Isolation of three cyanins from Lonicera caerulea L. fruits and its anticancer activity
来源期刊:中南大学学报(英文版)2017年第7期
论文作者:王亮生 李海普 罗洲飞 杨远
文章页码:1573 - 1581
Key words:cyanins; cyanidin 3-O-glucoside; isolation; anticancer; HSCCC; HPLC-ESI-MSn
Abstract: Lonicera caerulea L. fruit is an excellent source of bioactive compounds. An efficient separation method of cyanins is important for the development of many value-added products and functional food ingredients. High-speed counter-current chromatography (HSCCC) was applied to isolate cyanins from Lonicera caerulea fruits with a biphasic solvent system composed of methyl tert-butyl ether/n-butanol/acetonitrile/water/trifluoroacetic acid (2:2:1:5:0.01, volume ratio). 1.41 mg of cyanidin 3, 5-O-diglucoside, 1.08 mg of cyanidin 3-O-rutinoside and 6.38 mg of cyanidin 3-O-glucoside were obtained from 40 mg of crude extract. The purities of these compounds were 95.8%, 92.4% and 97.6%, respectively, as identified by high-performance liquid chromatography–diode array detection (HPLC-DAD) and high-performance liquid chromatography-electrospray ionization mass spectrometryn (HPLC-ESI/MSn). In addition, the dominant anthocyanin, cyanidin 3-O-glucoside was demonstrated cytotoxic response of human hepatocarcinoma SMMC-7721 cells, inducing live cancer cell apoptotic by flow cytometric analysis.
Cite this article as: LUO Zhou-fei, YANG Yuan, WANG Liang-sheng, LI Hai-pu. Isolation of three cyanins from Lonicera caerulea L. fruits and its anticancer activity [J]. Journal of Central South University, 2017, 24(7): 1573-1581. DOI: 10.1007/s11771-017-3562-1.
J. Cent. South Univ. (2017) 24: 1573-1581
DOI: 10.1007/s11771-017-3562-1
LUO Zhou-fei(罗洲飞)1, 2, YANG Yuan(杨远)1, WANG Liang-sheng(王亮生)2, LI Hai-pu(李海普)1
1. Center for Environment and Water Resources, College of Chemistry and Chemical Engineering,Central South University, Changsha 410083, China;
2. Key Laboratory of Plant Resources, Beijing Botanical Garden, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract: Lonicera caerulea L. fruit is an excellent source of bioactive compounds. An efficient separation method of cyanins is important for the development of many value-added products and functional food ingredients. High-speed counter-current chromatography (HSCCC) was applied to isolate cyanins from Lonicera caerulea fruits with a biphasic solvent system composed of methyl tert-butyl ether/n-butanol/acetonitrile/water/trifluoroacetic acid (2:2:1:5:0.01, volume ratio). 1.41 mg of cyanidin 3, 5-O-diglucoside, 1.08 mg of cyanidin 3-O-rutinoside and 6.38 mg of cyanidin 3-O-glucoside were obtained from 40 mg of crude extract. The purities of these compounds were 95.8%, 92.4% and 97.6%, respectively, as identified by high-performance liquid chromatography–diode array detection (HPLC-DAD) and high-performance liquid chromatography-electrospray ionization mass spectrometryn (HPLC-ESI/MSn). In addition, the dominant anthocyanin, cyanidin 3-O-glucoside was demonstrated cytotoxic response of human hepatocarcinoma SMMC-7721 cells, inducing live cancer cell apoptotic by flow cytometric analysis.
Key words: cyanins; cyanidin 3-O-glucoside; isolation; anticancer; HSCCC; HPLC-ESI-MSn
1 Introduction
Lonicera caerulea L. (L. caerulea) is an important fruit-bearing bush crop growing in Northern Russia, North Eastern Asia and Japan. The plant has strong tolerance to severe low-temperature and desiccation condition, so this crop could provide an additional opportunity in high latitudes and colder climates to diversify their production for high end specialty crop markets [1]. The berry of blue honeysuckle is an elongated oval shape about (2-6) cm× (1-3) cm in size and dark navy blue to purple in color. Evidenced by this deep color, it is a rich source of bioactive compounds including phenolic acids, flavonoids, proanthocyanidins and cyanins [2]. It was reported that blue honeysuckle berry contained up to 18.5% cyanins with crosstalk of anti-inflammatory and antioxidant effects [3].
Hepatocellular caicinoma is one of the most common malignancy tumors worldwide, especially highest incidence and clinical risk in China. It is essential to seek out techniques or remedies with efficient and less side effects for hepatocellular caicinoma. Numerous studies suggested that bioactive natural compounds found in common fruits could serve as alternatives to chemically designed anticancer agents [4, 5]. Cyanins are naturally chemical components, which are responsible for the intense color of many fruits, vegetables and flowers such as berries, purple cabbages, and petunia [6]. In addition to their contribution in pigmentation, they have been receiving considerable attention due to their crucial roles in antioxidation, anticancer, promotion of healthy lipid profile, prevention of cardiovascular disease and atherosclerosis, and improvement of night vision [7-9]. Cyanins are the most common anthocyanin, with a high inhibitory activity against tumor cancer cell growth and hepatic toxicity [10, 11]. Therefore, cyanins may be of great value in developing a potential cancer prevention agent from anthocyanin-rich blue honeysuckle fruits grown in China [12].
