
Effect of silicon on phase selection of ternary compounds during solidification of ZA84 magnesium alloy
ZHANG Chun-xiang1,2, GUAN Shao-kang3, LIU Tao2, LI Shao-hua2, LIU Zhong-xia1
1. Postdoctoral Research Station of Physics, Zhengzhou University, Zhengzhou 450001, China;
2. School of Materials Science and Engineering, Zhengzhou University, Zhengzhou 450001, China;
3. Graduate School, Zhengzhou University, Zhengzhou 450001, China
Received 15 March 2010; accepted 21 June 2010
Abstract: Effect of silicon on the phase selection between τ phase (Mg32(Al, Zn)49) and φ phase (Al2Mg5Zn2) in ZA84 (Mg-8Zn-4Al-0.3Mn) magnesium alloy produced by steel mold cast was studied using X-ray diffractometer, scanning electron microscope and differential scanning calorimeter. The results show that with increasing Si addition in ZA84 alloy, the liquidus temperatures of the alloys and the solidification temperature ranges decrease. The ternary compound in ZA84 alloy is mainly τ phase and a little φ phase. When adding Si to ZA84 alloy, the preferential precipitation sequence of the ternary compounds changes, φ phase preferentially forms, whereas τ phase is suppressed. The solidification kinetics study of phase selection indicates that there is a critical degree of undercooling of the melt. If the undercooling exceeds the critical degree, τ phase preferentially forms while φ phase is suppressed; otherwise, φ phase preferentially forms while τ phase is suppressed.
Key words: ZA84 alloy; silicon; phase selection; solidification kinetics
1 Introduction
At present, the Mg-Al system alloys, such as AZ91 and AM60, are most extensively used in automobile but their properties steeply fall when the work temperature is above 393 K. ZA84 alloy is a promising heat-resistant magnesium alloy for automobile application, containing no rare earths, it is strengthened mostly by ternary intermetallic compound τ phase (Mg32(Al, Zn)49) and a little φ phase (Al2Mg5Zn2)[1-2]. However, it is urgent to improve the castability of the alloy. Previous study indicated that the fluidity of ZA84 alloy was remarkably improved by the addition of silicon[3], and the Chinese script-type Mg2Si formed at low cooling rate can be modified by the additions of Ca[4], Sr[5] or AlP[3], etc. During the solidification of Si-alloying ZA84 alloy, the preferential precipitation sequence of τ phase and φ phase changes (called phase selection). Few studies were carried out on the phase selection of ternary intermetallic compounds in magnesium alloys. The aim of this work is to reveal the essential of the phase selection, which is significant for the strengthening-phase selection precipitation in this kind of alloys.
2 Experimental
The compositions of four experimental alloys were analyzed by ultraviolet-visible spectrophotometer as listed in Table 1. The alloys were prepared using the following materials: commercially pure Mg, Al and Zn (>99.9%), Al-50%Si and Al-20%Mn master alloys. The melting was carried out in an electric resistance furnace and protected by CO2+0.5%SF6 (volume fraction). After being refined by JDMJ flux, the melt was poured into a steel mold preheated at 250 °C, the size of test bars is d 25 mm×100 mm. The phases of the alloys were identified by X-ray diffraction spectroscopy using Cu Kα radiation. The microstructure analysis was carried out using a JSM-5801LV scanning electron microscopy. The chemical compositions of the micro-region were determined by Oxford energy X-ray dispersive spectrometer attached to SEM. The solidification process of the alloys was analyzed by Labsys differential
Table 1 Compositions of experimental alloys (mass fraction, %)
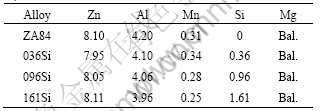
scanning calorimetry at a heating rate of 5 K/min.
