DOI: 10.11817/j.ysxb.1004.0609.2021-39715
铜离子对工业电解液中镍电结晶行为的影响
徐仰涛1, 2,刘志健1, 2,朱珍旭1, 2,王雅宁1, 2
(1. 兰州理工大学 省部共建有色金属先进加工与再利用国家重点实验室,兰州 730050;
2. 镍钴资源综合利用国家重点实验室,金昌 737100)
摘 要:研究铜离子浓度对工业电解液中镍电结晶过程的影响。采用传统三电极体系,通过阴极极化法、循环伏安法、计时电流法分析铜离子对镍电结晶初期行为的影响。利用电位阶跃和霍尔槽实验制备沉积层并通过SEM观察其微观组织形貌,通过XRD分析其择优生长取向。结果表明:不同质量浓度铜离子的加入使得镍电结晶过电位降低,镍的起始沉积电位发生正移,但并不改变镍形核/长大的生长方式。铜离子的加入使镍形核弛豫时间tm逐渐缩短,峰电流Im逐渐增大;当铜离子质量浓度为0.5和1.0 g/L、阶跃电位为-0.85 V时,镍电结晶处于三维连续形核和三维瞬时形核之间;当阶跃电位处于-0.90~-1.00 V时,接近于三维瞬时形核。随着铜离子浓度增大到1.5 g/L、2.0 g/L时,镍电结晶由三维连续形核转变为三维瞬时形核;镍-铜电解液在电沉积过程中更适用于三维瞬时形核/生长机制。随着铜离子的引入,镍和铜发生共沉积,沉积层晶粒尺寸变大;沉积层的形貌由最初的小圆球颗粒逐渐变为金字塔状,生长方式以螺旋位错生长方式为主,晶粒逐渐由(111)面转变为(111)、(220)面生长。
关键词:镍;工业电解液;铜离子;电结晶行为
文章编号:1004-0609(2021)-04-0984-11 中图分类号:TQ151.8 文献标志码:A
引文格式:徐仰涛, 刘志健, 朱珍旭, 等. 铜离子对工业电解液中镍电结晶行为的影响[J]. 中国有色金属学报, 2021, 31(4): 984-994. DOI: 10.11817/j.ysxb.1004.0609.2021-39715
XU Yang-tao, LIU Zhi-jian, ZHU Zhen-xu, et al. Effect of copper ions on electrocrystallization behavior of nickel in industrial electrolyte[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(4): 984-994. DOI: 10.11817/j.ysxb.1004.0609.2021-39715
金属镍具有优良的物理性质、化学稳定性、良好的机械加工性能和力学性能,广泛应用在冶金、石油化工、电子工业、航天航空、能源电池等领域[1-3]。硫化镍阳极隔膜电解是我国最主要的生产电解镍的工艺,利用该种方法生产的电解镍占镍总产量的90%以上[4]。硫化镍可溶阳极/硫酸盐体系工业电解液成分复杂,因为可溶阳极主要由Ni3S2组成,此外还含有少量其他金属合金相如Cu2S和CoS等,加之其生产工艺等原因,电解液中无法避免地会含有各种杂质离子(如Cu2+、Cl-、Na+、Co2+等)[5-6]。不同离子对镍的电沉积过程会产生不同的影响,如影响金属的析出速度、析出位置以及金属的结晶方式等。在镍的电沉积过程中,电解液体系对晶体形核及长大过程有很大的影响。此外镍电结晶的生长形态不仅与电解液的组成有关,而且还与基体、沉积条件和添加剂等因素有关[7-9]。
根据已报道的镍电结晶以及离子对镍电结晶行为影响的相关文章,发现国内外学者主要集中在对镍在实验室配制电解液如瓦特液、柠檬酸盐溶液等中电沉积特性的研究,还有在电解液中添加金属离子、改变工艺参数以及添加剂对镍的电结晶行为和沉积层微观组织结构的影响研究较多。
