Inhibition effect of a synthesized N, N′-bis(2-hydroxybenzaldehyde)-1, 3-propandiimine on corrosion of mild steel in HCl
来源期刊:中南大学学报(英文版)2013年第2期
论文作者:O. Ghasemi I. Danaee G.R. Rashed M. RashvandAvei M. H. Maddahy
文章页码:301 - 311
Key words:corrosion inhibitor; Schiff base; adsorption; Langmuir equation; atomic force microscope
Abstract: The corrosion inhibition of mild steel in 1 mol/L HCl by N, N′-bis (2-hydroxybenzaldehyde)-1, 3-propandiimine (2-HBP) has been investigated by using potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and chronoamperometry measurements. The experimental results suggest that this compound is an excellent corrosion inhibitor for mild steel and the inhibition efficiency increases with the increase in inhibitor concentration. Polarization curves reveal that this organic compound is a mixed type inhibitor. The effect of temperature on the corrosion behavior of mild steel with the addition of the Schiff base was studied in the temperature range from 25 °C to 65 °C. The experimentally obtained adsorption isotherms follow the Langmuir equation. Activation and thermodynamic adsorption parameters such as Ea, ΔH, ΔS, Kads and ΔGads were calculated by the corrosion currents at different temperatures and using the adsorption isotherm. The morphology of mild steel surface in the absence and presence of 2-HBP was examined by atomic force microscope (AFM) images.
J. Cent. South Univ. (2013) 20: 301–311
DOI: 10.1007/s11771-013-1488-9
O. Ghasemi1, I. Danaee1, G.R. Rashed1, M. RashvandAvei2, M. H. Maddahy
1. Abadan Faculty of Petroleum Engineering, Petroleum University of Technology, Abadan 63165–619, Iran;
2. Department of Chemistry, K. N. Toosi University of Technology, Tehran 15875–4416, Iran
 Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Abstract: The corrosion inhibition of mild steel in 1 mol/L HCl by N, N′-bis (2-hydroxybenzaldehyde)-1, 3-propandiimine (2-HBP) has been investigated by using potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and chronoamperometry measurements. The experimental results suggest that this compound is an excellent corrosion inhibitor for mild steel and the inhibition efficiency increases with the increase in inhibitor concentration. Polarization curves reveal that this organic compound is a mixed type inhibitor. The effect of temperature on the corrosion behavior of mild steel with the addition of the Schiff base was studied in the temperature range from 25 °C to 65 °C. The experimentally obtained adsorption isotherms follow the Langmuir equation. Activation and thermodynamic adsorption parameters such as Ea, △H, △S, Kads and △Gads were calculated by the corrosion currents at different temperatures and using the adsorption isotherm. The morphology of mild steel surface in the absence and presence of 2-HBP was examined by atomic force microscope (AFM) images.
Key words: corrosion inhibitor; Schiff base; adsorption; Langmuir equation; atomic force microscope
1 Introduction
Acid solutions with pH values below one are generally used for industrial acid cleaning, acid descaling, oil well acidizing, the pickling and removal of undesirable rust [1–3]. Mild steels which are extensively used in a lot of industrial processes, could be corroded during these acidic applications particularly with the use of HCl [4–5].
Corrosion prevention systems favor the use of corrosion inhibitors with low or zero environmental impacts. Inhibitors are chemicals that react with a metallic surface or the environment. Inhibitors decrease corrosion processes by increasing the anodic or cathodic polarization behavior, reducing the movement or diffusion of ions to the metallic surface and increasing the electrical resistance of the metallic surface [6].
The use of organic molecules as corrosion inhibitor is one of the most practical methods for protecting against the corrosion and it is becoming increasingly popular. These compounds in general are adsorbed on the metal surface, blocking the active corrosion sites. Four types of adsorption may take place by organic molecules at metal/solution interface: (1) electrostatic attraction between the charged molecules and the charged metal; (2) interaction of unshared electron pairs in the molecule with the metal; (3) interaction of π-electrons with the metal; (4) combination of (1) and (2).