3 Results and discussion
3.1 Phase selection of ternary compounds
The XRD pattern in Fig.1(a) shows that τ phase is the main ternary compound in ZA84 alloy, where a little φ phase exists; in 161Si alloy (Fig.1(b)), the ternary compound is only φ phase, and the peaks of τ phase can not be found. There is also Mg2Si phase in 161Si alloy due to Si addition. The results reveal that Si addition to ZA84 alloy facilitates the formation of φ phase and suppresses τ phase, namely, phase selection occurs. Furthermore, the XRD analyses of 096Si and 036Si alloys indicate that[3], φ phase is the main ternary compound and τ phase is little in 096Si alloy, while the volume fractions of τ and φ phases in 036Si alloy is nearly the same as ZA84
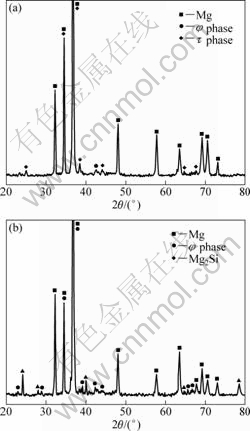
Fig.1 XRD patterns of as-cast alloys: (a) ZA84; (b) 161Si
alloy, because a little amount of Si is not enough to influence the phase selection.
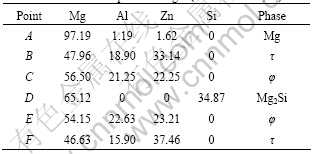
Fig.2 shows the SEM morphologies of the alloys. The chemical composition of the phases in Fig.2 is given in Table 2. Fig.2(a) shows that the bone-type τ phase distributes along grain boundary; in Fig.2(b), the bone-type phase is φ and there is beehive or fish-bone shape loose microstructure in φ, which indicates τ phase is more compact than φ phase. Moreover, there is Chinese script-type Mg2Si phase in 096Si alloy. The SEM and EDS results also verify the phase selection between τ and φ phases caused by Si addition to ZA84 alloy.
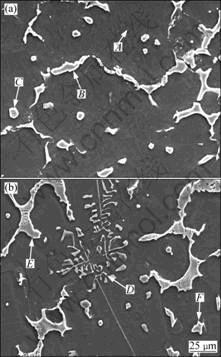
Fig.2 SEM images of alloys: (a) ZA84; (b) 096Si
Table 2 EDS results of points in Fig.2 (molar fraction, %)
3.2 DSC analysis on solidification process of alloys
Fig.3 shows the heating DSC curves of the experimental alloys. The present interpretation of these thermal curves is based on the Mg-Zn-Al ternary diagram[6-7], since the Mg-Zn-Al-Si phase diagram is not available. This procedure is justified by the fact that a
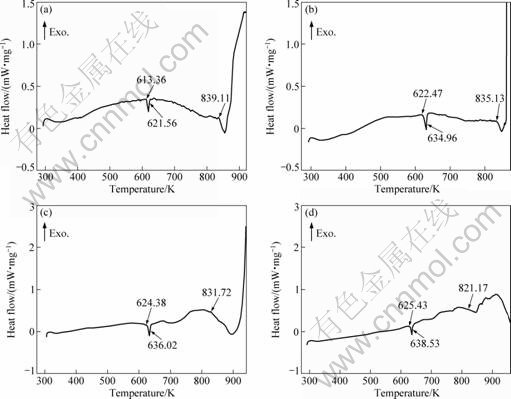
Fig.3 DSC curves of alloys at heating rate of 5 K/min: (a) ZA84; (b) 036Si; (c) 096Si; (d) 161Si
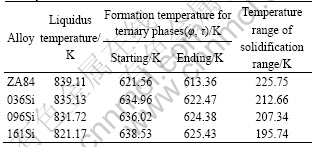
minor amount of Si is introduced in ZA84 alloy and Si does not take part in the reactions of ternary phases τ and φ. The first thermal valley in DSC curves in Fig.3 is the formation of τ or φ phase, and the second corresponds to α-Mg reaction. The character temperature values of the alloys are listed in Table 3. It can be seen that with increasing Si addition in ZA84 alloy, the liquidus temperatures of the alloys fall, the starting and ending temperatures of the ternary phases reaction increase, and the solidification temperature ranges decrease.
It is worthy pointing out that the holding and pouring temperatures of all the melts are the same in the course of test-bar preparation, which are 1 043 K and 1 008 K, respectively. Since the liquidus temperatures of Si-alloying ZA84 alloys are lower than that of ZA84 alloy (Table 3), the superheating degree of Si-alloying
Table 3 Temperatures of liquidus and ternary phase formation of alloys
ZA84 alloys is higher than that of ZA84 alloy, the cooling rate during solidification of Si-alloying ZA84 alloys is slower than that of ZA84 alloy, which also results from the effect of crystallization latent heat of Mg2Si phase. The slower cooling rate results in the reduction of undercooling degree at every phase- precipitation stage during the solidification of Si-alloying ZA84 alloys.
