Trans. Nonferrous Met. Soc. China 23(2013) 1028-1032
Preparation and electrochemical performance of 2LiFe1-xCoxPO4-Li3V2(PO4)3/C cathode material for lithium-ion batteries
Jia-feng ZHANG, Bao ZHANG, Xue-yi GUO, Xing OU, Jian-long WANG, Chun-li PENG, Jun-chao ZHENG, He-zhang CHEN, Chao SHEN
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 7 May 2012; accepted 5 September 2012
Abstract: 2LiFe1-xCoxPO4-Li3V2(PO4)3/C was synthesized using Fe1-2xCo2xVO4 as precursor which was prepared by a simple co-precipitation method. 2LiFe1-xCoxPO4-Li3V2(PO4)3/C samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and electrochemical measurements. All 2LiFe1-xCoxPO4-Li3V2(PO4)3/C composites are of the similar crystal structure. The XRD analysis and SEM images show that 2LiFe0.96Co0.04PO4-Li3V2(PO4)3/C sample has the best-ordered structure and the smallest particle size. The charge-discharge tests demonstrate that these powders have the best electrochemical properties with an initial discharge capacity of 144.1 mA·h/g and capacity retention of 95.6% after 100 cycles when cycled at a current density of 0.1C between 2.5 and 4.5 V.
Key words: LiFePO4; Li3V2(PO4)3; Co doping; lithium-ion batteries
1 Introduction
The applications of electric vehicles (EVs) and hybrid electric vehicles (HEVs) require lithium-ion batteries to have the qualities of high energy density and power density, long cycling life, etc. Olivine-type LiFePO4 [1], as a promising cathode material for lithium-ion rechargeable batteries, has been extensively studied owing to its environmental friendliness, low costs, and good cycling stability. However, its low-electronic conductivity and low lithium-ion diffusivity within particles [2,3] result in a poor rate capability, which prevents it from being applied more widely. Many efforts including metal doping [4,5], coating with the electronically conductive materials like carbon, metal, and metal oxide [6-9], and optimization of particles with suitable preparation procedures [10,11] have been made to improve the performance of LiFePO4 cathode materials.
Compared with LiFePO4, monoclinic Li3V2(PO4)3 possesses higher ionic diffusion coefficient, better rate capability, higher discharge voltage and energy density [12,13]. Moreover, Li3V2(PO4)3 exhibits two pairs of redox plateaus around 3.62, 3.68, 4.08, and 4.55 V. It can extract/insert two Li ions reversibly between 3.0 and 4.3 V based on the V3+/V4+ redox coupled with an average operation voltage of 3.8 V. Three Li ions may be completely extracted when charged to 4.8 V with a mean intercalation voltage up to 4.0 V [14,15].
WANG et al [16] found that 2LiFePO4- Li3V2(PO4)3/C compound has great electrochemical performance, and it is highly desirable to investigate the valence of V in these LiFePO4 compounds. Moreover, an increase in the conductivity by the chemical substitution of Co for Fe resulting in LiFexCo1-xPO4 compositions has been reported [17].
In this work, a series of 2LiFe1-xCoxPO4- Li3V2(PO4)3/C samples were prepared using Fe1-2xCo2xVO4 as precursor compounds, with which LiCoPO4 formed together as an additive during the heating process. The electrochemical characterization of all the samples was carried out by galvanostatic charge– discharge experiments and cyclic voltammetry. Besides enhancement of conductivity and mean operation voltage, a high capacity was also expected for the LiFePO4-based Li-ion batteries.
2 Experimental
2LiFe1-xCoxPO4-Li3V2(PO4)3/C(x=0.01, 0.02, 0.03, 0.04, 0.05) samples were synthesized by means of co-precipitation method using Fe1-2xCo2xVO4 as precursor. The Fe1-2xCo2xVO4 precursor was prepared by a simple co-precipitation method, details of which are described as follows: a 0.2 mol/L NH4VO3 solution was pumped into a continuously stirred reactor at 80 °C, and 0.2 mol/L Fe(NO3)3 and Co(NO3)2 solution were added drop by drop at pH 6. The resultant slurry was aged in a sealed container at 25 °C for 12 h. The precipitate was collected by filtration, air-drying and then calcined at 600 °C for 6 h. Thereafter, the obtained Fe1-2xCo2xVO4 powder was mixed with a stoichiometric amount of Li2CO3, NH4H2PO4, glucose in acetone according to the carbon contents in 2LiFe1-xCoxPO4-Li3V2(PO4)3/C composite, and then ball milled for 4 h. After evaporating the acetone at 100 °C, the mixture was heat-treated in a horizontal quartz tube from ambient temperature to 750 °C with the heating rate of 5 °C/min and kept at that temperature for 12 h in N2 atmosphere. Eventually, 2LiFe1-xCoxPO4-Li3V2(PO4)3/C composite was synthesized.
