidly, leading to its stable potential of -1.404 V in 3.5% NaCl+0.5 mol/L AlCl3 solution. So, it can be concluded from Fig.2 that Mg-5%Pb alloy can be activated in NaCl solutions containing Al3+ ions which can also improve its depolarization effect.

Fig.2 Galvanostatic curves of Mg-5%Pb alloys at current density of 180 mA/cm2 in 3.5% NaCl solution and 3.5% NaCl +0.5 mol/L AlCl3 solution at 25 °C
Fig.3 shows the galvanostatic polarization curves of Mg-6%Al alloys in 3.5% NaCl solution and 3.5% NaCl + PbCl2 saturated solution at 80 °C. As PbCl2 is insoluble in water at room temperature, the mixture of PbCl2 and water is heated up to 80 °C in order to obtain the clear solution above the PbCl2 sedimentation. This solution is used as the electrolyte and the test temperature is 80 °C.

Fig.3 Galvanostatic curves of Mg-6%Al alloys at current density of 180 mA/cm2 in 3.5% NaCl solution and 3.5% NaCl + PbCl2 saturated solution at 80 °C
According to Fig.3, the electrochemical activation of Mg-6%Al alloy in 3.5% NaCl + PbCl2 saturated solution is stronger than that in 3.5% NaCl solution as the mean potential of Mg-6%Al alloy in 3.5% NaCl + PbCl2 saturated solution is -1.334 V while that in 3.5% NaCl solution at 80 °C is -1.325 V. But the polarization curve of Mg-6%Al alloy in 3.5% NaCl + PbCl2 saturated solution is not smooth and vibrates acutely. Even at the end of the test its potential exceeds that in 3.5% NaCl solution. The reason might be that the activation and depolarization effect of Pb2+ ions in the NaCl solutions
are not distinct or worse than that of Al3+ ions for Mg-5%Pb alloy.
3.2 Elemental distribution of magnesium anode
Fig.4 shows the SEM image of Mg-6%Al-5%Pb alloy and its corresponding elemental distributions of magnesium and the alloying elements. The micrographs of Mg-6%Al and Mg-5%Pb alloys are similar to that of Mg-6%Al-5%Pb alloy. According to Fig.4, Mg-6%Al-5%Pb alloy is solid solution under the experimental condition and the elements of aluminium and lead distribute homogeneously in the magnesium matrix.
3.3 Chemical compositions of magnesium anodes and electrolyte solutions after galvanostatic tests
Table 1 lists the chemical compositions of Mg-6%Al, Mg-5%Pb and Mg-6%Al-5%Pb alloys. Table 2 lists the chemical compositions of electrolyte solutions after galvanostatic tests. All these chemical compositions were determined by atomic absorption spectrometry. In order to investigate the activation mechanism of the magnesium alloys, only Mg, Al and Pb were considered while other elements were ignored. According to Table 2, the contents of Mg2+ ions in solutions 1-3 are almost the same while that in solution 4 is much higher than those in solutions 1-3. Solution 1 is the solution after galvanostatic test of Mg-6%Al alloy in 3.5% NaCl solution; solution 2 is the solution after

Fig.4 SEM image (a) and corresponding elemental distributions of magnesium(b), aluminium (c) and lead(d) of Mg-6%Al-5%Pb alloy
galvanostatic test of Mg-5%Pb alloy in 3.5% NaCl solution; solution 3 is the solution after galvanostatic test of Mg-6%Al-5%Pb alloy in 3.5% NaCl solution; solution 4 is the solution after galvanostatic test of Mg-5%Pb alloy in 3.5% NaCl+0.5 mol/L AlCl3 solution. The content of Al3+ ions in solution 3 is only 0.4 mg/L which is lower than that of solution 1, indicating that Al3+ ions can precipitate easily on the surface of magnesium alloy with the existence of lead. The contents of Pb2+ ions in solution 3 and solution 4 are higher than those in solution 2. The reason might be that with the existence of aluminium in the magnesium matrix and the electrolyte solutions, the main corroded products, Mg(OH)2, can peel off easily from the surface of alloys, leading to the desquamation of precipitated lead. According to Table 2, the contents of Pb2+ ions in these solutions are still low and cannot be very harmful to the environment.
Table 1 Chemical compositions of Mg-6%Al, Mg-5%Pb and Mg-6%Al-5%Pb alloys (mass fraction, %)