3.3 Solidification kinetics analysis on phase selection of ternary compounds
Phase selection exists at every phase-precipitation stage during solidification. The formation predominance of a phase during solidification depends on its advantageous nucleation or growth rate. In general, phase selection is not only associated with the relative nucleation rate but also the relative growth rate. But when the growth characters of the two phases are similar, the competition of nucleation rates plays a decisive role in phase selection[8-9].
Since both τ phase and φ phase are Mg-Zn-Al ternary intermetallic compounds with complicated crystal structure and similar growth characters, the phase selection between φ and τ mainly depends on their nucleation condition[10-12]. The formulas of nucleation rate vs undercooling degree need to be calculated in order to decide the preferential precipitation sequence of φ and τ.
The nucleation rate of τ and φ in the undercooling melt is calculated using the mathematical model of heterogeneous nucleation[13-15]:
 (1)
(1)
where JS is the nucleation rate of heterogeneous nucleation; Nn is the quantity of latent heterogeneous nucleation particles in unit volume melt; da is the average atom diameter of nucleation solid phase; XL,eff is the effective alloy concentration, and its value is always less than 1. XL,eff approaching 1 shows the composition of nucleation solid phase approaches that of the melt. For binary alloy, XL,eff=XL,A/XS,A, when A is rich in crystal nucleus, here XL,A presents the atom concentration of element A at the solid/liquid interface of crystal nucleus. XL,eff=XL,B/XS,B when B is rich in crystal nucleus[16]. Since the effective alloy concentration of ternary alloy is not available, the average values of the effective alloy concentrations of Zn and Al are used as the effective alloy concentrations of φ or τ; θ is the contact angle of heterogeneous nucleation[17]; kB is the Boltzmann invariance; DL is the diffusion coefficient of solute atom in melt, that is
 (2)
(2)
The melt viscosity η(T) is expressed as
η(T)=10-3.3exp[3.34TL/(T-Tg)] (3)
where TL is the liquidus temperature; Tg is the ideal glass-transformation temperature, Tg=(0.5-0.65)TL; σm is the mole solid/liquid interface energy
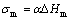 (4)
(4)
where α is the solid structural parameter; ΔHm is the mole fusion enthalpy, ΔHm=TmΔSm, where Tm is the liquidus temperatures of φ phase and τ phase, ΔSm is the fusion entropy of compound phase.
In Eq.1, a0 is the atom leap distance; f(θ) is the heterogeneous nucleation parameter; Rg is the gas constant; T is the undercooling temperature (absolute temperature).
There is
 (5)
(5)
where ΔG* is the critical nucleation energy; Tr=T/Tm, Tm is the melting point of solid phase; ΔTr is the no-dimension undercooling degree and ΔTr=1-Tr.
The chosen and calculated results of the parameters are given in Table 4.
The nucleation rates of τ phase and φ phase in the undercooling melt at a certain temperature can be worked out by combining Eqs.(1)-(5), in which the thermodynamic parameters of τ and φ phases are replaced by the values in Table 4.
Fig.4 shows the relationship of τ or φ nucleation rate with temperature in the undercooling melt. The results indicate that there is a critical degree of undercooling the ZA84 alloy melt (Fig.4(a)). If the undercooling exceeds the critical degree, the nucleation rate of τ phase is higher than that of φ phase, and τ phase preferentially forms while φ phase is suppressed; otherwise, φ phase preferentially forms while τ phase is suppressed.
The competition result of nucleation is that the phase with faster nucleation rate preferentially forms, in other words, during the solidification of ZA84 alloy, the undercooling (relative to the starting temperature of ternary phase reaction) exceeds the critical degree, so τ phase preferentially forms while φ phase is suppressed, which makes the volume fraction of τ phase is higher than that of φ phase in ZA84 alloy. Finally, the main ternary compound is τ phase in ZA84. The critical degree of undercooling is about 9 K (622-613 K), as shown in Fig.4(a).
096Si alloy is analyzed to explain the relationship of τ or φ nucleation rate with the temperature of Si-alloying ZA84 alloys in undercooling melt, as shown in Fig.4(b). The results show that there is a critical degree of the undercooling of 096Si alloy melt, which is similar to ZA84 alloy. The critical degree is about 12 K (636-624 K).