The adsorption ability of inhibitors onto metal surface depends on the nature and surface charge of metal, the chemical composition of electrolytes, and the molecular structure and electronic characteristics of inhibitor molecules. It was shown that organic compounds containing heteroatoms such as nitrogen, sulphur, oxygen and phosphor are capable of forming coordinate covalent bond with metal owing to their free electron pairs and thus acting as inhibitor. π electrons in triple or conjugated double bonds also exhibit good inhibitive properties [7–9]. Schiff bases (with RC=NR′ as general formula) have the both features combined with their structure which may then give rise to particularly potential inhibitors [10]. Schiff bases are condensation products of an amine and a ketone or aldehyde and the first Schiff base metal complexes were prepared and described in the literature by Schiff in 1864. An interesting phenomenon is that Schiff bases systematically display considerably stronger corrosion inhibition efficiencies than the corresponding amines. Schiff bases have been recently reported as effective corrosion inhibitors for steel, aluminum and copper in acidic media. The greatest advantages of Schiff bases are [11–12]: (1) they can be synthesized conveniently from inexpensive raw materials; (2) they contain the electron cloud on the aromatic ring or, the electronegative atoms such as nitrogen and oxygen in the relatively long chain compounds; (3) harmless for environment.
The purpose of the present work is to investigate the effect of synthesized Schiff base corrosion inhibitor named: N, N′-bis (2-hydroxybenzaldehyde)-1, 3- propandiimine (2-HBP) on the corrosion of mild steel in 1 mol/L HCl, evaluated by Tafel polarization data and electrochemical impedance spectroscopy (EIS). Effects of concentration and temperature on the inhibition efficiencies of the selected Schiff base have been studied systematically. Thermodynamic parameters such as enthalpy, entropy and Gibbs free energy were evaluated from experimental data of the inhibition process at different temperatures.
2 Experimental
2.1 Material preparation
All chemicals used in the present work were of reagent-grade Merck product and used as received without further purification. 2-HBP was prepared according to the described procedure [13]. 2-hydroxybenzaldehyde (0.122 g, 2 mmol) was added to a stirred ethanolic solution (20 mL) of 1, 3-diaminopropane (0.074 g, 1 mmol). The light yellow solution was stirred and heated to reflux for 6 h. Then, the solvent of reaction was evaporated in a rotary evaporator. And the reaction mixture was cooled in the refrigerator for several days to induce precipitation. A bright yellow precipitate was obtained and was then washed with diethyl ether and dried. Analysis was conducted for C17H18N2O2(282.3): 72.32% C, 6.42% H, 9.92% N. Experimentally obtained: 72.45% C, 6.56% H, 9.89% N. Identification of structure of synthesized Schiff base was performed by IR, 1HNMR and 13C-NMR spectroscopic techniques. The Schiff base used in this work is presented in Fig. 1.
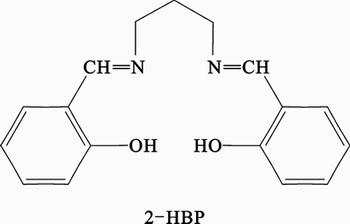
Fig. 1 Chemical structure of 2–HBP Schiff base
In the DFT calculations, the geometry of 2-HBP Schiff base was fully optimized in the gas phase without any symmetry constraints and using the non-local hybrid density functional B3LYP [14], combining Becke’s three-parameter hybrid exchange functional with the correlation functional of LEE et al [15]. The standard basis set of atomic functions 6-31G (d, p) was used for this optimization [16–17]. All the calculations were carried out with the Gaussian-03 program package.
2.2 Electrochemical measurements
Working electrodes were prepared from mild steel specimens of the chemical composition (mass fraction, %) C 0.16, Si 0.32, Mn 0.35, P 0.03, S 0.02, and the balance Fe. Samples were cut from a cylindrical rod with a cutter machine. The exposed surface area of each electrode is equal to 0.81 cm2. These specimens were used as working electrode in electrochemical measurements and the exposed areas of the electrodes were mechanically abraded with 220, 400, 600, 800, 1 000 and 1 200 grades of emery paper, degreased with acetone and rinsed by distilled water before each electrochemical experiment.
Electrochemical measurements were carried out in a conventional three-electrode system. A saturated calomel electrode (SCE) was used as the reference electrode and a platinum sheet was used as the counter electrode. Before each experiment, the working electrode was immersed in the test cell for 30 min until reaching steady state condition. The electrochemical measurements were carried out using computer controlled Auto Lab potentiostat/galvanostat (PGSTAT 302 N). Polarization curves were recorded at constant sweep rate of 1 mV/s in 1 mol/L HCl solution. All tests were carried out at constant temperature by controlling the cell temperature using a water bath. All the experiments was performed in quiescent conditions and solutions were open to the atmosphere under unstirred conditions.
Corrosion current Icorr was calculated from Tafel extrapolation methods. The measurements were repeated three times for each condition and the average values were presented. The polarization resistance was calculated from the slope of the potential versus current plots.