EL BORAEI等[10]研究了L-脯氨酸对镍离子还原过程的影响,L-脯氨酸的加入使沉积电位变正起到催化作用,但不改变镍形核模式并得到细晶粒镀层。ALIMADADI等[11]在瓦特液中通过不同沉积参数研究了电沉积镍的织构演变,不同pH及电流密度下镀层的织构变化可能与界面能最小化以及孪晶的重复有关。而电沉积镍织构随电解液温度变化的原因可能与电解液中存在的 Cl-、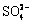 等化学物质在镍沉积层表面的吸附和解吸有关[12]。LIEZL等[13]研究认为,镍电解过程中添加Al3+在一定浓度时有利于镍的沉积,减小了镀镍过程中产生的内应力,并一定程度上促进了镍的形核。潘秉锁等[14]在研究Ce3+对镍电结晶过程的影响时发现,电解液加入铈离子后增加了镍沉积的阴极极化程度,同时使电结晶成核速率常数增大,晶体向外生长速度下降。唐徐情等[15]通过金属杂质离子对电镀镍层的影响研究发现,电解液中引入Co2+、Cr3+、Mn2+、Fe2+、Zn2+等离子,会对镀层形貌、厚度均匀性和质量产生影响。
等化学物质在镍沉积层表面的吸附和解吸有关[12]。LIEZL等[13]研究认为,镍电解过程中添加Al3+在一定浓度时有利于镍的沉积,减小了镀镍过程中产生的内应力,并一定程度上促进了镍的形核。潘秉锁等[14]在研究Ce3+对镍电结晶过程的影响时发现,电解液加入铈离子后增加了镍沉积的阴极极化程度,同时使电结晶成核速率常数增大,晶体向外生长速度下降。唐徐情等[15]通过金属杂质离子对电镀镍层的影响研究发现,电解液中引入Co2+、Cr3+、Mn2+、Fe2+、Zn2+等离子,会对镀层形貌、厚度均匀性和质量产生影响。
硫化镍可溶阳极/硫酸盐体系电解液(以下简称工业电解液)中杂质离子种类和含量较多,对该复杂体系中铜离子对镍形核和长大方式及其规律的研究较少。因此,本文对工业电解液中铜离子对镍形核/长大方式的影响进行研究,同时分析其对镍晶粒生长取向的影响。
1 实验
电化学实验采用传统的三电极体系,在普林斯顿电化学工作站(多通道系统PARSTAT MC)上进行测试,实验温度保持在(35±1) ℃,由恒温水浴装置控制。实验过程中选用镍电极作为工作电极,对电极为铂电极,饱和甘汞电极(SCE)用作参比电极,涉及到的所有电极电位均相对于SCE表示。电解液组成如下:取样于国内某公司生产线上的工业电解液,溶液pH=5.3,其成分为Ni2+ 70~75 g/L、Cl- 70 g/L、Na+ 40 g/L、Cu2+ 3 mg/L、Fe2+ 4 mg/L、Co2+ 20 mg/L、H3BO3 5 g/L、Zn2+ 0.35 mg/L。工业电解液作为基础电解液,含不同浓度铜离子的电解液通过向基础电解液中添加自配不同质量浓度(0.5、1.0、1.5和2.0 g/L)的硫酸铜溶液后配制而成,添加铜离子的工业电解液以下简称为镍-铜电解液。实验用无水硫酸铜(CuSO4)为分析纯,实验用水为自制蒸馏水。
在实验前,进行工作电极的处理,步骤如下:1) 工作电极在电极专用5000#、7000#砂纸上研磨至电极表面光滑;2) 0.5 μm高纯氧化铝粉末在电极抛光机上抛光;3) 用蒸馏水清洗其表面氧化铝粉末;4) 蒸馏水超声清洗;5) 酒精超声清洗;6) 蒸馏水超声清洗;7) 吹干。
单程线性伏安实验中,扫描电位范围为-0.1~ -1.0 V,扫描速率为30 mV/s;在循环伏安实验中,电位范围为0.2~-1.4 V,扫描速率40 mV/s;根据循环伏安曲线,在-0.85~-1.0 V范围内进行计时电流实验,同时使用JSM6700F型场发射电子显微镜观察电极上沉积层的表面微观形貌。
选用267 mL霍尔槽制备电沉积镍层,其配套的铜片作为阴极,阳极使用的是3 mm×60 mm×70 mm规格的纯镍,实验时先在稀盐酸中浸泡5 min并用蒸馏水处理干净,设定沉积温度为50 ℃,沉积时间10 min。使用扫描电子显微镜观察沉积层的表面微观形貌。通过D8-ADVANCE 型多晶X射线衍射仪分析镍沉积层的晶体结构以及生长方向,扫描范围10°~100°,扫描速度2 (°)/min。霍尔槽实验沉积层观察部分均选取自各阴极片相同部位。
2 结果与讨论
2.1 阴极极化曲线
图1所示为工业电解液及镍-铜电解液的阴极极化曲线对比图。由图1可知,在工业电解液中,镍的起始沉积电位出现在-0.82 V左右,添加不同质量浓度的铜离子后,通过局部放大图可以看出,在-0.34 V左右出现了一个还原峰,应为铜的还原峰,随着电位继续负向扫描时,在-0.72 V左右对应着为镍的沉积电位。通过对比工业电解液,随着铜离子的加入,不同质量浓度的铜离子均可以使得镍的沉积电位发生正移,电位由原来的-0.82 V正移到-0.72 V。由此可知,镍在电沉积过程中,阴极极化电位发生正移,表明其阴极过电位减小。从沉积动力学分析,随着过电位的减小,形核速率减小,生长速率增大[16]。