The results above indicate that the undercooling degree of 096Si alloy melt is less than that of ZA84, and the undercooling is smaller than the critical degree when ternary phases (τ or φ) precipitate during 096Si alloy solidification, so φ phase preferentially forms while τ phase is suppressed. Finally, the main ternary compound is φ phase in 096Si alloy.
Actually, since τ (Mg32(Al, Zn)49) phase is a typical 1/1 approximant of Mg-Zn-Al icosahedral quasicrystal (called I phase)[10, 18], if the undercooling degree of the melt is further increased, quasicrystal phase is obtained. For instance, a large quantity of I phase and a little τ
Table 4 Thermodynamic parameters of τ and φ phases in alloys
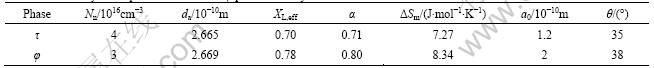
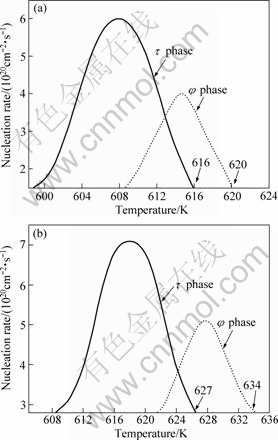
Fig.4 Steady-state nucleation rate of τ and φ phases vs temperature in alloy melt: (a) ZA84; (b) 096Si
phase form in die-cast thin-wall diecasting (about thickness 3 mm) casting with 096Si alloy.
4 Conclusions
1) The liquidus temperatures of the alloys and the solidification temperature ranges decrease with increasing Si addition in ZA84 alloy.
2) In ZA84 alloy with steel mold cast, τ phase is the main ternary compound while φ phase is little. Si addition in ZA84 alloy facilitates φ phase while suppresses τ phase.
3) The solidification kinetics study of phase selection indicates that there is a critical degree of undercooling of the melt. If the undercooling exceeds the critical degree, τ phase preferentially forms while φ phase is suppressed; otherwise, φ phase preferentially forms while τ phase is suppressed.
References
[1] ZHANG Jing, GUO Zheng-xiao, PAN Fu-sheng, LI Zhong-sheng, LUO Xiao-dong. Effect of composition on the microstructure and mechanical properties of Mg-Zn-Al alloys [J]. Materials Science and Engineering A, 2007, 456(1/2): 43-51.
[2] ZHANG Z, TREMBLAY R, DUBE D. Microstructure and mechanical properties of ZA104 (0.3~0.6Ca) die-casting magnesium alloys [J]. Materials Science and Engineering A, 2004, 385(6): 286-291.
[3] ZHANG Chun-xiang, GUAN Shao-kang, ZHAO Hong-liang. Effects of silicon and AIP on microstructure and properties of Mg-8Zn-4Al-0.3Mn alloy [J]. Materials Science Forum, 2005, 488-489(6): 197-200.
[4] YUAN Guang-yin, LIU Man-ping, DING Wen-jiang, INOUE A. Microstructure and mechanical properties of Mg-Zn-Si-based alloys[J]. Materials Science and Engineering A, 2003, 357(1/2): 314-320.
[5] YANG Ming-bo, PAN Fu-sheng, SHEN Jia, BAI Liang. Comparison of Sb and Sr on modification and refinement of Mg2Si phase in AZ61-0.7Si magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(2): 287-292.
[6] ZHANG Z, TREMBLAY R, DUBE D. Microstructure and creep resistance of Mg-10Zn-4Al-0.15Ca permanent moulding alloy [J]. Materials Science and Technology, 2002, 18(4): 433-437.
[7] ZHANG Z, TREMBLAY R, DUBE D, COUTURE A. Solidification microstructure of ZA102, ZA104 and ZA106 magnesium alloys and its effects on creep deformation [J].Canadian Metallurgical Quarterly, 2000, 39(4): 503-512.
[8] HERLACH D M, GAO J, HOLLAND-MORITZ D, VOLKMANN T. Nucleation and phase-selection in undercooled melts [J]. Materials Science and Engineering A, 2004, 375-377(15): 9-15.