Electrochemical impedance spectroscopy (EIS) measurements were carried out in frequency range from 100 kHz to 10 mHz with amplitude of 10 mV peak-to- peak using AC signals at open circuit potential. Fitting of experimental impedance spectroscopy data to the proposed equivalent circuit was done by means of home written least square software based on the Marquardt method for the optimization of functions and Macdonald weighting for the real and imaginary parts of the impedance [18–19].
Atomic force microscopy (AFM), a very high-resolution type of scanning probe microscopy, with demonstrated resolution on the order of fractions of a nanometer, was carried out as a surface topography. Before AFM analyses, the steel specimens were immersed in solutions (with and without inhibitor separately) for 1 d at 25 °C. The exposed steel specimens were determined by commercial atomic force microscope (Nanosurf easyScan2 AFM, Switzerland).
3 Results and discussion
3.1 Potentiodynamic polarization measurements
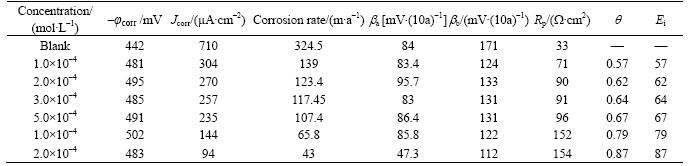
Figure 2 shows anodic and cathodic polarization plots of mild steel in 1 mol/L HCl in the absence and presence of 2-HBP inhibitor of different concentrations at 25 °C. Table 1 gives the electrochemical corrosion kinetic parameters such as corrosion potential (φcorr vs. SCE), corrosion current density (Jcorr), cathodic and anodic Tafel slopes (βa, βb), the degree of surface coverage (q) and inhibition efficiency (Ei) obtained by extrapolation of the Tafel lines. The degree of surface coverage and inhibition efficiency for different concentrations of inhibitor are calculated using the following equations [7–20]:
 (1)
(1)
 (2)
(2)
where Ei is the inhibition efficiency, 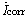 and Icorr are the corrosion current densities determined by the intersection of the extrapolated Tafel lines and the corrosion potential for mild steel in uninhibited and inhibited acid solution, respectively.
and Icorr are the corrosion current densities determined by the intersection of the extrapolated Tafel lines and the corrosion potential for mild steel in uninhibited and inhibited acid solution, respectively.
As expected, both anodic and cathodic reactions of mild steel electrode corrosion were inhibited with the increase of the inhibitor concentration. This result suggests that the addition of the inhibitor reduces anodic dissolution and also retards the hydrogen evolution reaction [21]. The electrochemical processes on the metal surface are likely to be closely related to the adsorption of the inhibitor and the adsorption is known to depend on the chemical structure of the inhibitor. The best inhibition efficiency was about 87% at concentration of 2×10–3 mol/L. It can be seen that the corrosion rate decreased and inhibition efficiency (Ei) increased by increasing inhibitor concentration. Figure 2 shows that the inhibitor causes change in the cathodic Tafel slopes. No definite trend was observed in the shift of φcorr values in the presence of different concentrations of the inhibitor, suggesting that this compound behaves as mixed-type inhibitor [22]. However, the influence is more pronounced in the cathodic polarization plots compared to that in the anodic polarization plots. It is clear that the addition of the inhibitor shifts the cathodic curves to a greater extent toward the lower current density when compared to the anodic curves. The φcorr value is also shifted to the more negative side with an increase in the inhibitor concentration [23]. Polarization resistance (Rp) values were determined from the slope of the polarization curve and calculated using Stern–Geary equation which is given below [24–25]:
 (3)
(3)
By increasing the inhibitor concentration, the polarization resistance increases in the presence of compound, indicating adsorption of the inhibitor on the metal surface to block the active sites efficiently and inhibit corrosion [26].

Fig. 2 Anodic and cathodic polarization curves for mild steel in 1 mol/L HCl without and with various concentration of inhibitor at 25 °C:
Table 1 Potentiodynamic polarization parameters for corrosion of mild steel in 1 mol/L HCl solution in absence and presence of different concentrations of 2-HBP at 25 °C
3.2 Electrochemical impedance spectroscopy
Figure 3 shows Nyquist plots recorded for the corrosion of steel in 1 mol/L HCl solution without and with different concentrations of inhibitor obtained at φcorr. The plots show a depressed capacitive loop which arises from the time constant of the electrical double layer and charge transfer resistance. The impedance of the inhibited steel increases with increasing the inhibitor’s concentration and consequently the inhibition efficiency increases. The equivalent circuit compatible with the Nyquist diagram recorded in the presence of inhibitor is depicted in Fig. 4.