High-speed counter-current chromatography (HSCCC) is a liquid-liquid partition method which uses no support matrix. A series of simultaneous mixing and settling zones along the length of the tubing is created by the planetary spinning motion, which results in high resolution purifications together with transfer of target compounds between the phases [13]. It has significant advantages over the conventional liquid–solid chromatography by eliminating all complications caused by the solid support matrix such as reduction of adsorptive loss, deactivation of samples, tailing of solute peaks and contamination, and the maximum capacity with an excellent sample recovery [14]. Since the 1980s, this method has been widely used for the separation and purification of various natural compounds. Many reports have been published on the separation of kinds of cyanins from many plants in nature using HSCCC method [15-17].
The purpose of this work is to establish a method to purify cyanins from blue honeysuckle fruits using HSCCC. The chemical structures of compounds were identified by high-performance liquid chromatography- electrospray ionization massn (HPLC-ESI/MSn). Cyanidin 3-O-glucoside(Cy3G) effect on human hepatocarcinoma cell viability and induced live cancer cell apoptosis based on flow cytometric analysis in vitro were investigated.
2 Materials and methods
2.1 Chemicals
Cyanidin 3-O-glucoside (Cy3G), Cyanidin 3, 5-O- diglucoside(Cy3,5G), cyanidin 3-O-rutinoside(Cy3R) standards were purchased from Shanghai Tauto Biotechnology Company, China. All solvents used for preparation of crude extract and HSCCC were of analytical grade (Beijing Chemical Works, China). Methanol and acetonitrile used for high performance liquid chromatography (HPLC) were of chromatographic grade (Alltech Scientific Company, China). Ultrapure water (resistivity of 18.2 MΩ) was obtained from Milli-Q System (Millipore, Billerica, USA). Trifluoroacetic acid was purchased from Merck (Hohenbrunn, Germany). Dimethyl sulfoxide (DMSO) and MTT were purchased from Sigma–Aldrich Chemicals, USA.
Cell lines SMMC-7721 and L02 were cultured in Roswell Park Memorial Institute (RPMI) on DMEM medium (Sigma, Saint Louis, USA) supplemented with 10% fetal calf serum, 2 mmol/L glutamine, 100 μg/mL penicillin, and 100 mg/mL streptomycin at 37 °C under a humidified atmosphere of 5% CO2.
2.2 Plant materials
The ripe fruits of L. caerulea were purchased from Mohe, Heilongjiang Province, north of China (N 121°07′, E 52°10′). The fruits were hand-picked in July, 2012 and stored in plastic container -25 °C in the laboratory for chemical analysis.
2.3 Cyanins extract of blue honeysuckle fruits
Blue honeysuckle fruits (100 g) were ground in liquid nitrogen, with the addition of 1000 mL extract solution (1.0% formic acid ethanol) for ultrasonic 60 min at room temperature (25 °C) three times. The combined extract was filtered and evaporated to residue. The residue was ready for subsequent HSCCC purification.
2.4 Determination of partition coefficients
Two phase solvent systems were selected according to the partition coefficient (K) of each target component. The values were determined by HPLC as follows: 2 mL of the crude extract was dissolved in 3 mL of pre- equilibrated two-phase solvent system and mixed thoroughly. The upper and lower phases were analyzed by HPLC to obtain the K-values after the solution was separated for a moment, all components of which were expressed as the peak area from the upper phase divided by the lower phase.
2.5 HSCCC separation
The HSCCC instrument was a model CCC TBE-300A (Tauto Biotechnology Company, Shanghai, China) equipped with PTFE (polytetrafluoroethylene) multilayer coils (inner diameter of tubing: 2.6 mm; total volume: 300 mL) and 20 mL sample loop. The solvent was pumped into the column with a model  KTA Prime (Amersham Pharmacia Biotechnique Group, Uppsala, Sweden) at a flow rate of 2.0 mL/min, while the apparatus was run at a revolution speed of 800 r/min. The elution mode was headed to tail with the less dense layer being the stationary phase. Prime View Chromatography workstation collected the data and UV-Vis detector at 280 nm.
KTA Prime (Amersham Pharmacia Biotechnique Group, Uppsala, Sweden) at a flow rate of 2.0 mL/min, while the apparatus was run at a revolution speed of 800 r/min. The elution mode was headed to tail with the less dense layer being the stationary phase. Prime View Chromatography workstation collected the data and UV-Vis detector at 280 nm.