[9] BALDISSIN D, BARICCO M, BATTEZZATI L. Microstructures in rapidly solidified AISI 304 interpreted according to phase selection theory [J]. Materials Science and Engineering A, 2007, 449-451: 999-1002.
[10] SUN W, LINCOLN F J, SUGIYAMA K, HIRAGA K. Structure refinement of (Al, Zn)49Mg32-type phases by single-crystal X-ray diffraction [J]. Materials Science and Engineering A, 2000, 294-296(15): 327-330.
[11] BOURGEOIS L, MUDDLE B C, NIE J F. The crystal structure of the equilibrium φ phase in Mg-Zn-Al casting alloys [J]. Acta Materialia, 2001, 49(14): 2701-2711.
[12] ZHANG Jing, ZUO Ru-lin, CHEN You-xing, PAN Fu-sheng, LUO Xiao-dong. Microstructure evolution during homogenization of τ-type Mg-Zn-Al alloy [J]. Journal of Alloys and Compounds, 2008, 448(1-2): 316-320.
[13] SHAO G, TSAKIROPOULOS P. Prediction of phase select in rapid solidification using time dependent nucleation theory [J]. Acta Metall Mater, 1994, 42(9): 2937-2441.
[14] BA Fa-hai. Phase composition and kinetics study of rapid solidification Ni-Al alloy [D]. Beijing: University of Science and Technology Beijing, School of Materials Science and Engineering, 2001: 79-84. (in Chinese)
[15] HEINEN O, HOLLAND-MORITZ D, HERLACH D M. Phase selection during solidification of undercooled Ti-Fe, Ti-Fe-O and Ti-Fe-Si-O melts-Influence of oxygen and silicon [J]. Materials Science and Engineering A, 2007, 449-451: 662-665.
[16] FAN Jian-feng, LIU Xin-bao, XIE Hui, WANG Jin-cheng, SONG Guang-sheng, YANG Gen-cang. Phase selection in undercooled melt of Al72Ni12Co16 quasicrystal-forming alloy [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(5): 1088-1091. (in Chinese)
[17] LU Yi-ping, LI Ting-ju, FU Ya-bo, SUN Jian-bo, LUO Da-wei, YANG Gen-cang, ZHOU Yao-he. Phase selection during solidification of undercooled Ni70.2Si29.8 eutectic alloy [J]. Progress in Natural Science, 2009, 19(11): 1619-1624.
[18] VOGEL M, KRAFT O, DEHM G, ARZT E. Quasi-crystalline grain-boundary phase in the magnesium die-cast alloy ZA85 [J]. Scripta Materialia, 2001, 45(5): 517-524.
Si对ZA84镁合金凝固过程中三元化合物相选择的影响
张春香1,2,关绍康3,刘 涛2,李少华2,刘忠侠1
1. 郑州大学 物理学博士后流动站,郑州 450001;
2. 郑州大学 材料科学与工程学院,郑州 450001;
3. 郑州大学 研究生院,郑州 450001
摘 要:借助X射线衍射仪、扫描电镜和差示扫描量热仪等分析手段研究了Si对金属型铸造ZA84 (Mg-8Zn-4Al-0.3Mn)镁合金中τ(Mg32(Al, Zn)49)和φ(Al2Mg5Zn2)两相之间的相选择影响。结果表明,随着ZA84合金中Si加入量的增加,合金的液相线温度下降,并且凝固区间减小。ZA84合金中的三元金属间化合物主要是τ相,φ相只占少量。当在ZA84合金中加入一定量的元素Si时,三元金属间化合物的优先析出次序发生改变,φ相优先析出而τ相被抑制。对相选择现象进行凝固动力学分析表明,在过冷熔体中存在临界过冷度,τ相与φ相析出时若熔体的过冷度大于此临界值,则τ相优先析出而φ相受到抑制,否则,φ相优先析出而τ相被抑制。
关键词:ZA84镁合金;Si;相选择;凝固动力学
(Edited by FANG Jing-hua)
Foundation item: Project(50571092) supported by the National Natural Science Foundation of China
Corresponding author: GUAN Shao-kang; Tel: +86-371-67780051; E-mail: skguan@zzu.edu.cn
DOI: 10.1016/S1003-6326(11)60677-8