The samples were characterized by X-ray diffraction (XRD, Rigaka D/MAX 2500V) using Cu Kα radiation (Kα=0.154 nm) to identify the crystalline phase of the prepared Fe1-2xCo2xVO4 and 2LiFe1-xCoxPO4- Li3V2(PO4)3/C samples. The data were collected in the range of 10°-90° in scanning rate of 8 (°)/min. The surface morphologies of the samples were observed with a scanning electron microscope (SEM, JSM-6380LV).
Electrochemical properties of 2LiFe1-xCoxPO4- Li3V2(PO4)3/C electrodes were measured after assembling them into coin cells (type CR2032) in an argon-filled glove box. The cathode was prepared by spreading a mixture of active material (80% in mass fraction), acetylene black (10%), and poly(vinylidene fluoride) binder (10%) dissolved in N-methyl pyrrolidone onto an aluminum foil current collector with thickness of 0.2 mm and dried in a vacuum oven at 120 °C. The cathode was separated from the lithium anode by a separator (Celgard 2300). The electrolyte was 1 mol/L LiPF6 dissolved in a mixture of ethylene carbonate (EC), dimethyl carbonate (DMC) and methyl–ethyl carbonate (EMC) with a volume ratio of 1:1:1. The cells were cycled between 2.5 and 4.5 V at ambient temperature using a battery testing system (Neware BTS-2000) at 0.1C current rate.
3 Results and discussion
3.1 Characterization of Fe1-2xCo2xVO4
The XRD patterns of the Fe1-2xCo2xVO4 samples are shown in Fig. 1. As can be seen, all the peaks can be readily indexed as a triclinic structure corresponding to FeVO4 and no impurity-phase peaks exist, indicating that the structure of the samples has not been changed with Co doping. The diffraction peaks are found to be very narrow, revealing that the samples have good crystallinity.
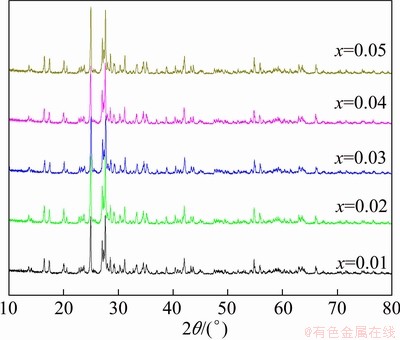
Fig. 1 XRD patterns of Fe1-2xCo2xVO4 samples
SEM micrographs of Fe1-2xCo2xVO4 samples with different Co contents are presented in Fig. 2. It can be clearly observed that all the samples consist of irregular primary particles. Besides, the size of irregular primary particles increases when the Co is doped. However, there is no difference in the micrographs of Fe1-2xCo2xVO4 with different Co contents. It is indicated that Co doping has little influence on the structure and micrographs of Fe1-2xCo2xVO4.
3.2 Structure and morphology of 2LiFe1-xCoxPO4- Li3V2(PO4)3/C
To prepare 2LiFe1-xCoxPO4-Li3V2(PO4)3/C, the Fe1-xCoxVO4 precursor was mixed with Li2CO3, NH4H2PO4 and glucose and then the mixture was treated by a high-temperature calcination process.
The XRD patterns of the 2LiFe1-xCoxPO4- Li3V2(PO4)3/C powders are shown in Fig. 3. All main peaks can be indexed as crystallized LiFePO4 phase and crystallized Li3V2(PO4)3 phase. The narrow diffraction peaks reveal that the samples have good crystallinity. These results indicate that solid-state reaction method and experimental conditions can produce 2LiFe1-xCoxPO4-Li3V2(PO4)3/C with high crystallinity and purity, which are key factors for a high performance electrode material. It has also been found that there is no visible difference between the XRD patterns of pristine 2LiFePO4-Li3V2(PO4)3/C and low Co-doped samples (x=0.01, 0.02), which indicates that Co has been doped into the host lattice or the amount of the composite formed from cobalt is too small. In contrast, for those high-level Co-doped samples (x=0.03, 0.04, 0.05), the diffraction intensity of peaks of LiFePO4 decreases to a certain extent, indicating that the structure of the material has a small change.
