新颖两步法提高阿希高硫金精矿生物氧化效率
来源期刊:中国有色金属学报(英文版)2015年第12期
论文作者:刘新星 王国华 霍强 谢建平 李寿朋 武海艳 郭玉洁
文章页码:4119 - 4125
关键词:金精矿;生物氧化法;pH调控;两步氧化法
Key words:gold concentrate; bio-oxidation; pH control; two-step oxidation
摘 要:为提高阿希难处理金精矿的生物氧化效率,利用两步氧化法(高温化学氧化与随后的生物氧化)处理阿希金精矿,并优化了生物氧化阶段的溶液pH。结果表明,两步法生物氧化阶段最优pH范围为1.0~1.2,在此条件下,既能保持微生物的氧化活性又能减少铁钒沉淀的生成,可以有效提高氧化效率;铁、硫的氧化率分别提高了12.50%与15.49%,提金率提高了21.02%。因此,两步氧化法联合pH调控是一种高效氧化阿希金精矿的方法,在今后处理复杂难处理金精矿领域中具有广阔的应用前景。
Abstract: In order to improve the bio-oxidation efficiency of Axi refractory gold concentrate, a two-step process including a high temperature chemical oxidation and a subsequent bio-oxidation, combined with pH control during the bio-oxidation step was used. The results revealed that the optimum mode was to maintain solution pH at 1.0-1.2 during the biological oxidation stage. Under this condition, the activity of mixed culture could be sustained and the formation of jarosite could be diminished, thus the oxidation efficiency was improved. The oxidation levels of iron and sulfur were improved by 12.50% and 15.49%, and the gold recovery was increased by 21.02%. Therefore, the two-step process combined with pH control is an effective method for oxidizing the biohydrometallurgical process of Axi gold concentrate, and it will have a broad prospect of application in dealing with complex refractory gold concentrate.
Trans. Nonferrous Met. Soc. China 25(2015) 4119-4125
Xin-xing LIU1,2, Guo-hua WANG1,2, Qiang HUO1,2, Jian-ping XIE1,2, Shou-peng LI1,2, Hai-yan WU1,2, Yu-jie GUO1,2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biohydrometallurgy, Ministry of Education, Central South University, Changsha 410083, China
Received 17 January 2015; accepted 2 June 2015
Abstract: In order to improve the bio-oxidation efficiency of Axi refractory gold concentrate, a two-step process including a high temperature chemical oxidation and a subsequent bio-oxidation, combined with pH control during the bio-oxidation step was used. The results revealed that the optimum mode was to maintain solution pH at 1.0-1.2 during the biological oxidation stage. Under this condition, the activity of mixed culture could be sustained and the formation of jarosite could be diminished, thus the oxidation efficiency was improved. The oxidation levels of iron and sulfur were improved by 12.50% and 15.49%, and the gold recovery was increased by 21.02%. Therefore, the two-step process combined with pH control is an effective method for oxidizing the biohydrometallurgical process of Axi gold concentrate, and it will have a broad prospect of application in dealing with complex refractory gold concentrate.
Key words: gold concentrate; bio-oxidation; pH control; two-step oxidation
1 Introduction
Bioprocessing of the refractory concentrates is considered to be low capital cost, environmental friendly compared with the conventional technologies [1-3]. But the average duration of the bio-oxidation process under industrial conditions is 4-6 d [4,5], the long residence time often causes excessive operational cost, thus it is desirable to improve the kinetics of the bio-oxidation process.
Chemical oxidation via ferric ion solution at high temperature (80-100 °C) can elevate the oxidation rate [6]. And ferric ion solution is available in bio-oxidation plant. However, high temperature would be detrimental for the microorganisms, and the regeneration of ferric ion would be influenced. As a result, it was not reasonable to carry out the bio-oxidation process at high temperatures by adding ferric ion solution. FOMCHENKO et al [7] proposed a two-stage chemical-bacterial oxidation process in 2010. In the first stage, the refractory gold-bearing sulfide concentrate was leached by the microbially-produced ferric sulfate solution under high temperature (80 °C). In the second stage, the reaction temperature was reduced to 40-45 °C, ferric ion was re-produced by the microorganisms and sulfides were oxidized. The separation of chemical leaching step and bio-oxidation step created the favorable conditions for chemical leaching process and microbial activity.