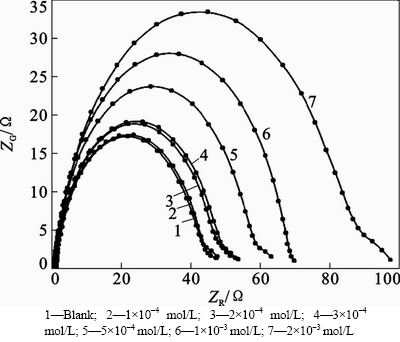
Fig. 3 Nyquist plots for mild steel in 1 mol/L HCl without and with various concentrations mol/L of 2-HBP at 25 °C:
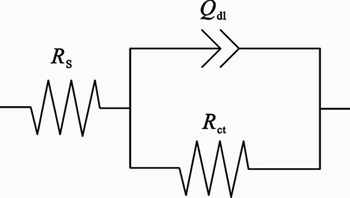
Fig. 4 Equivalent circuit compatible with experimental impedance data for corrosion of steel electrode in different inhibitor concentrations
The simplest approach requires the theoretical transfer function Z(ω) to be represented by a parallel combination of a resistance Rct and a capacitance C, both in series with another resistance Rs [27]:
 (4)
(4)
where ω is the frequency in rad/s, ω=2πf and f is frequency in Hz.
To obtain a satisfactory impedance simulation of steel, it is necessary to replace the capacitor (C) with a constant phase element (CPE) Q in the equivalent circuit. The most widely accepted explanation for the presence of CPE behavior and depressed semicircles on solid electrodes is microscopic roughness, causing an inhomogeneous distribution in the solution resistance as well as in the double-layer capacitance [28]. Constant phase element Cdl, Rs and Rct can be corresponded to double layer capacitance, solution resistance, and charge trasfer resistance, respectively. To corroborate the equivalent circuit, the experimental data are fitted to equivalent circuit and the circuit elements are obtained. Table 2 illustrates the equivalent circuit parameters for the impedance spectra of corrosion of steel in 1 mol/L HCl solution. The results demonstrate that the presence of 1×10–4– 2×10–3 mol/L inhibitor enhances the value of Rct obtained in the pure medium while that of Qdl is reduced. The decrease in Qdl is caused by adsorption of inhibitor, indicating that the exposed area decreases. On the other hand, a decrease in Qdl, which can result from a decrease in local dielectric constant and/or an increase in the thickness of the electrical double layer, suggests that Schiff base inhibitor acts by adsorption at the metal/solution interface.
Table 2 Impedance data for mild steel in 1 mol/L HCl solution without and with different concentrations of 2-HBP at 25 °C
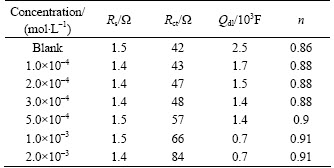
As the Qdl exponent (n) is a measure of the surface heterogeneity, values of n indicates that the steel surface becomes more and more homogeneous as the concentration of inhibitor increases as a result of its adsorption on the steel surface and corrosion inhibition. The increase in values of Rct and the decrease in values of Qdl with increasing the concentration also indicate that Schiff base acts as primary interface inhibitor and the charge transfer controls the corrosion of steel under the open circuit conditions.