Table 2 Chemical compositions of electrolyte solutions after galvanostatic tests (mg/L)

3.4 Corroded products of magnesium anodes after galvanostatic test
Fig.5(a) shows the XRD pattern of the corroded products of Mg-6%Al alloy after galvanostatic test in 3.5% NaCl solution at 25 °C. The corroded products are Mg(OH)2 and 2Mg(OH)2·Al(OH)3, indicating that Al3+ ions precipitate on the surface of Mg-6%Al alloy in the form of Al(OH)3 and peel the Mg(OH)2 film in the form of 2Mg(OH)2·Al(OH)3. Fig.5(b) shows the Mg-6%Al alloy after galvanostatic test in 3.5% NaCl+PbCl2 saturated solution at 80 °C. Metallic lead exists in the corroded products, indicating that Pb2+ ions precipitate on the surface of the alloy in the form of metallic lead when the concentration of Pb2+ ions in the solution is high. The metallic lead on the surface of the alloy cannot peel off easily. Besides, Mg(OH)2, PbO and many aluminium hydroxides also exist in the corroded products.

Fig.5 XRD patterns of corroded products of Mg-6%Al alloy after galvanostatic test in 3.5% NaCl solution at 25 °C (a), Mg-6%Al alloy after galvanostatic test in 3.5% NaCl + PbCl2 saturated solution at 80 °C (b), Mg-5%Pb alloy after galvanostatic test in 3.5% NaCl solution at 25 °C (c) and Mg-6%Al-5%Pb alloy after galvanostatic test in 3.5% NaCl solution at 25 °C (d)
These corroded products cannot peel off easily, leading to the weak activation of magnesium anode. Fig.5(c) shows the XRD pattern of the corroded products of Mg-5%Pb alloy after galvanostatic test in 3.5% NaCl solution at 25 °C. The corroded products are mainly Mg(OH)2. Besides, there are also many sorts of lead oxides in the corroded products, indicating that Pb2+ ions precipitate on the surface of magnesium alloy in the form of lead oxides when the concentration of Pb2+ ions in the solution is low. These corroded products cannot peel off easily and the activation of magnesium anode is not prominent. Fig.5(d) shows the XRD pattern of the corroded products of Mg-6%Al-5%Pb alloy after galvanostatic test in 3.5% NaCl solution at 25°C. It can be seen that its corroded products are similar to those of Mg-6%Al alloy after galvanostatic test in 3.5% NaCl solution. The difference is that PbO2 exists in the corroded products of Mg-6%Al-5%Pb alloy.
Fig.6 shows the corroded surface morphologies and corresponding EDS results of Mg-6%Al alloy after galvanostatic test in 3.5% NaCl solution. It can be seen from Figs.6(a) and (b) that the corroded products are thin and have many cracks, therefore, they cannot protect the surface of the alloy and the activation can be improved. According to the XRD pattern in Fig.5(a), the corroded products are mainly Mg(OH)2 and 2Mg(OH)2·Al(OH)3. According to the EDS results in Fig.6(c), the content of aluminium on the corroded surface of Mg-6%Al alloy is only 0.59% (mass fraction), indicating that Al3+ ions cannot precipitate on the surface of magnesium alloy easily without the existence of lead. So, the content of Al3+ ions in the electrolyte solution is high, which can be confirmed by the chemical compositions of solution 1 listed in Table 2. The Al3+ ions precipitate in the form of Al(OH)3 and can peel the corroded products easily in the form of 2Mg(OH)2·Al(OH)3, that is the reason why the corroded products on the surface of magnesium alloy are thin and have many cracks and the activation of Mg-6%Al alloy in 3.5% NaCl solution is strong.
Fig.7 shows the corroded surface morphologies and corresponding EDS results of Mg-5%Pb alloy after galvanostatic test in 3.5% NaCl solution. It can be seen from Figs.7(a) and (b) that the corroded products are very thick and compact, so they cannot peel off from the surface of the alloy easily, making the galvanostatic polarization curve of Mg-5%Pb alloy polarized severely. According to the XRD pattern in Fig.5(c), most of the corroded products are Mg(OH)2 and many sorts of lead oxides. According to the EDS results in Fig.7(c), the content of lead on the corroded surface of the Mg-5%Pb alloy is 9.35% (mass fraction), which exceeds that in the magnesium matrix. So, it can be concluded that a great deal of Pb2+ ions precipitate on the surface of the alloy in the form of lead oxides during the galvanostatic test, leading to the low content of Pb2+ ions in solution 2 (see Table 2). But the precipitated lead oxides cannot peel the corroded products easily, and this is the reason why the corroded products are very thick and compact and the