Bio-oxidation of Axi high-sulfur refractory gold concentrates was an acid producing process as shown in Eq. (1). In bio-oxidation process, the solution pH was often controlled to enhance the efficiency of this process [8-10]. However, when the solution pH was adjusted, the passivation layer because of ferric ion precipitates (such as jarosite) may deposit on the surface of sulfide concentrates impeding their oxidation [11]. Thus, it was necessary to optimize the solution pH during the bio-oxidation process.
FeS2+Fe2(SO4)3+3O2+2H2O→3FeSO4+2H2SO4 (1)
According to the comments above, the purpose of this work was to investigate the effect of a two-stage process combined with pH control on the bio-oxidation efficiency of high-sulfur refractory gold concentrate.
2 Experimental
2.1 Microorganisms and growth conditions
A mixed culture of moderately thermophilic microbes obtained from the industrial bioreactor of Axi gold factory in Xinjiang Autonomous Region of China was used for bio-oxidation experiments. In this study, the presence of Sulfobacillus thermosulfidooxidans, Acidithiobacillus caldus and Ferroplasma acidiphilum was detected by cloning and sequencing the amplified 16S rDNA. The mixed culture was cultured in 100 mL 9K basal medium [12] supplemented with 0.02% yeast extract (YE) in 250 mL Erlenmeyer flasks at 45 °C on a rotary shaker (170 r/min). The solution pH of enrichment medium was adjusted to 1.5.
2.2 Minerals components
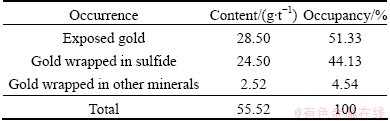
The mineral samples used in this study were gold concentrates from the Axi Gold Factory in Xinjiang Autonomous Region of China. Screen analysis showed that 90% of the samples were finer than 44 μm with gold mainly in pyrite phase. The contents of the elements of the gold concentrate were as follows: 55.52 g/t Au, 23.68% Fes (sulfidic iron), 2.25% Ass, trace S0, and 24.53% Ss (sulfidic sulfur). The content of gold was 55.52 g/t. Mineralogical analyses showed that the concentrate contained pyrite (58.77%) as the major sulfide phase. The mode of occurrence of gold (Table 1) was supplied by the Axi Gold Factory in Xinjiang Autonomous Region of China.
Table 1 Mode of occurrence of gold
2.3 Chemical oxidation of concentrate (first step)
In the two-step process, the concentrate was first leached by ferric ion containing solution (collected from the industrial bioreactors of Axi Gold Factory) prior to bio-oxidation. Chemical leaching was conducted in a 1 L reactor that contained 500 mL pulp. The stirring rate was 330 r/min, and the temperature was maintained at 80 °C by a thermostat. The concentrate was loaded and mixed with the ferric ion containing solution at a pulp density of 10%. The concentration of ferric ion in the chemical leaching solution was 20 g/L with pH 1.25. When the concentration of ferric ion and pH were steady, the residues were filtrated and washed with distilled water (pH 1.5) twice and then subjected to the bio-oxidation process.