3.3 Effect of temperature
The adsorption phenomenon has been successfully explained by thermodynamic parameters. To elucidate the inhibition properties of inhibitor, the kinetic model was a useful tool to explain the mechanism of corrosion inhibition for the inhibitor [29–30]. The change of the corrosion rate with the temperature was studied in the absence and presence of 2-HBP in 1 mol/L HCl. For this
purpose, polarization readings were performed at different temperatures from 25 °C to 65 °C in absence and presence of different concentrations of 2-HBP (Figs. 5 and 6). For 45 °C and 65 °C, the electrochemical parameters were extracted and summarized in Tables 3 and 4. It is obvious that the values of Jcorr increases by increasing the temperature in both solutions and the efficiency value decreases from 87% to 75%. Figures 5 and 6 show that raising the temperature has no significant effect on the corrosion potentials, but leads to a higher corrosion rate (Jcorr). According to the Arrhenius equation, the apparent activation energy (Ea) of metal corrosion in both media (Blank and inhibited) can be calculated from the following equation [31]:
 (5)
(5)
where Ea represents the apparent activation energy, R is the gas constant, A is the pre-exponential factor, and T is the absolute temperature. Figure 7 shows the logarithm of Jcorr against the reciprocal of temperature (1/T) in the absence and presence of 2-HBP. The activation energy Ea is calculated from the slope of the plots (–Ea/R). The calculated value of Ea in the absence of inhibitor is 60.5 kJ/mol, while in the presence of 2×10–3 mol/L 2-HBP, it is 73.65 kJ/mol. It has been reported that higher Ea in presence of inhibitor for mild steel in comparison with blank solution typically shows physisorption [32]. Enthalpy and entropy of activation (△Ha, △Sa) are calculated from the transition state theory [33]:
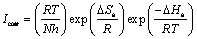 (6)
(6)
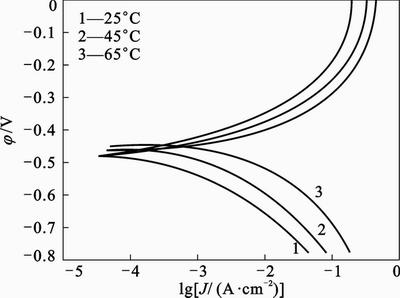
Fig. 5 Anodic and cathodic polarization curves for mild steel in 1 mol/L HCl without inhibitor at different temperatures
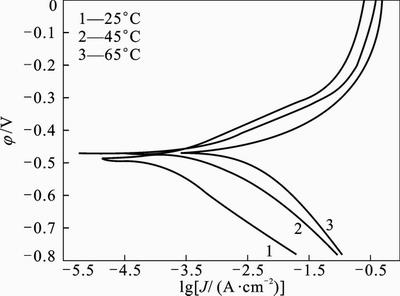
Fig. 6 Anodic and cathodic polarization curves for mild steel in 1 mol/L HCl with 2×10–3 mol/L of 2-HBP at different temperatures
Table 3 Electrochemical parameters for corrosion of mild steel in 1 mol/L HCl solution in absence and presence of different concentrations of 2-HBP at 45 °C
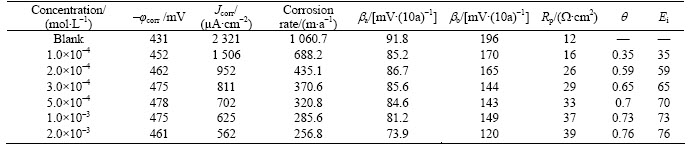
Table 4 Electrochemical parameters for corrosion of mild steel in 1 mol/L HCl solution in absence and presence of different concentrations of 2-HBP at 65 °C
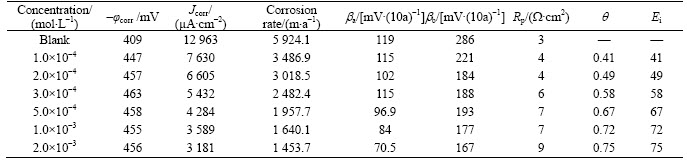
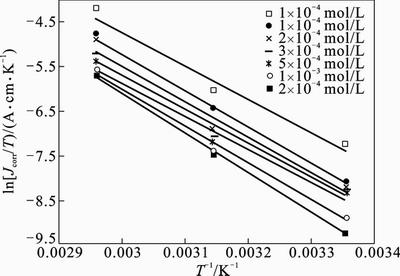
Fig. 7 Typical Arrhenius plots of lnJcorr vs. 1/T for mild steel in 1 mol/L HCl at different concentrations of 2-HBP
where h is the Plank constant and N is the Avogadro’s number. A plot of ln(Icorr/T) versus 1/T gave straight lines as shown in Fig. 8 for mild steel dissolution in 1 mol/L HCl in the absence and presence of different concentrations of inhibitor. Straight lines are obtained with a slope of –△Ha/R and an intercept of ln(R/Nh)+△Sa/R. The values of Ea, A, △Ha and △Sa are calculated and given in Table 5. The positive values of △Ha mean that the dissolution reaction is an endothermic process and that the dissolution of steel is difficult. Practically, Ea and △Ha are of the same order. Also, the entropy △Sa increases more positively with the presence of the inhibitor than in the presence of the non-inhibited one. This reflects the formation of an ordered stable layer of the inhibitor on the steel surface.