During each separation process, mixture layered thoroughly in a funnel at room temperature to obtain the upper phase and lower phase. The upper phase (stationary phase) and the lower phase (mobile phase) were pumped into the multiplayer-coiled column according to the volume ratio of 60:40, at the same time, the HSCCC run at a positive speed of 800 r/min. An HX 1050 constant-temperature circulator (Beijing Boyikang Lab Instrument Company, China) was used to control the separation temperature using water as the circulating medium. The separation temperature was controlled at 25 °C. After hydrodynamic equilibrium, 40 mg of the crude extract (dry power) was dissolved in 15 mL of a mixture of the upper and lower phases (10:5, volume ratio) and injected into the system by a loop injection. The data were collected immediately after sample injection. Each peak fraction was collected manually according to the HSCCC chromatogram and evaporated to dryness under reduced pressure. The preparative compounds were dissolved in methanol for HPLC analysis.
2.6 HPLC analysis and LC-MS identfication
The crude sample and each peak fraction obtained through HSCCC were analyzed by HPLC. The HPLC analysis was performed with a Dionex system (Sunnyvale, CA, USA) equipped with a P680 HPLC pump, an UltiMate 3000 autosampler, a TCC-100 thermostatted column compartment and a Dionex PDA100 photodiode array detector. The analytical column was a C18 ODS 80Ts QA (d4.6 mm×150 mm, Tosoh, Tokyo, Japan) protected by a Transgenomic CARB Sep Coregel 87C Guard Cartridge (Transgenomic, Omaha, NE, USA). An aliquot of 10 μL solution was injected. Chromatograms were acquired at 525 nm for cyanins, while photodiode array spectra were recorded from 200 to 800 nm. Gradient program for cyanins analysis was applied. The eluents were: phase A, 10% formic acid in water and phase B, 15% methanol in acetonitrile (0.1% TFA). The applied gradient program was: phase B: 5%-15% at 0-5 min; 15%-15.4% at 5- 20 min; 15.4%-25% at 20-25 min; 25%-5% at 25-30 min, the flow rate was 0.8 mL/min, and the temperature was 35 °C.
Further identfication of HSCCC peak fractions were performed by an Agilent-1100 HPLC system coupled with a LC/MSD Trap VL electrospray ion mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). The chromatographic separation condition was the same with the HPLC-DAD analysis described above. The MS conditions were listed as follows: positive ion mode; gas (N2) temperature: 350 °C;flow rate: 8 L/min; nebulizer pressure: 241.32 kPa; HV voltage: 4 kV; octopole RF amplitude: 150 V; skim 1 voltage: 37.5 V; skim 2 voltage: 6.0 V; capillary exit: 111.8 V; cap exit offset: 79.6 V and scan range, mass-to-charge ratio (m/z) 0–1200, and using LC-MSD Trap software to analyze the results.
2.7 Cell viability assay
To evaluate the cytotoxic activity of Cy3G toward important model of human hepatocellular carcinoma cell line (SMMC-7721) and human normal liver cell line (L02), an MTT assay was performed to determine the cell viability. Cy3G from L. caerulea was dissolved in DMSO as a stock solution, and diluted with medium before each experiment. 2×104 cells were treated with Cy3G at different concentrations for 24 h in a 96-well microplate. Then MTT solution was added and further incubated for 4 h. After dissolving the MTT-formazan product, the amount of formazan was determined by measuring the absorbance at 490 nm with a microplate reader. The results were expressed as the contents of treated cells to that of blank. Blank groups were cultivated under the same condition without Cy3G and DMSO, and control groups were added equal volume of DMSO instead of Cy3G with other conditions unchanged.
2.8 Apoptosis assay
Apoptotic cells were detected using FITC-conjugated Annexin V (Caltag laboratories, Burlingame, CA, USA) and propidium iodide (PI). SMMC-7721 cells were grown to 60% confluence and then treated with Cy3G as described above. Cells were washed twice with cold phosphate buffer saline (PBS) and resuspended in annexin V-binding buffer (10 mmol/L of 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (HEPES); 140 mmol/L of NaCl; and 5 mmol/L of CaCl2) at a concentration of 1× 106 cells/mL. 100 μL (1×105 cells) of this suspension were added to a 5 mL culture tube, 5 μL of Annexin V-FITC and 10 μL of PI were added to it, and the tube was gently vortexed and incubated for 15 min in the dark at room temperature. Thereafter, binding buffer (400 μL) was added to each tube, and the cells were analyzed with a flow cytometer.
3 Result and discussion
3.1 Selection of two-phase solvent system of HSCCC
During HSCCC, successful separation depends on the selection of a suitable two-phase solvent system, which requires the following consideration: the K-value of the target compound should between 0.4 and 2.5. While small K-value usually results in a poor peak resolution, large K-value tends to produce excessive sample band broadening. According to Refs. [18, 19], different solvent systems were tested for a better separation of cyanins from the extraction from L. caerulea, and the K values of cyanins were summarised in Table 1.