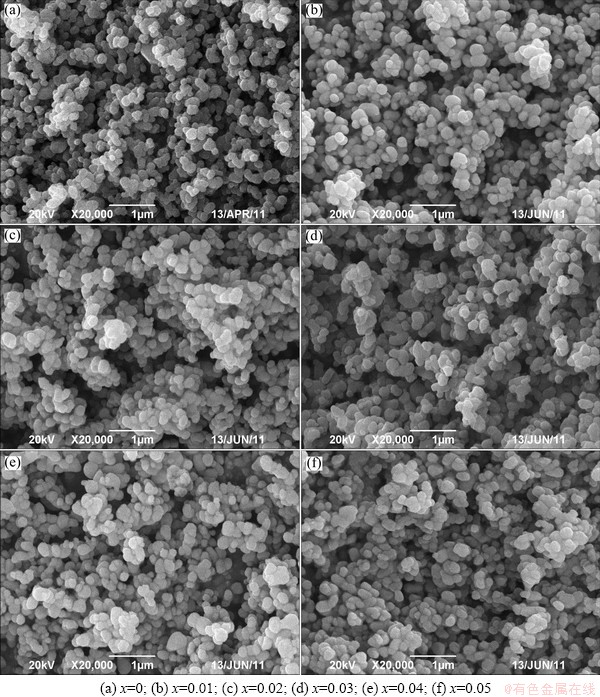
Fig. 2 SEM images of Fe1-2xCo2xVO4 samples prepared
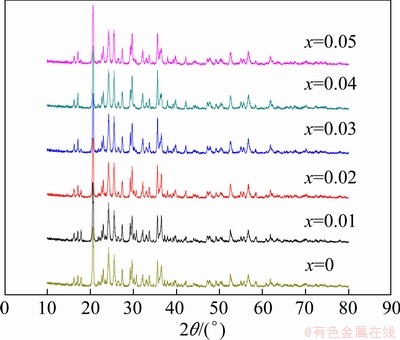
Fig. 3 XRD patterns of 2LiFe1-xCoxPO4-Li3V2(PO4)3/C samples
Figure 4 shows SEM images of 2LiFe1-xCoxPO4- Li3V2(PO4)3/C composites. The powders consist of agglomerates of primary particles. The morphology of composites is slightly changed with different amounts of Co doping. The particle size of pristine decreases when x is below 0.04, whereas that of pristine with x=0.05 increases and it presents irregular blocks with a wide size distribution.
3.3 Electrochemical performance of 2LiFe1-xCoxPO4- Li3V2(PO4)3/C
Figure 5 shows the initial charge-discharge voltage profiles of 2LiFe1-xCoxPO4-Li3V2(PO4)3/C composites synthesized with different Co doping amounts in the cut-off potential range of 2.5-4.5 V at 0.1C rate. The initial discharge capacities of composites with different cobalt contents of 0.01, 0.02, 0.03, 0.04 and 0.05 are 135.7, 140.1, 142.4, 144.1 and 139.4 mA·h/g, respectively. During the calculation of the discharge capacity of 2LiFe1-xCoxPO4-Li3V2(PO4)3/C samples, the active materials includes 10% carbon in the composites, but does not include the acetylene black added as electric conductor in the electrode fabrication.
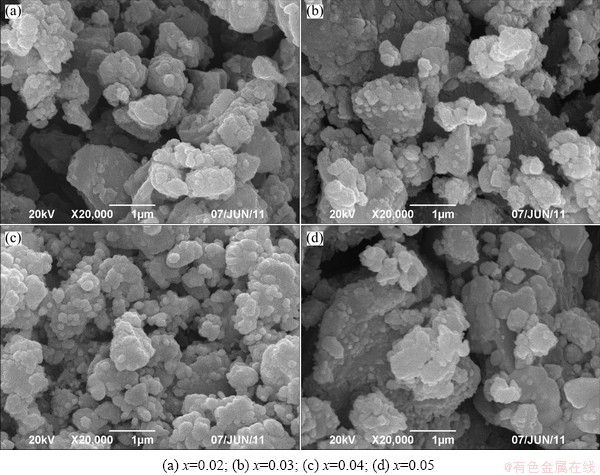
Fig. 4 SEM images of 2LiFe1-xCoxPO4-Li3V2(PO4)3/C samples
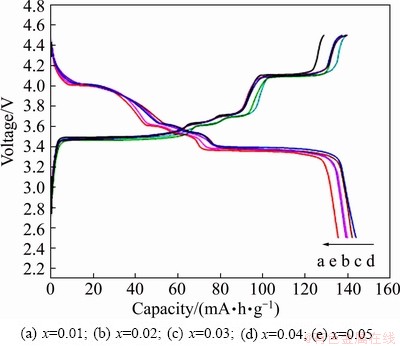
Fig. 5 Initial charge-discharge curves of 2LiFe1-xCoxPO4- Li3V2(PO4)3/C composites
Obviously, all the charge-discharge profiles exhibit four redox plateaus around 3.38, 3.62, 3.68 and 4.08 V, which are ascribed to one Li+ extracted based on the Fe2+/Fe3+ redox potential for LiFePO4 and three Li+ reversibly extracted based on the V3+/V4+ redox couple for Li3V2(PO4)3. Moreover, the redox plateaus around 3.38, 3.62 and 3.68 V of 2LiFe1-xCoxPO4- Li3V2(PO4)3/C (x=0.03, 0.04, 0.05) are higher than those of the samples with x=0.01 or 0.02. So, we can conclude that the proper content of Co-doping can effectively improve the discharge capacity and discharge potential plateau.