图1 添加不同质量浓度铜离子电沉积镍的阴极极化曲线
Fig. 1 Cathodic polarization curves of electrodeposited nickel with different mass concentrations of copper ions
2.2 循环伏安曲线分析
图2(a)所示为工业电解液的循环伏安曲线,从0.2 V开始负向扫描,到达-1.4 V之后开始正向扫描至0.2 V。电位开始从负向扫描,当扫描到-0.82 V左右电流逐渐增大,此时对应的是镍起始沉积电位,电位继续负向扫描时,即从-0.82 V起始,镍开始形核并长大。当电位开始往正方向扫描的时候,从局部放大图中可以看出(见图2(a)中插图),在-0.9 V左右出现了一个交叉点,即所谓的“电流环”,这说明镍电沉积经历了形核、长大的过程;在-0.4 V左右出现了一个氧化峰,应是镍的溶解峰。
图2(b)所示为不同质量浓度的镍-铜电解液和工业电解液的循环伏安曲线对照图。在镍-铜电解液中,当电位从负向扫描的时候,在-0.34 V左右都出现了小的还原峰,可能为铜的沉积峰;随着电位继续向负向扫描时,在-0.72 V左右电流逐渐增大,对应镍开始沉积,这与前面阴极极化曲线得到的结果相同。随着电位从正向扫描时,从局部放大图中(见图2(b)中插图),同样出现了感抗性电流环,说明镍结晶过程仍然按照形核长大方式;随着电位继续正向扫描时,出现了2个阳极反应峰,据推测是金属铜与镍的溶解峰。因此引入不同质量浓度的铜离子均降低了镍电结晶的过电位,这与之前文献报道的结果相符[17-18]。其原因可能为在镍的电沉积过程中,铜的标准电极电位较镍的要正,沉积过程中首先铜析出,紧接着镍析出;随着反应的进行,镍和铜发生了共沉积,使得原来镍的起始沉积电位发生了正移,从而促使了镍电结晶过程的发生。
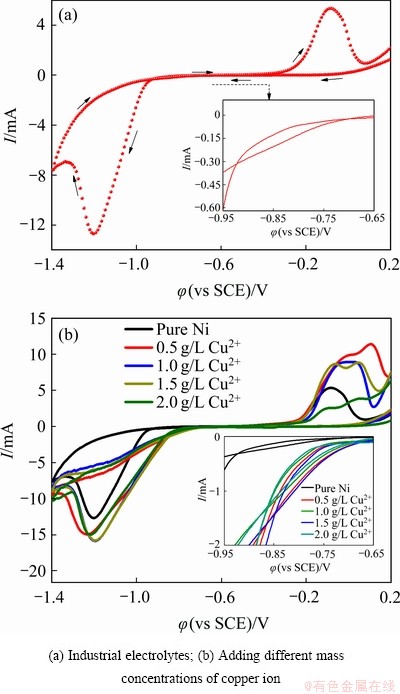
图2 电沉积镍的循环伏安曲线
Fig. 2 Cyclic voltammetry curves of electrodeposited nickel
2.3 计时电流曲线

图3 不同阶跃电位下电沉积镍的I-t曲线
Fig. 3 I-t curves of electrodeposition of nickel at different step potentials
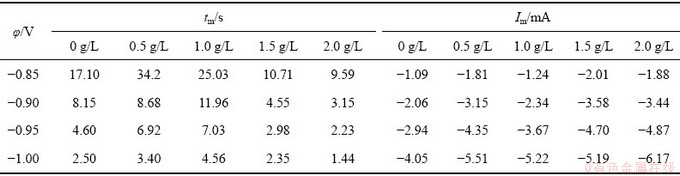
图3(a)所示为工业电解液中施加不同阶跃电位下的恒电位I-t曲线。从图3(a)可以看出:I-t曲线在不同阶跃电位下电流均随时间的延长而逐渐上升,达到一个最大值后逐渐出现衰减,然后逐渐趋于稳定,最后达到一个稳定值。当其阶跃电位由-0.85~-1.0 V发生负移时,即随着过电位的增加,电流值Im增加,相应的tm(峰值电流发生的时间)有规律地缩短。这表明随着过电位的攀升,成核速率逐渐增加,与此同时,成核的诱导时间逐渐减少。在高过电位下,电流在开始很短的时间内迅速攀升到一个最大值,在达到最大值前电流上升速率逐渐减缓,当达到峰电流时,电流开始逐渐减小,最后趋于一个恒定值。在不同的阶跃电位下,镍的形核生长表现出经典的扩散控制下三维生长的特征。在整个反应中,当沉积时间较短时,晶粒尺寸较小,此时,整个电极表面反应表现为扩散控制;随着沉积时间的延长,晶粒尺寸逐渐变大,离子在电极表面的电结晶过程,动力学方面的因素不可忽略,同时表面电场的作用下,当晶核尺寸不断增大,在达到一定的尺寸时,整个电极表面反应不仅表现为扩散控制,而且是扩散与电化学反应的混合控制[19]。
图3(b)所示为工业电解液添加不同质量浓度铜离子后电沉积镍的I-t曲线对比图。通过对比可以看出,在含2.0 g/L铜离子工业电解液中,其形核弛豫时间tm均逐渐减少,峰电流Im逐渐增大。在相同的阶跃电位下,含2.0 g/L铜离子的镍-铜电解液形核的峰电流比工业电解液的形核峰电流值大,形核弛豫时间tm逐渐减少,随着阶跃电位的负移,形核弛豫时间tm逐渐减少,峰电流Im逐渐增大。在实验范围内,2.0 g/L铜离子的存在降低了镍的结晶过电位,这个结果与之前所得的结论相一致。