Table 1 Partition coefficients(K) of cyanins in HSCCC solvent system

Solvent system 1 was used and resulted in contamination of the stationary phase. The retention of the stationary phase was less than 25%, which were hostile for HSCCC purification. Solvent system 2 was employed, small K-values were produced and the target peaks appeared too early to separate three cyanins from impurities. When solvent system 3 was used, the K value of compound I was 0.26 and a sub-optimal target peak appeared to separate three kinds of cyanins. Solvent system 4 was a polar solvent system which can be described as organic solvent-organic modifier-aqueous modifier-H2O. Appropriate K-values of the target compounds were within the range of 0.5-2.0, respectively. The retention of stationary phase was 48.3%. Therefore, solvent system 4 was chosen for purfication cyanins by HSCCC.
3.2 Flow rate and rotational speed of HSCCC
During the subsequent studies, different constant flow rates (1.5 and 2.0 mL/min) were tested and investigated, the results indicate that reducing flow rate could improve the reservation of the stationary phase in some degree [20]. While the mobile phase was pumped into the column at 1.5 mL/min, three kinds of target compounds were separated, the mobile phase was better set at 1.5 mL/min than at 2.0 mL/min. The rotation speed of 800 r/min was resulted in good separation of sample within a decent time period. The temperature has significant effect on K-values, the retention fraction of stationary phase and the mutual solvency of the two-phase. When the separation temperature was controlled at 25 °C, the HSCCC was carried out at a flow rate of 1.5 mL/min, with a rotational speed of 800 r/min, good separation results could be obtained and the separation time was acceptable.
The separation of cyanins from extract by optimised HSCCC system chromatogram is given in Fig. 1. Solvent system: methyl tert-butyl ether–n-butanol–acetonitrile– water–trifluoroacetic acid (2:2:1:5:0.01, volume ratio); flow rate of the mobile phase: 1.5 mL/min; detection wavelength: 280 nm; revolution speed: 800 r/min; column temperature: 25 °C; retention of stationary phase: 48.3%; sample size: 40 mg. Peak I shows cyanidin 3, 5-O-diglucoside collected during 101–108 min; Peak II shows cyanidin 3-O-rutinoside collected during 142– 152 min; Peak III shows cyanidin 3-O-glucoside collected during 194–215 min. The results show that three peaks could be separated at different retention time. The order of elution is not identical with HPLC data, revealing that in the case of HSCCC the order of elution and is cyanidin 3, 5-O-diglucoside, cyanidin 3-O- rutinoside, and cyanidin 3-O-glucoside. Here we can find that the major advantage of HSCCC technique is obvious, slight polarity differences between cyanidin 3-O- rutinoside and cyanidin 3-O-glucoside are sufficient to achieve complete separation on preparative scale. The major compounds were obtained and yielded 1.41 mg of fraction I, 1.08 mg of fraction II and 6.38 mg of fraction III. To its high purity, the proposed method is reproducible well as the HSCCC technique suggested by repeated experiments.
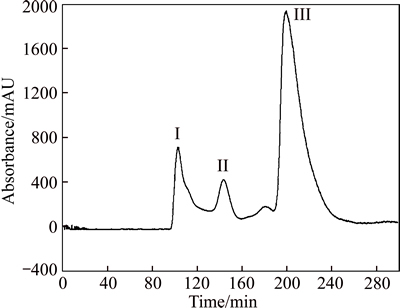
Fig. 1 HSCCC Chromatogram of extract from L. caerulea
HSCCC is an effective methodology for selective preparation of pure cyanins-done on a laboratory scale, and the result of this work is now available for further biological studies [15]. The present study demonstrates that a single chromatographic separation by HSCCC is able to yield pure three cyanins from a complex matrix of natural fruits extract. In this case, for isolating polar cyanins pigments, time consuming clean-up procedures are done before HSCCC separation, i.e. done by size- exclusion chromatography on Sephadex LH-20, or absorbance to AB-8 resin material was not necessary [21]. By improvement of HSCCC separation conditions through reducing flow rates, three kinds of cyanins were successfully obtained from crude extract. Therefore, valuable attributes of one step separating cyanins by HSCCC compared to other preparative methods are simple, effective and high sample recovery [22].
3.3 Analysis of crude sample and identification of cyanin fractions by HPLC-MS
Cyanin fractions from HSCCC, and together with the crude methanol extract were further identfied by their retention times on HPLC, UV-Vis spectra and comparison of MS spectra with previously published data [23]. Figure 2(a) shows a typical HPLC chromatogram of methanol extract of cyanins recorded at 520 nm, cyanidin 3-O-glucoside is represented 89.3% of the total cyanins. The results reveal that the purities of them are 95.8%, 92.4% and 97.6%, respectively, as determined by HPLC shown in Figs. 2(b)-(d).