3.4 Adsorption isotherm
Adsorption isotherms provide information about the interaction of the adsorbed molecules with the metal surface [1]. The adsorption of organic compounds can be expressed by two main types of interactions: physical adsorption and chemical adsorption. There are some factors that influence the adsorption processes including the nature and charge of metal, the chemical of inhibitor, and the type of electrolyte [29]. The adsorption of an organic adsorbate at metal/solution interface can be presented as a substitution adsorption process between the organic molecules in aqueous solution, Org(sol), and the water molecules on metallic surface, H2O (ads) [20]:
Org(sol)+xH2O(ads) Org(ads)+xH2O(sol) (7)
Org(ads)+xH2O(sol) (7)
where Org(sol) and Org(ads) are the organic species dissolved in the aqueous solution and adsorbed onto the metallic surface, respectively, H2O(ads) is the water molecule adsorbed on the metallic surface, H2O(sol) is water molecule in solution, and x is size ratio and represents the number of molecules of water replaced by inhibitor molecules. For the studied inhibitor, it was found that the experimental data obtained from polarization readings could be fitted by Langmuir’s adsorption isotherm. According to this isotherm, the surface coverage q is related to inhibitor concentration by [34–35]
 (8)
(8)
Rearranging Eq. (8) gives
 (9)
(9)
where Kads is the equilibrium constant of the inhibitor adsorption process, c is the inhibitor concentration and q is the surface coverage that was calculated by Eq. (1).
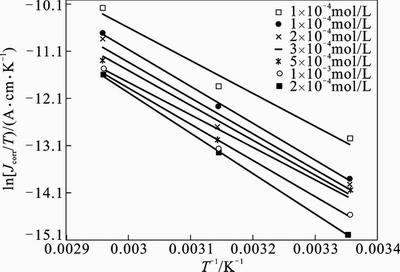
Fig. 8 Typical Arrhenius plots of ln (Jcorr/T) vs. 1/T for mild steel in 1 mol/L HCl at different concentrations of 2-HBP:
Table 5 Activation parameters of dissolution of steel in 1 mol/L HCl solution in absence and presence of 2-HBP
A fitted straight line is obtained for the plot of c/q versus c with slopes close to 1 as seen in Fig. 9. The strong correlation (R2>0.99) suggests that the adsorption of inhibitor on the mild steel surface obeys Langmuir’s adsorption isotherm. This isotherm assumes that the adsorbed molecules occupy only one site and there are no interactions with other adsorbed species [1]. The Kads values can be calculated from the intercept lines on the c/q -axis. This is related to the standard free energy of adsorption (△Gads) with the following equation:
 (10)
(10)
where R is the gas constant and T is the absolute temperature. The constant value of 55.5 is the concentration of water in solution in mol/L. The enthalpy and entropy of adsorption (△Hads and △Sads) can be calculated using the following equations [9]:
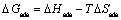 (11)
(11)
 (12)
(12)
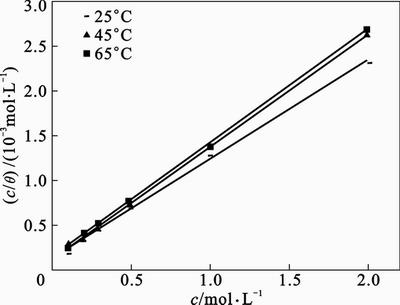
Fig. 9 Langmuir adsorption isotherm (c/q vs. c of inhibitor in 1 mol/L HCl at different temperatures
Figure 10 represents the plots of lnKads versus 1/T for adsorption 2-HBP. The lines obtained represent a slope of (–△Hads/R) and intercept of [–ln(55.5)+△Sads/R]. The values of Kads and △Gads are listed in Table 6. The negative sign of △Gads demonstrates that the inhibitor is spontaneously adsorbed onto the metal surface. Normally, the magnitude of △Gads around –20 kJ/mol or less negative is assumed for electrostatic interactions existing between inhibitor and the charged metal surface (physisorption). Those around –40 kJ/mol or more negative are indication of charge sharing or transferring from organic species to the metal surface to form a coordinate type of metal bond (chemisorption). The values of △Gads are between these two, but modestly closer to –40 kJ /mol. Therefore, it can be interpreted that the adsorption of this inhibitor on the steel surface takes place through both chemical and physical adsorption namely mixed type inhibitor [29]. Valuable information about the mechanism of corrosion inhibition can be provided by the values of thermodynamic parameters for the adsorption of inhibitor. The calculated values of △Hads and △Sads are –14.38 kJ/mol and 62.07 J/(mol·K), respectively. An endothermic adsorption process (△Hads>0) is due to chemisorption while an exothermic adsorption process (△Hads<0) may be attributed to physisorption, chemisorption or a mixture of both. When the process of adsorption is exothermic, physisorption can be distinguished from chemisorption according to the absolute value of △Hads. For physisorption processes, this is usually lower than –40 kJ/mol while its value is around –100 kJ/mol for chemisorption. Base on the results of the present work, the calculated △Gads and △Hads values for 2-HBP show that adsorption mechanism is not completely physical or chemical and a combination of physisorption and chemisorption exists between the inhibitor and metal surface. The positive sign of △Sads arises from substitution process, which can be attributed to the increase in the solvent entropy and more positive water desorption entropy. It is also interpreted with increase of disorders due to the more water molecules which can be desorbed from the metal surface by one inhibitor molecule [25].