对添加0.5~2.0 g/L的铜离子所得的I-t曲线进行数据处理,处理后的数据如表1所列。由表1可知,工业电解液和添加不同质量浓度铜离子工业电解液都遵循同样的规律,即随着施加过电位的升高,其形核的弛豫时间tm均逐渐减小,所对应的最大电流Im均逐渐增大。在施加过电位为-0.85 V、-0.90 V时,添加0.5 g/L、1.0 g/L铜离子时的形核弛豫时间均大于工业电解液的形核弛豫时间。这说明在添加0.5 g/L、1.0 g/L铜离子对镍的结晶形核产生了抑制作用,随着铜离子质量浓度增加到1.5 g/L和2.0 g/L时,其形核弛豫时间均小于工业电解液的形核弛豫时间,其对镍结晶形核促进作用逐渐增强。
表1 添加不同铜离子浓度电解液的I-t数据
Table 1 I-t data of electrolytes with different copper ion concentrations
根据SCHARIFKER提出的经典半球状晶核理论模型(SH模型),可以推导出恒电位暂态电流的无因次关系式[20]如下:
瞬时成核
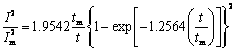 (1)
(1)
连续成核
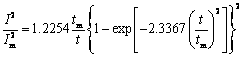 (2)
(2)
式中:Im代表峰值电流;tm代表峰值电流所对应的时间。将实验数据按照式(1)和(2)进行无量纲处理,计算得出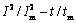 曲线,并同时绘制三维晶核瞬时成核和连续成核的
曲线,并同时绘制三维晶核瞬时成核和连续成核的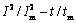 理论曲线作对比。
理论曲线作对比。
图4所示为工业电解液在不同阶跃电位下的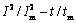 曲线,从图4可以看出,工业电解液中镍的电结晶过程基本遵循SH模型,近似半球状形核模式。当t<tm时,在-0.85~-0.95 V之间,实验曲线接近连续形核的理论模型曲线,在-1.0 V时,实验曲线略微发生了变化,实验曲线处在连续形核和瞬时形核曲线之间,并且接近连续形核曲线;在tm<t<2tm时,实验曲线接近于瞬时成核曲线;当t>2tm时,实验曲线逐渐偏离瞬时形核曲线,这可能是由于SH模型是理想的半球状,其生长控制因素完全是由扩散控制,由于工业电解液的复杂性,其控制方式并不是简单的扩散控制,而是由扩散控制与电化学反应的混合控制。
曲线,从图4可以看出,工业电解液中镍的电结晶过程基本遵循SH模型,近似半球状形核模式。当t<tm时,在-0.85~-0.95 V之间,实验曲线接近连续形核的理论模型曲线,在-1.0 V时,实验曲线略微发生了变化,实验曲线处在连续形核和瞬时形核曲线之间,并且接近连续形核曲线;在tm<t<2tm时,实验曲线接近于瞬时成核曲线;当t>2tm时,实验曲线逐渐偏离瞬时形核曲线,这可能是由于SH模型是理想的半球状,其生长控制因素完全是由扩散控制,由于工业电解液的复杂性,其控制方式并不是简单的扩散控制,而是由扩散控制与电化学反应的混合控制。
图5所示为含不同质量浓度铜离子工业电解液在不同阶跃电位下的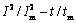 曲线。由图5可以看出,镍电结晶过程基本遵循SH模型,当铜离子质量浓度为0.5 g/L和1.0 g/L,施加阶跃电位为-0.85 V时,在t<tm时,实验曲线处于连续形核曲线和瞬时形核曲线之间;当阶跃电位处于-0.90~-1.00 V时,曲线接近于瞬时形核曲线,由此可知,施加电位的大小会对形核产生影响;在t>tm时,实验曲线靠近瞬时形核理论曲线,并逐渐偏离瞬时形核曲线。当添加1.5 g/L和2.0 g/L的铜离子时,在t<tm时,实验曲线接近瞬时形核曲线,在t>tm时,实验曲线为瞬时形核理论曲线,并逐渐偏离瞬时形核曲线。随着沉积时间的延长,铜离子的浓度不断增大,使得电极表面的形核位点增多,形核位点相互重叠覆盖,使得形核位点趋于饱和,不再产生新的形核位点,从而使得在不同的阶跃电位下电沉积时,镍形核方式由三维连续形核转变为三维瞬时形核。在镍的电沉积过程中,引入不同质量浓度铜离子的工业电解液更加符合三维生长瞬时成核的方式。