To further verify the identity of the cyanins compounds. HPLC-MSn analysis was performed in positive mode with the [M+H]+ ion subjected to fragmentation, which allowed the identification of cyanins core (aglycone) shown in Fig. 3. Fraction I:
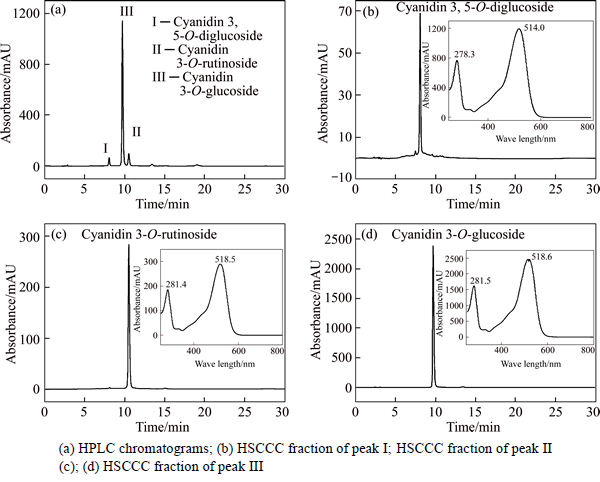
Fig. 2 HPLC analyses of sample of L. caerulea
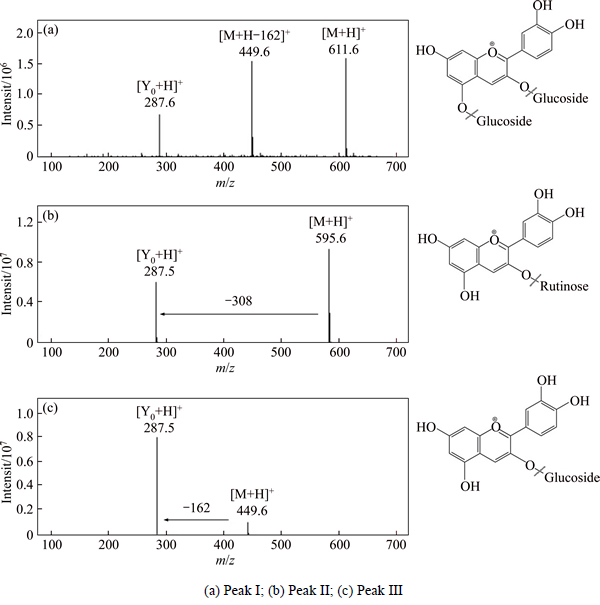
Fig. 3 Mass chromatogram of HSCCC fraction by ESI-MS
ESI-MS m/z: 611 [M+H]+, 449 [M+H-162]+ and 287 [Y0+ H]+, Rt = 8.058 min, and their UV λmax at 278 nm and 514 nm. The aglycon ion (287 m/z) indicates that it is cyanidin derivative, and if the cyanin contains two dehydrated hexoses (consequent loss of two 162), the two dehydrated hexoses would likely link to different positions of the aglycone, most likely at the 3- and 5- positions. Considering the preceding studies and MS fragmentation data, fraction I is tentatively identified as cyanidin 3, 5-O-diglucoside [24]. Fraction II: ESI-MS m/z: 595 [M+H]+, 287 [M+H-308]+, Rt=10.525 min, the MS fragmentation data (loss of 308 u) of fraction II suggested a rutinoside was the glycosyl substituent [25]. Plus the UV λmax at 281 nm (Band II) and 518 nm (Band I), fraction II was tentatively identified as cyanidin 3-O-rutinoside. Fraction III: ESI-MS m/z: 449 [M+H]+, 287 [M+H-162]+, Rt=9.717 min, fraction III and fraction II shared the similar UV λmax, but we can compare the different elution order and MS data with the reported literature, where ion at m/z 449 indicates the molecular mass of Cy3G. Amongst the ion products of HPLC-MSn, ion at m/z 287 shows high abundance and it corresponds to cyanin without the glucosemoiety. Fraction III is identified as cyanidin 3-O-glucoside [26]. In addition, fractions I, II and III are finally identified as cyanidin 3, 5-O-diglucoside, cyanidin 3-O-rutinoside and cyanidin 3-O-glucoside by co-elution with standards.
3.4 Effect of Cy3G on cell viability
The result suggests that Cy3G has potent cytotoxic activity against human hepatoma SMMC-7721 cell line but weak effect on normal liver cell lines L02 after 24 h treatment. The normal human hepatic cell strain, L02, acts as cytotoxicity reference. In contrast, only Cy3G is selectively cytotoxic to SMMC-7721 human liver cancer cells, while it does not show significant cytotoxicity to the survival of normal hepatic cells (Fig. 4). The SMMC-7721cell line decreases more susceptible to Cy3G as compared with that of L02 cell line. The cell viability of human hepatoma cells line SMMC-7721 significantly decreases in response to Cy3G and this decreases is more pronounced proportionately to increase in Cy3G concentration. Groups of higher Cy3G concentrations result in higher inhibitory activity on cancer cells proliferation, and groups with lower Cy3G concentrations result in lower inhibitory activity on cancer cell proliferation. The inhibition rate is then plotted against log concentration fitted in Fig. 4 and IC50 (half-maximal inhibitory concentration) value is calculated by linear regression analysis. Lower absorbance of the reaction mixture indicates higher free radical activity [27]. IC50 value (34.25 μg/L) is obtained for the human hepatoma SMMC-7721 cell viability after 24 h of blue honeysuckle anthocyanin treatment. Human breast carcinoma HS578T cells lines show similar sensitivities to the Cy3G, which suggests that the most abundant anthocyanin found in fruits shows a potent inhibition of the growth of cancer cells [10], indicating signficant inhibition effect of Cy3G on SMMC-7721 cells, and weak effect on normal liver cell lines L02 after the same treatment.