2.4 Bio-oxidation of concentrates (second step)
The research of the bio-oxidation of the concentrates was carried out using three series of experiments: 1) a one-step process without pH control (initial pH 1.5) in the whole process, 2) a two-step process without pH control (initial pH 1.5) in the process, 3) a two-step process with pH control (i.e., 0.8-1.0, 1.0-1.2, 1.2-1.4, 1.4-1.6) in the process. The experiments were conducted in 1 L reactors that contained 500 mL pulp. A 9K basal medium supplemented with 0.02% yeast extract (YE) was used as the liquid phase. The initial concentration of microorganisms was 1.0×107 cell/mL. The pulp density of Group (a) was 10%, and in Groups (b) and (c) residues of chemical leaching were added accordingly. The solution pH was adjusted twice a day in Group (c). Samples were taken every three days to determine total soluble iron, ferrous ion, redox potential (φh) and number of microbes. Sodium bicarbonate was utilized as the neutralizing reagent, because it can on one hand, neutralize the acid to maintain the pH, on the other hand, produce CO2 which is needed as the carbon source for the microorganisms[13]. The experiments were conducted at 45 °C with a stirring speed of 350 r/min and an aeration rate of 4 L/min. All of the experiments were carried out at the same time.
2.5 Analytical methods
Enumeration of the microbes was carried out by a direct count using a Thoma chamber of 0.1 mm in depth and 0.0025 mm2 in area with an optical microscope (Olympus, Japan). The pH was determined at room temperature with a pH meter (PHSJ-4A) calibrated with a low pH buffer. While the redox potential was monitored with a platinum electrode against saturated Hg/Hg2Cl2 reference electrode. The total iron concentrations in the suspension were measured spectrophotometrically with the 5-sulfosalicylic acid as an indicator [14]. Concentration of ferrous ion in solution was measured by titrating against 0.005 mol/L potassium dichromate with N-phenylanthranilic acid as an indicator. And Fe3+ concentration was determined by the subtraction of the total iron concentration and ferrous ion concentration. The sulfide iron and sulfur contents were measured using the phase methods. SEM-EDX (FEI Quanta-200) was used to detect the morphological changes and compositions of the chemical leaching residues.
The content of gold in the solid phase was determined by fire assay. Cyanide leaching of the oxidized residues as well as the untreated concentrate was carried out for gold recovery. All of the cyanidation tests were conducted in 100 mL flask at a working volume of 40 mL with 30% (w/v) solids in an orbital incubator shaker at 25 °C and the rotation rate of 180 r/min for 48 h. The pH of the cyanidation pulp was maintained at 10.5-11.0 (adjusted using 10 mol/L NaOH). The cyanide concentration was maintained in the range of 1.0-3.0 g/L as determined by titration.
2.6 Calculation formula
The oxidation levels of iron and sulfur, Oi, were evaluated by using Eq. (2) because the analyses were done on undissolved leach residues, where X0 and Xi are the contents (mass fraction) of sulfide iron and sulfur in the initial feed and undissolved leach residues, respectively, and m0 and mi are the masses of initial feed and undissolved residues.
 (2)
(2)
3 Results and discussion
3.1 Chemical oxidation of concentrate
In two-step process, the concentrate was first leached by a ferric ion containing solution to remove the most easily oxidized sulfides. Under the parameters mentioned above, chemical leaching occurred within 7 h, because the concentration of ferric ion and pH in the solution decreased to a steady value within 7 h. In the process of chemical leaching, solution pH decreased significantly from 1.25 to 0.31 and concentration of ferric ion decreased from 20.00 to 2.19 g/L (Fig. 1). The composition of the leaching residue was shown in Table 2. The oxidation levels of iron and sulfur were 16.58% and 14.89%, respectively. The oxidation level of pyrite was 14.23%. In the process of chemical leaching, though the temperature (80 °C) was conducive to form jarosite, only small amount of jarosite formed, because of the low pH of solution (Fig. 1).