曲线。由图5可以看出,镍电结晶过程基本遵循SH模型,当铜离子质量浓度为0.5 g/L和1.0 g/L,施加阶跃电位为-0.85 V时,在t<tm时,实验曲线处于连续形核曲线和瞬时形核曲线之间;当阶跃电位处于-0.90~-1.00 V时,曲线接近于瞬时形核曲线,由此可知,施加电位的大小会对形核产生影响;在t>tm时,实验曲线靠近瞬时形核理论曲线,并逐渐偏离瞬时形核曲线。当添加1.5 g/L和2.0 g/L的铜离子时,在t<tm时,实验曲线接近瞬时形核曲线,在t>tm时,实验曲线为瞬时形核理论曲线,并逐渐偏离瞬时形核曲线。随着沉积时间的延长,铜离子的浓度不断增大,使得电极表面的形核位点增多,形核位点相互重叠覆盖,使得形核位点趋于饱和,不再产生新的形核位点,从而使得在不同的阶跃电位下电沉积时,镍形核方式由三维连续形核转变为三维瞬时形核。在镍的电沉积过程中,引入不同质量浓度铜离子的工业电解液更加符合三维生长瞬时成核的方式。

图4 工业电解液的无因次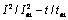 曲线
曲线
Fig. 4 Dimensionless 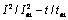 curve of industrial electrolyte
curve of industrial electrolyte
2.4 镍沉积层微观形貌分析
在工业电解液中添加2.0 g/L铜离子,设定阶跃电位为-0.95 V,沉积时间为1 s、2 s、4 s、50 s,分别观察各沉积层的微观形貌,得到如图6所示的SEM像。图6(a)中电极表面由随机分布的细小圆球形颗粒组成,圆球形颗粒之间存在着大量的空隙,沉积层较为疏松。图6(b)中随着沉积时间的延长,圆球形小颗粒逐渐增多,小颗粒相互连接,有的聚集成一个个小的团簇,在小团簇之间还存在着大量的空洞。在图6(c)中小圆颗粒慢慢覆盖住整个表面,颗粒发生了堆叠,沉积层变得更为致密,空洞逐渐减少。随着沉淀时间的增加,在图6(d)中沉积50 s的图中可以看出,镀层表面有类似鱼苗状的小颗粒,实际为棱锥状的细小颗粒组成,这些小颗粒沿着其箭头状顶点方向快速生长,相邻小颗粒之间发生聚集,小颗粒之间形成一个主干,向四周扩散。当沉积时间继续延长的时候,颗粒主干部位相互融合,进一步长大,最后合并成为类似金字塔状,说明生长方式为螺旋位错生长方式[21]。
对图6(a)和(d)进行EDS能谱分析,如图7所示。通过面扫描分析可以发现,沉积1 s、50 s所得到的沉积层,镍和铜均匀地分布在镍电极表面。这说明在电沉积初期阶段,铜离子加入后,镍和铜发生共沉积。随着沉积时间的增加,铜原子和镍原子的晶核相互合并,在同一个晶核长大,使得晶胞参数变大,进而晶粒尺寸变大。

图5 含不同质量浓度铜离子电沉积镍的无因次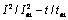 曲线
曲线
Fig. 5 Dimensionless 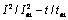 curves of electrodeposited nickel with different mass concentrations of copper ions
curves of electrodeposited nickel with different mass concentrations of copper ions
采用霍尔槽实验制备镍沉积层,观察其微观组织形貌。图8所示为工业电解液以及添加0.5 g/L、1.0 g/L、1.5 g/L、2.0 g/L 铜离子的镍-铜电解液中所得沉积层的SEM像。从图8(a)中可以看出,沉积层晶粒的形状为大小不均一的棱锥状,大晶粒和小晶粒相互交叠,小晶粒逐渐被大晶粒覆盖,在表面可见一些突出的大颗粒,说明发生了择优生长。沉积层表面呈现棱锥状,可知其通过螺旋位错生长方式进行[22]。在图8(b)、(c)、(d)、(e)中,随着铜离子质量浓度的增大,晶粒尺寸逐渐变大。晶粒仍然呈现出棱锥状,说明并没有改变其形核生长方式,依然是螺旋位错方式生长,铜离子的加入使得工业电解液中镍晶粒尺寸变大。由图8(a')可以看出,在基体表面零星的分布着一些形核位点,随着铜离子浓度的增大,由8(b′)、(c′)、(d′)、(e′)图像中可以看出,形核位点逐渐增多,这与前面电化学实验所得的结论相符,这些形核位点相互叠加覆盖,逐渐铺满整个基体表面。当铜离子质量浓度达到2.0 g/L时,小颗粒已经完全覆盖了基体表面。