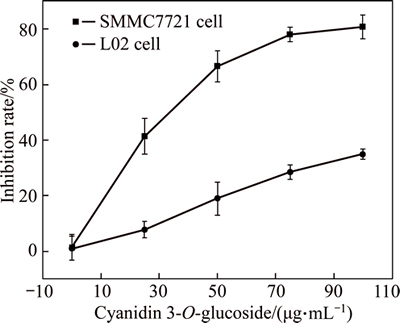
Fig. 4 MTT assay on inhibition rate of SMMC-7721 cells after treatment with Cy3G for 24 h
3.5 Flow cytometric analysis of Cy3G induced SMMC-7721 cells apoptosis
To confirm whether Cy3G induces SMMC-7721 cells apoptosis, SMMC-7721 cells are stained with Annexin V-fluorescein isotuiocyanate (FITC) and propidium iodide, and subsequently analyzed by flow cytometry. As shown in Fig. 5, in the left lower quadrant (Q3) staining is FITC-/PI-cells, the cells are stained with PI-FITC+/ as the early apoptotic cells, and the right upper quadrant Q2 staining is FITC+/propidium iodide (PI)+ for Q4. The detection results with flow cytometry show that the anti-proliferative effect of Cy3G for 24 h is mediated by the induction of apoptosis and highly correlated with Cy3G concentrations. Different concentrations of Cy3G are necessary to induce apoptosis in SMMC-7721 cells, even the lowest concentration tested is sufficient to increase the number of cells displaying features of apoptosis are necessary to trigger apoptosis in SMMC-7721 cells, even the lowest concentration tested is sufficient to increase the number of cells displaying features of apoptosis. After treatment with higher doses, the fraction of apoptotic cells increases not significantly. In summary, it is obviously suggested that a diphenylpropane-based polyphenolic ring structure contributes to the cell proliferation inhibitory activity against human cancer cell line studied [28]. The significantly induce apoptosis shows a moderately dose-dependent of Cy3G.
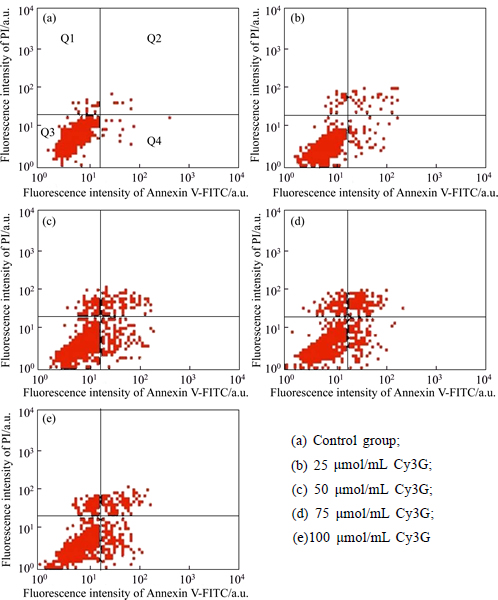
Fig. 5 Cell cycle distribution of SMMC7721 cell with treatment and after incubation different concentration of Cy3G for 24h
Cyanins extracts from plant foods are proved to have a series of benefits on various chronic diseases [29]. Cy3G is the most common cyanin with a glycoside. High-purity Cy3G as a monomer of cyanin is extracted from blue honeysuckle and purified by HSCCC, which has many merits including lower cost, higher speed, and greater loading of samples compared with the other purification methods. Apoptotic cell death is a consequence of a series of precisely controlled events that are frequently altered in cancer cells. Abrogation of apoptotic pathways is frequently found in diverse malignant cells arising from a complex interplay of genetic aberrations and misregulated pathways [29, 30]. Cy3G shows high cytotoxicity to SMMC-7721 cell spredominantly through induction of apoptosis, thus potentiating the clinical use of Cy3G in the treatment of tumors.
Not that many studies have been done on the molecular mechanisms involved in cyanins effect on hepatic protection. There is need to study the pure compounds in order to confirm some aspects regarding those mechanisms and the effect on them of different changes on the cyanidin structure. Until now, several studies have used cyanidins, aglycons, and hydroxyls as standard molecules for those studies [31]. However, molecular mechanisms of Cy3G act inside the live cancer cells as such powerful anticancer bioactivity in vivo has not been confirmed so far. As far as we know, cyanins are mainly present in animal or human plasma and tissues in their glycosylated form, glucuronidated or sulphated [32]. Additionally, the study of mechanistic differences of cyanins seems the most promising research line in this area.