Specific capability is one of the important electrochemical characteristics of a lithium secondary battery for storage application. Figure 6 shows the cycling performance of 2LiFe1-xCoxPO4-Li3V2(PO4)3/C composites synthesized with different Co contents at 0.1C rate between 2.5 and 4.5 V at room temperature. 2LiFe1-xCoxPO4-Li3V2(PO4)3/C samples with x of 0.01, 0.02, 0.03, 0.04 and 0.05 keep the capacity retention of 90.3%, 91.4%, 92.2%, 95.6% and 92.8%, respectively after 100 cycles.
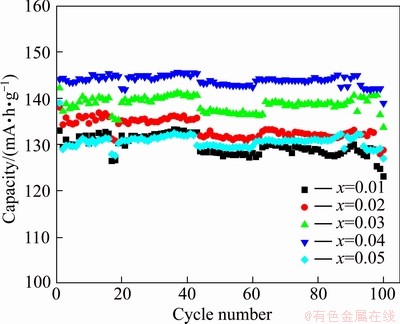
Fig. 6 Cycle performance of 2LiFe1-xCoxPO4-Li3V2(PO4)3/C with various amounts of Co doping at 0.1C
When Co content is less than 0.04, 2LiFe1-xCoxPO4-Li3V2(PO4)3/C composites show better cycling performance with the increase of carbon content because addition of cobalt enhances the conductivity of the composites [17]. Meanwhile, addition of cobalt can decrease the sizes of particles and thus reduce the volume change of composite particles during the charge-discharge cycles. So, addition of cobalt enhances the structural stability of composites in charge-discharge process and improves the cycling performance. However, when Co content is 0.05, the composites show a worse cycling performance. Among these cathodes, 2LiFe0.96Co0.04PO4-Li3V2(PO4)3/C shows the highest initial discharge capacity of 144.1 mA·h/g, and 95.6% capacity after 100 cycles.
4 Conclusions
1) 2LiFe1-xCoxPO4-Li3V2(PO4)3/C samples with different Co doping contents can be synthesized using Fe1-2xCo2xVO4 as precursor.
2) Co doping has little effect on structure and morphology of the precursor. All 2LiFe1-xCoxPO4- Li3V2(PO4)3/C composites are of the similar crystal structure with crystallized LiFePO4 phase and crystallized Li3V2(PO4)3 phase.
3) The maximum capacity of 144.1 mA·h/g is observed in 2LiFe0.96Co0.04PO4-Li3V2(PO4)3/C sample at 0.1C. The capacity retention of the sample remains at 95.6% after 100 cycles at 0.1C.
References
[1] PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. New cathode materials for rechargeable lithium batteries: The 3-D framework structures Li3Fe2(XO4)3(X=P, As) synthesis, redox potential evaluation and electrochemical characteristics of nasicon-related-3D framework compounds [J]. J Electrochem Soc, 1997, 144: 1188-1190.
[2] OU X Q, LIANG G C, LI W, XU S Z, ZHAO X. Effects of magnesium doping on electronic conductivity and electro-chemical properties of LiFePO4 prepared via hydrothermal route [J]. J Power Sources, 2008, 184: 543-547.
[3] GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries [J]. Chem Mater, 2010, 22(3): 587-603.
[4] YANG G, NI H, LIU H D, GAO P, JI H M, ROY S, PINTO J, JIANG X F. The doping effect on the crystal structure and electrochemical properties of LiMnxM1-xPO4 (M=Mg, V, Fe, Co, Gd) [J]. J Power Sources, 2011, 196: 4747-4755.