2.5 择优生长取向
图9所示为通过霍尔槽实验在工业电解液中添加不同质量浓度的铜离子后所得沉积层的XRD谱。由图9可以看出,镍沉积层的晶体结构为简单面心立方结构。在工业电解液中,在44.51°出现了一个明显的衍射峰,分别对应(111)晶面。电沉积镍的生长方向主要沿(111)晶面,其峰型窄且尖锐,由此可知其产物较纯且结晶度较好。从添加了不同浓度的铜离子的衍射峰可以看出,不同的晶面峰值强度变化反映了晶粒存在择优取向,且随着铜离子浓度的变化而发生改变。当添加0.5 g/L的铜离子时,镍在(111)面的衍射强度增强;当添加1.0 g/L、1.5 g/L的铜离子时,沿(111)、(200)面的衍射强度降低;当添加2.0 g/L的铜离子时,沿(111)面的衍射强度变得更低,在76.37°出现了一个明显的衍射峰,对应为(220)晶面,在(220)面的衍射强度增大。晶粒的择优取向由(111)面择优生长逐渐转变为(111)、(220)面生长。

图6 电极表面沉积1 s、2 s、4 s、50 s得到的沉积层的SEM像
Fig. 6 SEM images of nickel deposits at different deposition time
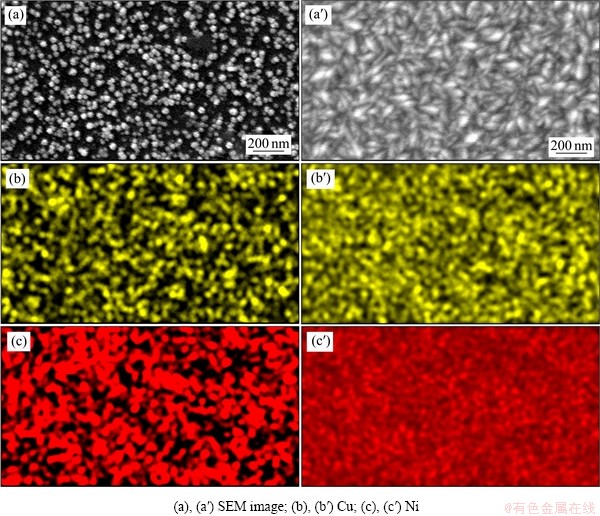
图7 电极沉积1 s、50 s时得到的沉积层的SEM像及元素面扫描分布
Fig. 7 SEM images and elemental map scanning distribution of deposits obtained from electrode deposited for 1 s((a), (b), (c)) and 50 s((a′), (b′), (c′))
图8 不同质量浓度铜离子电解液下沉积层的SEM像
Fig. 8 SEM images of deposits under copper ion electrolyte with different mass concentrations

图9 工业电解液中添加不同质量浓度铜离子后所得镍沉积层的XRD谱
Fig. 9 XRD spectrum of nickel deposits obtained by adding copper ions with different mass concentrations in industrial electrolyte
3 结论
1) 不同质量浓度铜离子的镍-铜电解液,在电位-0.34 V左右出现了铜的还原峰,铜离子的引入使得镍的起始沉积电位发生正移,降低了镍电结晶的过电位,促进了镍的形核生长。
2) 铜离子的加入并未改变镍的形核/长大机理。未引入铜离子的工业电解液中镍电结晶处于三维连续形核和三维瞬时形核之间,且接近于三维连续形核。在引入铜离子质量浓度为0.5 g/L、1 g/L,阶跃电位为-0.85 V的情况下,镍电结晶处于三维连续形核和三维瞬时形核之间,当阶跃电位处于-0.90~-1.00 V时,镍电结晶接近于瞬时形核。当铜离子浓度的增大到1.5和2 g/L时,其完全由三维连续形核/生长机制转变为三维瞬时形核。镍-铜电解液在电沉积过程中更加适用于三维瞬时成核/生长机制。
3) 铜离子引入后,镍、铜均匀地分布在基体表面,沉积层的形貌由最初的小圆球颗粒逐渐变为金字塔状,其生长方式仍以螺旋位错生长方式为主。在电沉积过程中,镍、铜发生共沉积,沉积层晶粒尺寸变大。镍的择优生长取向由(111)面转变为(111)、(220)面。
REFERENCES
[1] 侯晓川, 彭 俊. 电解镍应用于电镀中存在的质量问题及改进措施[J]. 稀有金属与硬质合金, 2012, 40(5): 70-72.