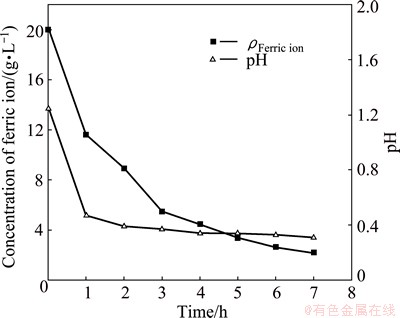
Fig. 1 Changes in concentration of ferric ion and pH during chemical leaching step
Table 2 Analytical data of chemical leaching residues
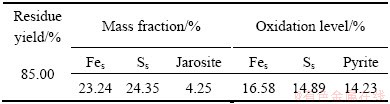
The outcome of chemical leaching was better than that by MURAVYOV and BULAEV [15]. That was probably because the pulp density in this study was lower than that in Ref. [15] but with the similar concentration of ferric ion. Pyrite could be oxidized by ferric ion solution rapidly because of the high temperature [16]. After chemical leaching, we found that the oxidation levels of sulfur were lower than those of iron (Table 2), which was consistent with the mechanism of pyrite oxidation. Moreover, SEM images of the concentrate and oxidizing residues were shown in Fig. 2. Some leaching products were observed on the mineral surface (Fig. 2(b)). SEM-EDX analysis also indicated that a sulfur-rich layer might be formed in the chemical leaching stage [17-19].
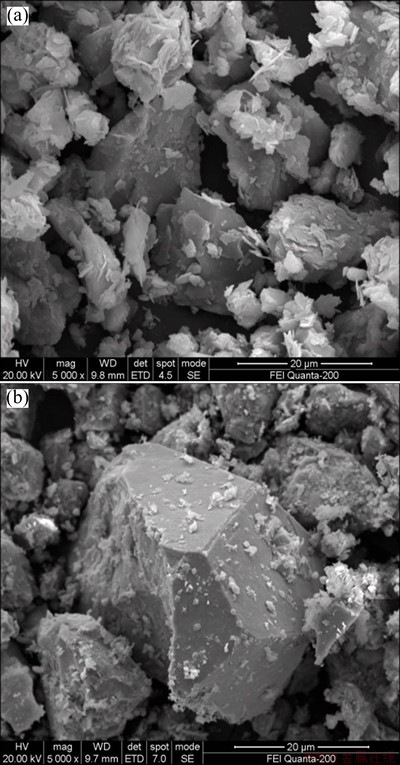
Fig. 2 SEM images of Axi gold concentrate (a) and oxidizing residues (b)
3.2 Biological oxidation of concentrates
The calculated oxidation levels of iron and sulfur were used for assessment of the efficiency of the oxidation of the concentrate. The results of analytical data from one-step and two-step process without pH control were listed in Table 3. It was obvious that the oxidation levels of iron and sulfur with two-step process were higher than those in the one-step process. The oxidation levels of iron and sulfur in the one-step process were 50.25% and 48.71%, and the oxidation level of pyrite was only 47.23%. However, in the two-step process, these values increased to 69.88%, 70.03% and 78.67%. It had been stated that insufficient gas transfer together with microbial cell damage due to high shear force at high pulp density influenced the bio-oxidation efficiency [20,21]. After chemical leaching, the pulp density declined to 8.5% in the biological leaching step, while in the one-step process, the pup density was 10%. As a result, the negative effect of high pulp density was diminished partially after chemical leaching. During the bio-oxidation step, the solution φh and microbial concentration increased more quickly than the one-step process. This was the evidence of active bacteria oxidation of sulfide. Additionally, the chemical leaching kinetics of pyrite via ferric ion was greatly influenced by temperature [22-24]. The reaction rate increased as temperature rose, and it would be relatively fast at high temperature. In the chemical leaching step the reaction temperature could be elevated to 80 °C, which would improve the leaching rate significantly, and the oxidation levels of iron and sulfur could reach 16.58% and 14.89% within 7 h. By applying physical separation of the chemical leaching and biological leaching step, we could take advantage of chemical leaching and bio-oxidation, and obtain favorable conditions for both chemical leaching process and microbial activity in the biological leaching step. Thus, the two-steps process improved the efficiency of bio-oxidation substantially.