[5] ZHANG B, LI X H, LUO W B, WANG Z X. Electrochemical properties of LiFe1-xMgxPO4 for cathode materials of lithium ion batteries [J]. Journal of Central South University: Science and Technology, 2006, 37(6): 1094-1097. (in Chinese)
[6] AKIRA K, SHINYA S, MASARU M. High-rate properties of LiFePO4/carbon composites as cathode materials for lithium-ion batteries [J]. Ceramics International, 2008, 34(4): 863-866.
[7] LIN Y, GAO M X, ZHU D, LIU X F, PAN H G. Effects of carbon coating and iron phosphides on the electrochemical proper-ties of LiFePO4/C [J]. J Power Sources, 2008, 184: 444-448.
[8] JIN Y, YANG C P, RU X H, CHENG T, CHEN C H. V2O3 modified LiFePO4/C composite with improved electrochemical performance [J]. J Power Sources, 2011, 196: 5623-5630.
[9] YIN Y H, GAO M X, PAN, H G, SHEN L K, YE X, LIU Y F, FEDKIW P S, ZHANG X W. High-rate capability of LiFePO4 cathode materials containing Fe2P and trace carbon [J]. J Power Sources, 2012, 199: 256-262.
[10] DELACOURT C, POIZOT P, LEVASSEUR S, MASQUELIER C. Size effects on carbon-free LiFePO4 powders [J]. Electrochem Solid-State Lett A, 2006, 9: 352-355.
[11] LIU X, ZHAO Z. Synthesis of LiFePO4/C by solid-liquid reaction milling method [J]. Powder Technology, 2010, 197(3): 309-313.
[12] LI Y Z, ZHOU Z, REN M M, GAO X P, YAN J. Electrochemical performance of nanocrystalline Li3V2(PO4)3/carbon composite material synthesized by a novel so-gel method [J]. Electrochim Acta, 2006, 51: 6498-6499.
[13] HUANG H, YIN S C, KERR T, TAYLOR N, NAZAR L F. Nanostructured composites: A high capacity, fast rate Li3V2(PO4)3/carbon cathode for rechargeable lithium batteries [J]. Advanced Materials, 2002, 14: 1525-1526.
[14] YIN S C, GRONDEY H, STROBEL P, HUANG H, NAZAR L F. Charge ordering in lithium vanadium phosphates: Electrode materials for lithium-ion batteries [J]. Journal of the American Chemical Society, 2003, 125(2): 326-327.
[15] YIN S C, GRONDEY H, STROBEL P, ANNE M, NAZAR L F. Electrochemical property: Structure relationships in monoclinic Li3-yV2(PO4)3 [J]. Journal of the American Chemical Society, 2003, 125: 10402-10411.
[16] WANG L N, LI Z C, XU H J, ZHANG K L. Studies of Li3V2(PO4)3 additives for the LiFePO4-based Li ion batteries [J]. J Phys Chem C, 2008, 112: 308-312.
[17] RUFFO R, MARI C M, MORAZZONI F, ROSCIANO F, SCOTTI R. Electrical and electrochemical behaviour of several LiFexCo1-xPO4 solid solutions as cathode materials for lithium ion batteries [J]. Ionics, 2007, 13: 287-289.
2LiFe1-xCoxPO4-Li3V2(PO4)3/C锂离子电池正极材料的制备和性能
张佳峰,张 宝,郭学益,欧 星,王健龙,彭春丽,郑俊超,陈核章,沈 超
中南大学 冶金科学与工程学院,长沙 410083
摘 要:用共沉淀法合成的Fe1-2xCo2xVO4为原料制备2LiFe1-xCoxPO4-Li3V2(PO4)3/C。采用X射线衍射、扫描电镜以及电化学测试对2LiFe1-xCoxPO4-Li3V2(PO4)3/C样品进行表征。结果表明,所有2LiFe1-xCoxPO4- Li3V2(PO4)3/C 复合材料样品均有相似的晶体结构。X射线衍射和扫描电镜表明,2LiFe0.96Co0.04PO4-Li3V2(PO4)3/C具有最有序的晶体结构和细小、均匀的颗粒。充放电测试表明,2LiFe0.96Co0.04PO4-Li3V2(PO4)3/C具有最佳的电化学性能,在电压2.5~4.5 V,0.1C条件下首次放电比容量可达144.1 mA·h/g,循环100次后容量保持率仍可达到95.6%。
关键词: 磷酸铁锂;磷酸钒锂;钴掺杂;锂离子电池
(Edited by Hua YANG)
Foundation item: Project (51072233) supported by National Natural Science Foundation of China
Corresponding author: Bao ZHANG; Tel: +86-731-88836357; E-mail: csuzb@vip.163.com
DOI: 10.1016/S1003-6326(13)62562-5