Fig.6 SEM image and its corresponding EDS spectrum of corroded surface of Mg-6%Al alloy after galvanostatic test in 3.5% NaCl solution

Fig.7 SEM image and its corresponding EDS spectrum of corroded surface of Mg-5%Pb alloy after galvanostatic test in 3.5% NaCl solution
activation of Mg-5%Pb alloy in 3.5% NaCl solution is weak.
Fig.8 shows the corroded surface morphologies and corresponding EDS results of Mg-6%Al-5%Pb alloy after galvanostatic test in 3.5% NaCl solution. It can be seen from Figs.8(a) and (b) that the porous corroded products are thin and have wide circular cavity with many cracks, so they can peel off from the surface of the alloy easily and the activation can be improved. According to the XRD pattern in Fig.5(d), the corroded products are M(OH)2, 2Mg(OH)2·Al(OH)3 and PbO2. According to the EDS results in Fig.8(c), the content of aluminium on the corroded surface of Mg-6%Al-5%Pb alloy is 1.28% (mass fraction), which is higher than that of Mg-6%Al alloy, indicating that it is easy for the Al3+ ions to precipitate on the surface of magnesium alloy in the form of Al(OH)3 with the existence of lead oxides. It can also be seen from Fig.8(c) that the content of lead on the corroded surface is 5.40% (mass fraction), which is lower than that of Mg-5%Pb alloy. The reason might be that with the existence of the precipitated Al(OH)3, the Mg(OH)2 film can peel off easily in the form of 2Mg(OH)2·Al(OH)3 from the surface of the alloys, leading to the desquamation of precipitated lead oxides. That is why the corroded products are very thin and have many cracks and the activation of Mg-6%Al-5%Pb alloy is stronger than that of Mg-6%Al alloy.
Fig.9 shows the corroded surface morphologies and corresponding EDS results of Mg-5%Pb alloy after the galvanostatic test in 3.5% NaCl+0.5mol/L AlCl3 solution. It can be seen from Figs.9(a) and (b) that the corroded products are the thinnest among all specimens and have many cracks, therefore, they cannot protect the surface of the alloy, leading to the high activation of Mg-5%Pb alloy in 3.5% NaCl+0.5mol/L AlCl3 solution. According to the EDS results in Fig.9(c), the content of Al3+ ions precipitated on the surface of the alloy from the electrolyte is 6.85% (mass fraction), which is the highest among all the specimens. It can also be seen from Fig.9(c) that the content of lead on the corroded surface is only 0.70% (mass fraction), which is much lower than that of Mg-6%Al-5%Pb alloy. So, it can be concluded that with lead in the magnesium matrix, Al3+ ions in the electrolyte solution can precipitate on the surface of the alloy easily and peel the corroded products, leading to the desquamation of Mg(OH)2 film and precipitated lead oxides as well as the strongest activation of magnesium alloy.
Fig.10(a) shows the scanning electron micrograph of cross-section of Mg-6%Al-5%Pb alloy after galvanostatic test in 3.5% NaCl solution. It can be seen that the corroded products distribute on the surface of the alloy. Figs.10(b) and (c) show its corresponding elemental linear distribution from point A to point B. According to Fig.10(b) and the XRD pattern in Fig.5(d), most of the corroded products are Mg(OH)2. The