HOU Xiao-chuan, PENG Jun. Quality problems and improvement measures of electrolytic nickel in electroplating industry[J]. Rare Metals and Cemented Carbides, 2012, 40(5): 70-72.
[2] 杨 哲, 杨 晗, 程 伟, 等. 镍及镍合金产品标准现状分析及应用[J]. 有色金属加工, 2020, 49(2): 4-6, 11.
YANG Zhe, YANG Han, CHENG Wei, et al. Analysis and application on standard of nickel and nickel alloy product[J]. Nonferrous Metals Processing, 2020, 49(2): 4-6, 11.
[3] 吴 田. 镍基体系三维电极材料的制备及其电化学性能研究[D]. 北京: 中国科学院大学, 2018.
WU Tian. Synthesis and electrochemical performance study of nickel-based three-dimensional electrode materials[D]. Beijing: University of Chinese Academy of Sciences, 2018.
[4] 何焕华, 蔡乔方. 中国镍钴冶金[M]. 北京: 冶金出版社, 2009: 559-560.
HE Huan-hua, CAI Qiao-fang. Nickel and cobalt metallurgy in China[M]. Beijing: Metallurgical Press, 2009: 559-560.
[5] 陈自江. 镍电解精炼工[M]. 北京: 冶金工业出版社, 2016: 66-68.
CHEN Zi-jiang. Nickel electrolytic refiner[M]. Beijing: Metallurgical Industry Press, 2016: 66-68.
[6] 徐仰涛, 黄 凯, 朱珍旭. 工业电解液中镍阴极沉积和阳极溶出过程的EQCM研究[J]. 表面技术, 2019, 48(9): 293-299, 314.
XU Yang-tao, HUANG Kai, ZHU Zhen-xu. EQCM of nickel cathode deposition and anode dissolution in industrial electrolyte[J]. Surface Technology, 2019, 48(9): 293-299, 314.
[7] PISSOLATI N C, MAJUSTE D. Morphology, roughness and microhardness of nickel electrodeposits produced in sulfate media on 316L SS or Ti cathodes[J]. Hydrometallurgy, 2018, 175: 193-202.
[8] Lü Jin-long, LIANG Tong-xiang, WANG Chen. Effect of electrodeposition temperature on grain orientation and corrosion resistance of nanocrystalline pure nickel[J]. Journal of Solid State Chemistry, 2016, 240: 109-114.
[9] KOLONITS T, JENEI P, PETER L, et al. Effect of bath additives on the microstructure, lattice defect density and hardness of electrodeposited nanocrystalline Ni films[J]. Surface & Coatings Technology, 2018, 349: 611-621.
[10] EL BORAEI N F, IBRAHIM M A M. Catalytic effect of L-proline on the reduction of Ni(Ⅱ) ions during nickel electrodeposition from a Watts-type nickel bath[J]. Surface & Coatings Technology, 2018, 347: 113-122.
[11] ALIMADADI H, FANTA A B, KASAMA T, et al. Texture and microstructure evolution in nickel electrodeposited from an additive-free Watts electrolyte[J]. Surface & Coatings Technology, 2016, 299: 1-6.
[12] KARAYANNIS H S, PATERMARAKIS G. Effect of the Cl- and SO42- ions on the selective orientation and structure of Ni electrodeposits[J]. Electrochimica Acta, 1995, 40(9): 1079-1092.
[13] LIEZL S, EMMANUEL N N, KATHRYN C S, et al. Effect of aluminium on polarisation parameters and deposit characteristics in typical nickel sulfate electrolyte for electrowinning applications[J]. Hydrometallurgy, 2020, 194.
[14] 潘秉锁, 方小红, 黄志强. 铈离子对镍电结晶过程的影 响[J]. 表面技术, 2010, 39(3): 23-25.
PAN Bing-suo, FANG Xiao-hong, HUANG Zhi-qiang. Effects of cerium ions on the electrocrystallization of nickel[J]. Surface Technology, 2010, 39(3): 23-25.
[15] 唐徐情, 任秀斌, 陆海彦, 等. 镀液中金属杂质离子对电镀镍层性能的影响[J]. 高等学校化学学报, 2016, 37(7): 1364-1371.
TANG Xu-qing, REN Xiu-bin, LU Hai-yan, et al. Effect of metallic impurities in the electroplating solution on nickel coatings[J]. Chemical Journal of Chinese Universities, 2016, 37(7): 1364-1371.