4 Conclusions
In this study, L. caerulea is proved to be an outstanding source for the isolation of cyanins. HSCCC is successfully used for the isolation and purfication three kinds of cyanins from L. caerulea, which yields 1.41 mg of cyanidin 3, 5-O-diglucoside, 1.08 mg of cyanidin 3-O-rutinoside, and 6.38 mg of cyanidin 3-O-glucoside obtained from 40 mg crude extract. HSCCC will get more broadly applied for the separation and purification of bioactive compounds from natural food due to its unique advantages. Studies have indicated that Cy3G has antiproliferative activity on liver cancer cells via the induction of apoptosis, and the precise mechanism of action of Cy3G remains unclear. Our promising results reveal the need for future investigation on the use of cyanins from L. caerulea in clinical settings on the inhibition of cancer in vivo. Because of the demand for healthy nutrition, cancer prevention and anticancer medication, much attention has been paid on the anticancer activity of L. caerulea and its related compounds as valuable natural foods in recent years.
References
[1] IMANISHI H T, SUZUKI T, MASUDA. Accumulation of raffinose and stachyose in shoot apices of Lonicera caerulea L. during cold acclimation [J]. Scientia Horticulturae, 1998, 72: 255-263.
[2] SVARCOVAA I, HEINRICHB J, VALENTOVA K. Berry fruits as a source of biologically active compounds: the case of Lonicera Caerulea [J]. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 2007, 151(2): 163-174.
[3] PALIKOVA I, VALENTOVA K, OBORNA I, ULRICHOVA J. Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage [J]. Journal of Agricultural and Food Chemistry, 2009, 57(15): 6584-6589.
[4] SUN Jie, CHU Yi-gang, WU Xian-zhong, LIU Rui-hai. Antioxidant and Antiproliferative Activities of Common Fruits [J]. Journal of Agricultural and Food Chemistry, 2002, 50(25): 744-745.
[5] DAI Jin, MUMPER R J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties [J]. Molecules, 2010, 15(10): 7313-7352.
[6] ZHANG Yan-jun, SEERAM N P, LEE R, FENG L, HEBER D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties [J]. Journal of Agricultural and Food Chemistry, 2008, 56(3): 670-675.
[7] YANG Xiao-lan, YANG Lei, ZHENG Hai-ying. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats [J]. Food and Chemical Toxicology, 2010, 48(8, 9): 2374-2379.
[8] DE PASCUAL-TERESA S, MORENO D A, GARCIA-VIGUERA C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence [J]. International Journal of Molecular Sciences, 2010, 11(4): 1679-703.
[9] KALT W, HANNEKEN A, MILBURY P, TREMBLAY F. Recent research on polyphenolics in vision and eye health [J]. Journal of Agricultural and Food Chemistry, 2010, 58(7): 4001-4007.
[10] CHEN Pei-ni, CHU Shu-chen, CHIOU Hui-ling, CHIANG Chui-liang, YANG Shun-fa, HSIEH Y S. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo [J]. Nutrition and Cancer, 2005, 53(2): 232-243.
[11] WANG Chau-jong, WANG Jin-ming, LIN Wea-lung, CHU Chia-yih, CHOU Fen-pi, TSENG Tsui-hwa. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats [J]. Food and Chemical Toxicology, 2000, 38: 411-416.
[12] SEERAM N P. Berry fruits for cancer prevention current status and future prospects [J]. Journal of Agricultural and Food Chemistry, 2008, 56(3): 630-635.
[13] ITO Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography [J]. Journal of Chromatography A, 2005, 1065(2): 145-168.
[14] GU Ming, OUYANG Fan, SU Zhi-guo. Comparison of high-speed counter-current chromatography and high-performance liquid chromatography on fingerprinting of Chinese traditional medicine [J]. Journal of Chromatography A, 2004, 1022(1, 2): 139-144.
[15] DU Qi-zhen, JERZ G, WINTERHALTER P. Isolation of two anthocyanin sambubiosides from bilberry (Vaccinium myrtillus) by high-speed counter-current chromatography [J]. Journal of Chromatography A, 2004, 1045(1, 2): 59-63.
[16] DEGENHARDT A, KNAPP H, WINTERHALTER P. Separation and purification of anthocyanins by high-speed countercurrent chromatography and screening for antioxidant activity [J]. Journal of Agricultural and Food Chemistry, 2000, 48(2): 338-343.
[17] LI Bo, DU Wen-kai, QIAN Deng-yong, DU Qi-zhen. Combination of high-speed countercurrent chromatography and reversed phase C18 chromatography for large-scale isolation of cyanidin-3-O-β-d- glucoside from black rice bran extract [J]. Industrial Crops and Products, 2012, 37(1): 88-92.
[18] SALAS E, DUENAS M, SCHWARZ M, WINTERHALTER P, CHEYNIER V, FULCRAND H. Characterization of pigments from different high speed countercurrent chromatography wine fractions [J]. Journal of Agricultural and Food Chemistry, 2005, 53(11): 4536-4546.
[19] KUANG Peng-qun, SONG Dan, YUAN Qi-peng, LV Xin-hua, ZHAO Di, LIANG Hao. Preparative separation and purification of sulforaphene from radish seeds by high-speed countercurrent chromatography [J]. Food chemistry, 2013, 136(2): 309-315.