Fig.8 SEM image and its corresponding EDS spectrum of corroded surface of Mg-6%Al-5%Pb alloy after galvanostatic test in 3.5% NaCl solution

Fig.9 SEM image and its corresponding EDS spectrum of corroded surface of Mg-5%Pb alloy after galvanostatic test in 3.5% NaCl+ 0.5 mol/L AlCl3 solution
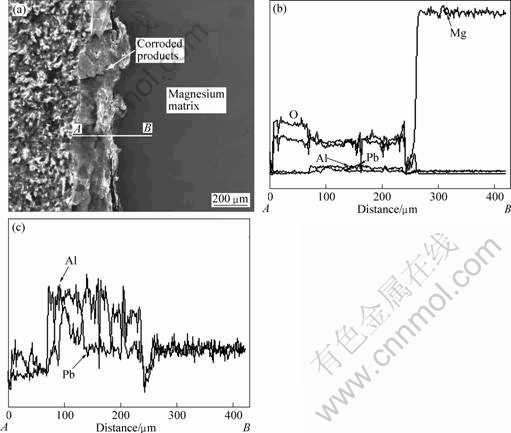
Fig.10 SEM image and its corresponding elemental linear distribution of cross-section of Mg-6%Al-5%Pb alloy after galvanostatic test in 3.5% NaCl solution
contents of aluminium and lead in corroded products are lower than those of Mg(OH)2. The Mg(OH)2 surface film on the magnesium alloy is probably formed by a precipitation reaction when Mg2+ ion concentration on the corroded surface exceeds the solubility limit[15-16]. Fig.10(c) shows the distribution of aluminium and lead. Aluminium and lead enrichments are found at the metal/corrosion layer interface, possibly produced by preferential magnesium dissolution during the galvanostatic test[17]. The content of aluminium at the metal/corrosion layer interface is higher than that of lead, indicating that lead can dissolve into the solution more easily than aluminium. This is the reason why the contents of Pb2+ ions in the solution and the precipitated lead on the corroded surface are higher than those of aluminium.
The activation reaction equations can be written as follows:
Mg(Al,Pb)→Mg2++Al3++Pb2++7e (1)
2H2O+2e→H2+2OH- (2)
Mg2++2OH-→Mg(OH)2 (3)
Pb2++yOH-→PbOx+ nH2O (4)
Al3++3OH-→Al(OH)3 (5)
Pb2+ ions precipitate on the surface of the alloy in the form of many sorts of lead oxides when the concentration of Pb2+ ions in the solution is low. These lead oxides are shown as PbOx. With the help of PbOx, Al3+ ions can precipitate easily in the form of Al(OH)3 on the surface of the alloy, peel the Mg(OH)2 film in the form of 2Mg(OH)2·Al(OH)3 and activate the magnesium matrix.
4 Conclusions
1) The activation of magnesium is not prominent when only aluminium or lead exists in the magnesium matrix as Mg-6%Al alloy has the stable potential of -1.325 V and Mg-5%Pb alloy has the mean potential of -1.154 V in 3.5% NaCl solution. The coexistence of the two elements can increase this effects as Mg- 6%Al-5%Pb alloy has the stable potential of -1.400 V in 3.5% NaCl solution and Mg-5%Pb alloy has the stable potential of -1.404 V in 3.5% NaCl +0.5 mol/L AlCl3 solution.
2) The activation mechanism of Mg-Al-Pb alloy is dissolving-reprecipitating and there is a synergistic effect between aluminium and lead: aluminium is the dominating element controlling the activation process of magnesium. The activation produced by lead is not prominent, but the precipitated lead oxides on the surface of the alloy can facilitate the precipitation of Al(OH)3, which can peel the Mg(OH)2 film in the form of 2Mg(OH)2·Al(OH)3 and activate the magnesium matrix.
References
[1] RENUKA R. Influence of allotropic modifications of surphur on the cell voltage in Mg-CuI(S) seawater activated batter [J]. Materials Chemistry and Physics, 1999, 59(1): 42-48.