Table 3 Analytical data of bio-oxidized residues by one-step and two-step process without pH control

However, in the bio-oxidation process, the solution pH declined more quickly in the two-step process than in the one-step process (Fig. 3). For one thing, it might be because the sulfur-rich layers were more convenient to be oxidized by the microorganisms; for another thing, most alkali gangue could be consumed because of the highly acidic environment. In addition, the content of pyrite was 58.77%, only a small part of the sulfide was oxidized in the chemical leaching stage. As a result, large amount of acid would be produced during the bio-oxidation process because of the high content of pyrite in the chemical leaching residues. However, the solution pH decreased to below 0.5 in these two groups, indicating that pH control should be used to further improve the efficiency.
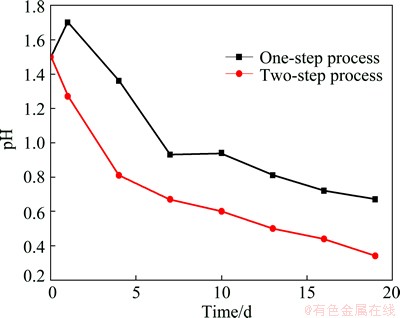
Fig. 3 Changes of pH value in solution during one-step and two-step processes without pH control
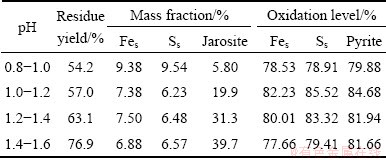
As the main sulfide mineral in the concentrate, it was now recognized that pyrite was acid-insoluble and could be oxidized by ferric ion in the solution. So, the iron-oxidizing acidophilic prokaryotes played an important role in the bio-oxidation process because they could regenerate the oxidant ferric ion. It was proposed that the optimum pH to carry out bio-oxidation process with moderate thermophiles was 1.5-1.6 [8,9,25], as the predominant ferrous ion-oxidizing microorganism of the mixed culture was Sulfobacillus thermosulfidooxidans. The concentration of microorganisms during operating time at pH 1.4-1.6 was higher than that at pH 1.0-1.2. The results of pH control were listed in Table 4, simply indicating that oxidation levels of iron and sulfur were increased when solution pH was controlled. The optimum pH to carry out bio-oxidation was 1.0-1.2. The oxidation levels of iron and sulfur were 82.23% and 85.52% at pH 1.0-1.2, which were only 77.66% and 79.41% at pH 1.4-1.6.
Table 4 Analytical data of bio-oxidized residues by two-step process with pH control
The leaching rate of pyrite was a function of redox potential [6,26]. The purpose of pH control was to keep the function of iron-oxidizing acidophilic prokaryotes in the bio-oxidation process with the aim of maintaining the environment of high redox potential. Results given in Fig. 4 showed that, contrasted with the redox potentials in pH 1.4-1.6, redox potentials (φh) were elevated in pH 1.0-1.2. In the bio-oxidation system, the ferric to ferrous iron ratio could be related to the solution redox potential using the Nernst equation (Eq. (3)). It was supposed that the ferric to ferrous ratio at pH 1.0-1.2 was higher than that at pH 1.4-1.6. Results presented in Fig. 5 revealed that the concentration of ferric ion at pH 1.0-1.2 was higher than that at pH 1.4-1.6. This indicated that ferric ion in the solution might be consumed largely at pH 1.4-1.6 when the alkaline reagents were added to adjust the solution pH.