[16] FLETCHER S. Some new formulae applicable to electrochemical nucleation/growth/collision[J]. Electrochimica Acta, 1983, 28: 917-923.
[17] 刘 宇, 谭澄宇, 贾志强, 等. 铜离子对镍在玻碳电极上电结晶行为的影响[J]. 中南大学学报(自然科学版), 2010, 41(1): 144-149.
LIU Yu, TAN Cheng-yu, JIA Zhi-qiang, et al. Effect of copper ions on behavior of nickel electro-crystallization on glassy carbon electrode[J]. Journal of Central South University(Science and Technology), 2010, 41(1): 144-149.
[18] 陈永言, 喻敬贤, 黄清安. 铜离子对镍电沉积行为的影响[C]// 中国机械工程学会. 1999年第二届表面工程国际会议论文集, 1999: 137-139.
CHEN Yong-yan, YU Jing-xian, HUANG Qing-an. Effect of copper ions on electrodeposition behavior of nickel[C]// Chinese Mechanical Engineering Society Proceedings of the Second International Conference on Surface Engineering in 1999, 1999: 137-139.
[19] 辜 敏, 杨防祖, 黄 令, 等. 氯离子对铜在玻碳电极上电结晶的影响[J]. 化学学报, 2002, 60(11): 1946-1950, 1892.
GU Min, YANG Fang-zu, HUANG Ling, et al. Effect of chloride ion on electrocrystallization of copper on glass carbon electrode[J]. Acta Chimicasinica, 2002, 60(11): 1946-1950, 1892.
[20] SCHARIFKER B, HILLS G. Theoretical and experimental studies of multiple nucleation[J]. Electrochimica Acta, 1983, 28: 879-889.
[21] 周绍民. 金属电沉积-原理与研究方法[M]. 上海: 上海科学技术出版社, 1987: 221-226.
ZHOU Shao-min. Electrodeposition of metals-principles and research methods[M]. Shanghai: Shanghai Science and Technology Press, 1987: 221-226.
Effect of copper ions on electrocrystallization behavior of nickel in industrial electrolyte
XU Yang-tao1, 2, LIU Zhi-jian1, 2, ZHU Zhen-xu1, 2, WANG Ya-ning1, 2
(1. State Key Laboratory of Advanced Processing and Recycling of Non-ferrous Metal, Lanzhou University of Technology, Lanzhou 730050, China;
2. State Key Laboratory for Comprehensive Utilization of Nickel and Cobalt Resources, Jinchang 737100, China)
Abstract: In order to study the effect of different concentrations of copper ions on the electrocrystallization behavior of nickel in industrial electrolytes, the traditional three-electrode system was used to analyze the effect of copper ions on the initial behavior of nickel electrocrystallization through cathodic polarization, cyclic voltammetry and chronoamperometry. The microstructure morphology was observed by SEM using chronoamperometry and Hall cell experiment, and the preferred growth orientation was analyzed by XRD. The results show that the addition of copper ions with different mass concentrations decreases the electrocrystallization overpotential of nickel and the initial deposition potential of nickel shifts positively, which does not change the mode of nickel through the way of nucleation/growth. The addition of copper ions gradually reduces the nucleation relaxation time tm, and the peak current Im gradually increases. When the copper ion mass concentrations are 0.5 and 1.0 g/L, and the step potential is -0.85 V, nickel electrocrystallization is between the three-dimensional continuous nucleation and the three-dimensional instantaneous nucleation; when the step potential is -0.90--1.00 V, nickel electrocrystallization is close to instantaneous nucleation. As the copper ion concentration increases to 1.5 g/L and 2.0 g/L, nickel electrocrystallization changes from the three-dimensional continuous nucleation to the three-dimensional instant nucleation. Nickel-copper electrolyte is more suitable for three-dimensional instantaneous nucleation/growth mechanism during electrodeposition. With the introduction of different mass concentrations of copper ions, nickel and copper are co-deposited, the grain size becomes larger. The morphology of the sedimentary layer gradually changes from the original small spherical particles to pyramid shape, and the growth mode is still dominated by screw dislocation growth. The grain orientation changes from (111) plane to (111), (220) plane.
Key words: nickel; industrial electrolyte; copper ions; electrocrystallization behavior
Foundation item: Project supported by the State Key Laboratory for Comprehensive Utilization of Nickel and Cobalt Resources, China
Received date: 2020-05-25; Accepted date: 2020-10-28
Corresponding author: XU Yang-tao; Tel: +86-931-2973905; E-mail: lanzhouxuyt@163.com
(编辑 龙怀中)
基金项目:镍钴资源综合利用国家重点实验室资助项目
收稿日期:2020-05-25;修订日期:2020-10-28
通信作者:徐仰涛,教授,博士;电话:0931-2973939;E-mail:lanzhouxuyt@163.com