[20] CAI Fan-fan, LI Yang, ZHANG Min, ZHANG Hong-yang, WANG Yue-rong, HU Ping. Combination of integrated expanded bed adsorption chromatography and countercurrent chromatography for the direct extraction and purification of pseudohypericin and hypericin from St. John’s wort (Hypericum perforatum L.) [J]. Journal of Separation Science, 2015, 38(15): 2588-2596.
[21] VALLS J, ,
, ,
,  , AROLA L. Advanced separation methods of food anthocyanins, isoflavones and flavanols [J]. Journal of Chromatography A, 2009, 1216(43): 7143-7172.
, AROLA L. Advanced separation methods of food anthocyanins, isoflavones and flavanols [J]. Journal of Chromatography A, 2009, 1216(43): 7143-7172.
[22] CHEN Liang, XIN Xiulan, LAN Rong, YUAN Qi-peng, WANG Xiao-jie, LI Ye. Isolation of cyanidin 3-glucoside from blue honeysuckle fruits by high-speed counter-current chromatography [J]. Food Chemistry, 2014, 152: 386-390.
[23] ZHENG Jie, DING Chen-xu, WANG Liang-sheng, LI Guo-liang, SHI Junyou, LI Hui, WANG Honglun, SUO Yourui. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau [J]. Food Chemistry, 2011, 126(3): 859-865.
[24] FISCHER U A, CARLE R, KAMMERER D R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn [J]. Food Chemistry, 2011, 127(2): 807-821.
[25] BICUDO M O, RIBANI R H, BETA T. Anthocyanins, phenolic acids and antioxidant properties of Jucara fruits (Euterpe edulis M.) along the on-tree ripening process [J]. Plant Foods for Human Nutrition, 2014, 69(2): 142-147.
[26] MYJAVCOVA R, MARHOL P, KREN V, SIMANEK V, ULRICHOVA J, PALIKOVA I, PAPOUSKOVA B, LEMR K, BEDNAR P. Analysis of anthocyanin pigments in Lonicera (Caerulea) extracts using chromatographic fractionation followed by microcolumn liquid chromatography-mass spectrometry [J]. Journal of Chromatography A, 2010, 1217(51): 7932-7941.
[27] ZHONG Zhi-hong, CHEN Wen-gui, LIU Yong-he, LI Qi-xing, QIU Yue. Inhibition of cell growth and induction of apoptosis in human hepatoma cell line HepG2 by tanshione IIA [J]. Journal of Central South University (Science and Technology), 2007, 32(1): 99-103. (in Chinese)
[28] DE PASCUAL-TERESA S. Molecular mechanisms involved in the cardiovascular and neuroprotective effects of anthocyanins [J]. Archives of Biochemistry and Biophysics, 2014, 559: 68-74.
[29] JIANG Xin-wei, GUO Hong-hui, SHEN Tian-ran, TANG Xi-lan, YANG Yan-yang, LING Wen-hua. Cyanidin-3-O-β-glucoside purified from black rice protects mice against hepatic fibrosis induced by carbon tetrachloride via inhibiting hepatic stellate cell activation [J]. Journal of Agricultural and Food Chemistry, 2015, 63(27): 6221-6230.
[30] WANG Jing, WANG Xiu-jie, JIANG Shu-jiang, LIN Ping, ZHANG Jie, LU Yan-rong, WANG Qi, XIONG Zhu-juan, WU Ya-ying, REN Jing-jing, YANG Hong-liang. Cytotoxicity of fig fruit latex against human cancer cells [J]. Food and chemical toxicology, 2008, 46(3): 1025-1033.
[31] HIDALGO M, MARTIN-SANTAMARIA S, RECIO I, SANCHEZ- MORENO C, DE PASCUAL-TERESA B, RIMBACH G, DE PASCUAL-TERESA S. Potential anti-inflammatory, anti-adhesive, anti-strogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites [J]. Genes & nutrition, 2012, 7(2): 295-306.
[32] CZANK C, CASSIDY A, ZHANG Q, MORRISON D J, PRESTON T, KROON P A, BOTTING N P, KAY C D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study [J]. The American journal of clinical nutrition, 2013, 97(5): 995-1003.
(Edited by FANG Jing-hua)
Cite this article as: LUO Zhou-fei, YANG Yuan, WANG Liang-sheng, LI Hai-pu. Isolation of three cyanins from Lonicera caerulea L. fruits and its anticancer activity [J]. Journal of Central South University, 2017, 24(7): 1573-1581. DOI: 10.1007/s11771-017-3562-1.
Foundation item: Project(KSCX2-YW-N-043) supported by the Knowledge Innovation Program of the Chinese Academy of Sciences, China
Received date:2016-02-29; Accepted date: 2016-10-06
Corresponding author:WANG Liang-sheng, Professor; Tel: +86-10-62836654; E-mail: wanglsh@ibcas.ac.cn; LI Hai-pu, Professor; Tel: +86-731-88876961; E-mail: lihaipu@csu.edu.cn