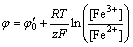 (3)
(3)
The effects of pH control on jarosite formation were listed in Table 4. The content of jarosite formed in the bio-oxidation process was proportional to the pH value. At pH 1.4-1.6, the content of jarosite formed in the bio-oxidation process was as high as 39.7%, which prevented the microorganisms and ferric ion from accessing the surface of the concentrate. In addition, large amount of ferric ion in the solution precipitated as jarosite, so the concentration of ferric ion in the solution at pH 1.4-1.6 was lower than that at pH 1.0-1.2. As a result, the oxidation levels were influenced accordingly. The formation of passive layers should be diminished, as it may impede the bio-oxidation reaction and consume large amount of ferric ion in the solution [19,27]. Even though the optimum pH to maintain the activity of microorganisms was at 1.4-1.6, the oxidation levels were not satisfied compared with that at pH 1.0-1.2. At pH 1.0-1.2, the content of jarosite was only 19.9%, and the redox potential value was higher than that at pH 1.4-1.6. This would necessitate operation probably at pH values below 1.4 at the expense of reduced activity of mixed culture to diminish the formation of detrimental jarosite [11,28]. However, the activity of the microorganisms must be taken into consideration, though the content of jarosite was only 5.8% at pH 0.8-1.0, the activity of microorgunisms decreased. As a result, the oxidation levels of iron and sulfur were only 78.53% and 78.91% at pH 0.8-1.0, respectively. It was obvious that the solution pH should be maintained at a certain range in the second step to further improve the oxidation efficiency.
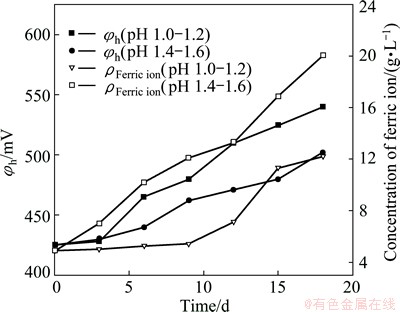
Fig. 4 Concentration of ferric ion and redox potential evolution during operating time with pH control
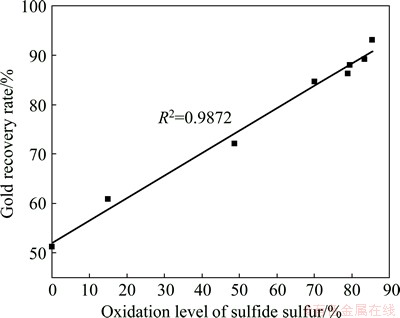
Fig. 5 Dependence of gold recovery from concentrate on oxidation level of sulfide sulfur
3.3 Cyanidation tests
The oxidized residues under various conditions were subjected to the cyanide leaching tests to determine the oxidation efficiency. Obviously, the gold recovery rate of gold from the head sample was low (51.22%). The results indicated that the two-step approach combined with pH control especially at pH 1.0-1.2 can improve the gold recovery rate. The gold recovery rate was elevated to 93.15%, about 21.02% greater than that of the one-step approach, 8.49% higher than that of the two-step approach without pH control, and 5.13% higher than that at pH 1.4-1.6 in the two-step process.
The dependence of gold recovery rate from the concentrate on the oxidation level of sulfide sulfur was shown in Fig. 5. The data presented that the gold recovery from the concentrate was linearly dependent on the sulfide sulfur oxidation level (the regression coefficient was greater than 0.98). It may be estimated that the oxidation level of the sulfide minerals is the most important factor that influences the cyanide leaching efficiency.
4 Conclusions
1) A chemical leaching prior the bio-oxidation process can enhance the leaching rates of iron and sulfur significantly in the concentrates. The oxidation levels of iron and sulfur were elevated to 69.88% and 70.03%, respectively; gold recovery was 84.66%. While the oxidation levels of iron and sulfur were only 50.25% and 48.71%, respectively in the one-step process; gold recovery by cyanidation was 72.13%.
2) The optimum pH range to carry out the bio-oxidation process in the two-step process was 1.0-1.2. The oxidation levels of iron and sulfur were further elevated to 82.23% and 85.52%, respectively with a gold recovery of 93.15%.
3) The effect of microbial activity and precipitation must be taken into consideration in the process of bio-oxidation. A novel two-step process combined with pH control can enhance the efficiency of bio-oxidation.
References
[1] BRIERLEY C. Biohydrometallurgical prospects [J]. Hydrometallurgy, 2010, 104(3): 324-328.