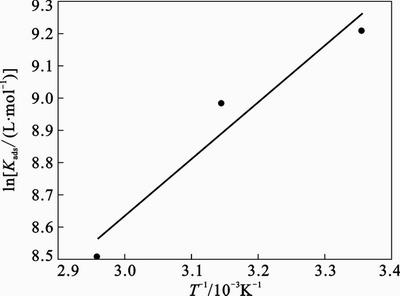
Fig. 10 lnKads vs. 1/T for adsorption 2-HBP on steel surface
Table 6 Thermodynamic and equilibrium adsorption parameters for adsorption 2-HBP on mild steel surface in 1 mol/L HCl solution
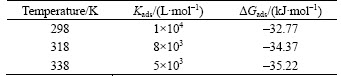
3.5 Effect of immersion time
The effect of immersion time was determined by exposing the mild steel with 1 mol/L HCl solution in the presence of 2-HBP. Electrochemical impedance method is a useful technique for long time tests, because it does not significantly disturb the system and it is possible to follow it over time [36]. Therefore, results were followed by EIS, and impedance spectra were measured at different exposure time. Time dependency of mild steel in 1 mol/L HCl solution in the presence of 2×10-3 mol/L of 2-HBP are presented in Fig. 11. The inhibition efficiency of studied Schiff base increases with increasing immersion time from 30 min to 24 h. This increase in Ei is probably due to the more complete and stable surface coverage of the electrode with inhibitor molecules and an improvement in the quality of the protective film with time [37].
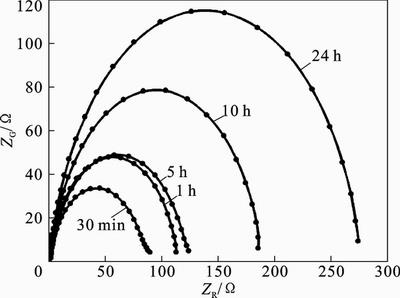
Fig. 11 effect of immersion time on mild steel in 1 mol/L HCl in presence of 2.0×10-3 mol/L 2-HBP at 25°C
3.6 Chronoamperometry
In order to gain more insight about the effect of inhibitor on the electrochemical behavior of steel in 1 mol/L HCl solution, potentiostatic current–time transients were recorded. Figure 12 shows the current transients of steel electrode at –0.4 V (vs. SCE) applied anodic potential. Initially the current decreases monotonically with time, and the decrease in the current density is due to the formation of corrosion products layer on the anode surface. However, in later time, the current increases tracing approximately a straight line to reach a steady state value depending on applied potential (Fig. 12). The increase in current is related to the dissolution of the steel and pit nucleation and pit growth. In presence of inhibitor, increasing current was not observed and electrode was inhibited from corrosion due to inhibitor adsorption.
3.7 Molecular structure and inhibitor properties
The mechanism of the inhibition process of the corrosion inhibitor under consideration is mainly the adsorption one. The process of adsorption is governed by different parameters almost depend on the chemical structure of the inhibitor [6]. Density functional theory (DFT) [14–15] was used to obtain optimized structure for 2-HBP Schiff base for this purpose. Figures 13(a) and (b) depict the optimized geometry obtained at the unrestricted B3LYP level for this complex. The DFT calculations suggest that 2-HBP Schiff base has a prefect inhibitive performance through the adsorption of aromatic rings and heteroatom centers and the interaction between the aliphatic moieties and metal surface in simulated atmospheric water medium.
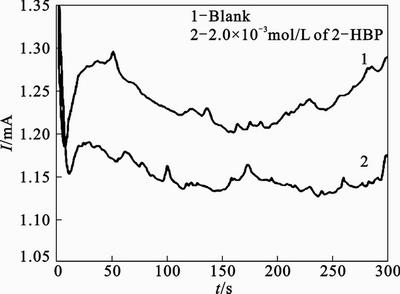
Fig. 12 Current transients of steel electrode at -0.4 V vs. SCE
The FT-IR spectrum of the Schiff-base ligand (Fig. 13(c)) shows two sharp bands appearing at 1 629 and 1 543 cm–1 which are attributable to the azomethine group of Schiff-base ligands and symmetric stretching vibrations of C=C band, respectively. An abroad band at the region of 3 400 cm–1 could be attributed to the symmetric stretching vibrations of phenolic OH. Also, the IR spectrum of the Schiff-base ligand shows major bands at 1 139 cm–1 (C—C) and 2 947 cm–1 (C—H). Figure 13(d) shows UV-visible spectrum of synthesis Schiff base 2-HBP. The absorption spectrum of the Schiff-base ligand consists of a higher energy band at 313 nm and a low energy band at 410 nm that are attributable to π→π* and n→π* transitions, respectively.