[2] FENG Yan, WANG Ri-chu, YU Kun, PENG Chao-qun, LI Wen-xian. Influence of Ga and Hg on microstructure and electrochemical corrosion behavior of Mg alloy anode materials [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(6): 1363-1366.
[3] RENUK A R. AgCl and Ag2S as additives to CuI in Mg-CuI seawater activated batteries [J]. Journal of Applied Electrochemistry, 1997, 27(12): 1394-1397.
[4] CAO Dian-xue, WU Lin, WANG Gui-ling, LU Yan-zhou. Electrochemical oxidation behavior of Mg-Li-Al-Ce-Zn and Mg-Li-Al-Ce-Zn-Mn in sodium chloride solution [J]. Journal of Power Sources, 2008, 183(2): 799-804.
[5] FENG Yan, WANG Ri-chu, PENG Chao-qun, WANG Nai-guang. Influence of Mg21Ga5Hg3 compound on electrochemical properties of Mg-5%Hg-5%Ga alloy [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(1): 154-159.
[6] FIRA S S, KIBL L, LIW L W. Water-activated disposable and long shelf-life microbatteries [J]. Sensors and Actuators A, 2004, 111: 79-86.
[7] VENKATESARA R K. Performance evaluation of Mg-AgCl batteries for under water propulsion [J]. Defense Science Journal, 2001, 5(2): 161-170.
[8] FIDEL G M, JUAN M F, RUBEN D R, GENESCA J. Electrochemical study on magnesium anodes in NaCl and CaSO4-Mg(OH)2 aqueous solutions [J]. Electrochimica Acta, 2006, 51: 1820-1830.
[9] SHI Zhi-ming, SONG Guang-ling, ATRENS A. Corrosion resistance of anodised single-phase Mg alloys [J]. Surface and Coatings Technology, 2006, 201: 492-503.
[10] PARDO A, MERION M C, COY A E, VIEJO F, ARRABAL R, FELIUJR S. Influence of microstructrue and composition on the corrosion behaviour of Mg/Al alloys in chloride media [J]. Electrochimica Acta, 2008, 53: 7890-7902.
[11] GU Xue-nan, ZHENG Yu-feng, CHENG Yan, ZHONG Sheng-ping, XI Ting-fei. In vitro corrosion and biocompatibility of binary magnesium alloys [J]. Biomaterials, 2009, 30: 484-498.
[12] SHI Zhi-ming, SONG Guang-ling, ATRENS A. Influence of the β phase on the corrosion performance of anodised coatings on magnesium-aluminium alloys [J]. Corrosion Science, 2005, 47: 2760-2777.
[13] CANDAN S, UNAL M, TURKMEN M, KOC E, TUREN Y, CANDAN E. Improvement of mechanical and corrosion properties of magnesium alloy by lead addition [J]. Materials Science and Engineering A, 2009, 501: 115-118.
[14] UDHAYAN R, BHATT D P. On the corrosion behavior of magnesium and its alloys using electrochemical techniques [J]. Journal of Power Sources, 1996, 63(1): 103-107.
[15] ZHAO Ming-chun, SCHMUTZ P, BRUNNER S, LIU Ming, SONG Guang-ling, ATRENS A. An exploratory study of the corrosion of Mg alloys during interrupted salt spray testing [J]. Corrosion Science, 2009, 51: 1277-1292.
[16] ZHAO Ming-chun, LIU Ming, SONG Guang-ling, ATRENS A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41 [J]. Corrosion Science, 2008, 50: 3168-3178.
[17] PARDO A, MERION M C, COY A E, ARRABAL R, VIEJO F, MATYKINA E. Corrosion behaviour of magnesium/aluminium alloys in 3.5 wt.% NaCl [J]. Corrosion Science, 2008, 50: 823-834.
Corresponding author: WANG Ri-chu; Tel/Fax: +86-731-88836638; E-mail: wrc@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(09)60312-5