[2] MA S, LUO W, MO W, SU X, LIU P, YANG J. Removal of arsenic and sulfur from a refractory gold concentrate by microwave heating [J]. Minerals Engineering, 2010, 23(1): 61-63.
[3] RAWLINGS D E, JOHNSON D B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia [J]. Microbiology, 2007, 153(2): 315-324.
[4] BRIL-RLEY C. How will biomining be applied in future? [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1302-1310.
[5] RAWLINGS D E, DEW D, PLESSIS C D. Biomineralization of metal-containing ores and concentrates [J]. Trends in Biotechnology, 2003, 21(1): 38-44.
[6] IGLESIAS N, CARRANZA F. Treatment of a gold bearing arsenopyrite concentrate by ferric sulphate leaching [J]. Minerals Engineering, 1996, 9(3): 317-330.
[7] FOMCHENKO N Y V, MURAVYOV M I, KONDRAT'EVA T F. Two-stage bacterial–chemical oxidation of refractory gold-bearing sulfidic concentrates [J]. Hydrometallurgy, 2010, 101(1-2): 28-34.
[8] GAHAN C S, SUNDKVIST J E, ENGSTR M F, SANDSTR M  . Utilisation of steel slags as neutralising agents in biooxidation of a refractory gold concentrate and their influence on the subsequent cyanidation [J]. Resources, Conservation and Recycling, 2011, 55(5): 541-547.
. Utilisation of steel slags as neutralising agents in biooxidation of a refractory gold concentrate and their influence on the subsequent cyanidation [J]. Resources, Conservation and Recycling, 2011, 55(5): 541-547.
[9] GAHAN C S, SUNDKVIST J E,  A. Use of mesalime and electric arc furnace (EAF) dust as neutralising agents in biooxidation and their effects on gold recovery in subsequent cyanidation [J]. Minerals Engineering, 2010, 23(9): 731-738.
A. Use of mesalime and electric arc furnace (EAF) dust as neutralising agents in biooxidation and their effects on gold recovery in subsequent cyanidation [J]. Minerals Engineering, 2010, 23(9): 731-738.
[10] WANG Yu-guang, SU Li-jun, ZENG Wei-min, QIU Guan-zhou, WAN Li-li, CHEN Xin-hua, ZHOU Hong-bo. Optimization of copper extraction for bioleaching of complex Cu-polymetallic concentrate by moderate thermophiles [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(4): 1161-1170.
[11] YAHYA A, JOHNSON D B. Bioleaching of pyrite at low pH and low redox potentials by novel mesophilic Gram-positive bacteria [J]. Hydrometallurgy, 2002, 63(2): 181-188.
[12] SILVERMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans II: Manometric studies [J]. Journal of Bacteriology, 1959, 78(3): 326-331.
[13] ASTUDILLO C, ACEVEDO F. Effect of CO2 air enrichment in the biooxidation of a refractory gold concentrate by Sulfolobus metallicus adapted to high pulp densities [J]. Hydrometallurgy, 2009, 97(1): 94-97.
[14] KARAMANEV D, NIKOLOV L, MAMATARKOVA V. Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions [J]. Minerals Engineering, 2002, 15(5): 341-346.
[15] MURAVYOV M I, BULAEV A G. Two-step oxidation of a refractory gold-bearing sulfidic concentrate and the effect of organic nutrients on its biooxidation [J]. Minerals Engineering, 2013, 45: 108-114.
[16] MOUSAVI S, YAGHMAEI S, SALIMI F, JAFARI A. Influence of process variables on biooxidation of ferrous sulfate by an indigenous Acidithiobacillus ferrooxidans. Part I: Flask experiments [J]. Fuel, 2006, 85(17): 2555-2560.
[17] CHERNYSHOVA I V. An in situ FTIR study of galena and pyrite oxidation in aqueous solution [J]. Journal of Electroanalytical Chemistry, 2003, 558(1): 83-98.
[18] HOLMES P R, CRUNDWELL F K. The kinetics of the oxidation of pyrite by ferric ions and dissolved oxygen: An electrochemical study [J]. Geochimica et Cosmochimica Acta, 2000, 64(2): 263-274.
[19] XIA J L, YANG Y, HE H, ZHAO X J, LIANG C L, ZHENG L, MA C Y, ZHAO Y D, NIE Z Y, QIU G Z. Surface analysis of sulfur speciation on pyrite bioleached by extreme thermophile Acidianus manzaensis using Raman and XANES spectroscopy [J]. Hydrometallurgy, 2010, 100(3-4): 129-135.
[20] JIN Jian, SHI Shao-yun, LIU Guo-liang, ZHANG Qing-hua, CONG Wei. Comparison of Fe2+ oxidation by Acidithiobacillus ferrooxidans in rotating-drum and stirred-tank reactors [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(3): 804-811.
[21] CIFTCI H, AKCIL A. Effect of biooxidation conditions on cyanide consumption and gold recovery from a refractory gold concentrate [J]. Hydrometallurgy, 2010, 104(2): 142-149.
[22] LIANG Chang-li, XIA Jin-lan, NIE Zhen-yuan, YU Shui-jing, XU Bao-quan. Effect of initial pH on chalcopyrite oxidation dissolution in the presence of extreme thermophile Acidianus manzaensis [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1890-1897.
[23] CHANDRA A P, GERSON A R. Redox potential (Eh) and anion effects of pyrite (FeS2) leaching at pH 1 [J]. Geochimica et Cosmochimica Acta, 2011, 75(22): 6893-6911.
[24] GAO G, LI D, ZHOU Y, SUN X, SUN W. Kinetics of high-sulphur and high-arsenic refractory gold concentrate oxidation by dilute nitric acid under mild conditions [J]. Minerals Engineering, 2009, 22(2): 111-115.
[25] PLUMB J, MUDDLE R, FRANZMANN P. Effect of pH on rates of iron and sulfur oxidation by bioleaching organisms [J]. Minerals Engineering, 2008, 21(1): 76-82.
[26] BREED A W, HARRISON S T L, HANSFORD G S. A preliminary investigation of the ferric leaching of a pyrite/arsenopyrite flotation concentrate [J]. Minerals Engineering, 1997, 10(9): 1023-1030.
[27] ZHU X, LI J, WADSWORTH M E. Characterization of surface layers formed during pyrite oxidation [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1994, 93: 201-210.
[28] QIN W, LIU K, DIAO M, WANG J, ZHANG Y, YANG C, JIAO F. Oxidation of arsenite (As (III)) by ferric iron in the presence of pyrite and a mixed moderately thermophilic culture [J]. Hydrometallurgy, 2013, 137: 53-59.
刘新星1,2,王国华1,2,霍 强1,2,谢建平1,2,李寿朋1,2,武海艳1,2,郭玉洁1,2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083
摘 要:为提高阿希难处理金精矿的生物氧化效率,利用两步氧化法(高温化学氧化与随后的生物氧化)处理阿希金精矿,并优化了生物氧化阶段的溶液pH。结果表明,两步法生物氧化阶段最优pH范围为1.0~1.2,在此条件下,既能保持微生物的氧化活性又能减少铁钒沉淀的生成,可以有效提高氧化效率;铁、硫的氧化率分别提高了12.50%与15.49%,提金率提高了21.02%。因此,两步氧化法联合pH调控是一种高效氧化阿希金精矿的方法,在今后处理复杂难处理金精矿领域中具有广阔的应用前景。
关键词:金精矿;生物氧化法;pH调控;两步氧化法
(Edited by Xiang-qun LI)
Foundation item: Project (2010CB630901) supported by the National Basic Research Program of China; Project (2013M531814) supported by the 53rd China Postdoctoral Science Foundation
Corresponding author: Xin-xing LIU; Tel: +86-731-88716592; E-mail: xxlcsu@163.com
DOI: 10.1016/S1003-6326(15)64063-8