3.8 Surface metallography
Figure 14 shows the variation in the surface topography of the mild steel sample after holding the electrode at the specified potential of –0.4 V (vs. SCE) for 1 000 s with and without inhibitor. Figure 14(a) shows that the corroded surface appeared to dissolve uniformly with deep terraces in 1 mol/L HCl solution. Figure 14(b) shows clearly that as 2×10–3 mol/L of 2-HBP inhibitor was added to 1 mol/L HCl, the metal surface appeared to be less corroded, concluding that mild steel surface was covered with a layer containing the molecules of 2-HBP.
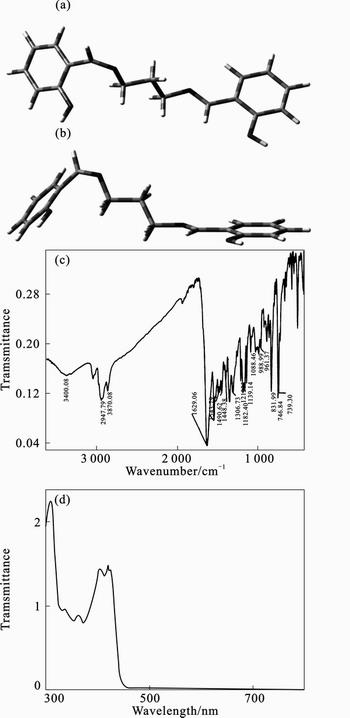
Fig. 13 Optimized structure (a) and “side on view” (b) of 2-HBP Schiff base obtained at B3LYP level; (c) FT-IR spectrum and UV-vis spetrum (d) of Schiff-base ligand
The two- (2D) and three-dimensional (3D) AFM images of mild steel surface after exposure to 1 mol/L HCl solution for 1 d are shown in Fig. 15. As it is shown in Fig. 15, the surface of mild steel electrode exposed to corrosive solution has a considerably porous structure with large and deep pores. The roughness of surface is 2.65 μm. However, the surface is smoother in the presence of 2×10–3 mol/L 2-HBP (Fig. 16), which suggests adsorption of inhibitor molecules on the mild steel surface and reduces the corrosion rate. The surface roughness of mild steel after the addition of 2×10–3 mol/L 2-HBP is 793 nm [35, 38–39].
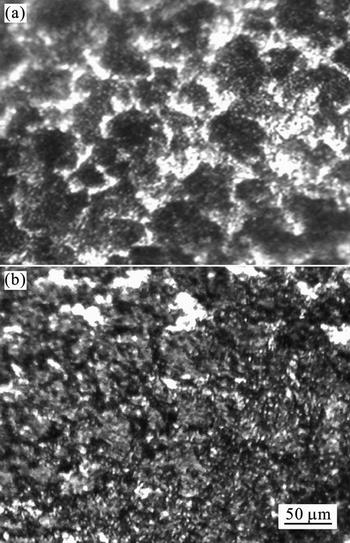
Fig. 14 Optical photomicrographs of mild steel surface immersed in 1 mol/L HCl (a) and 1 mol/L HCl+ 2.0×10–3 mol/L of 2-HBP(b)
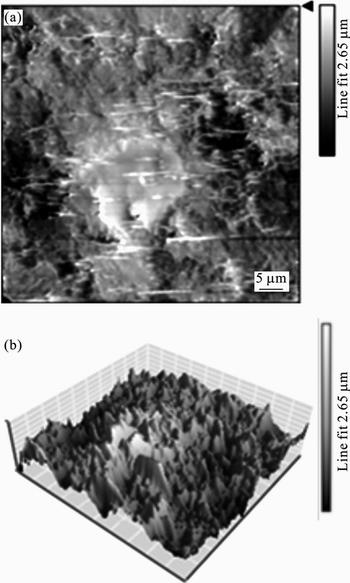
Fig. 15 2D (a) and 3D (b) AFM images of mild steel exposed to 1 mol/L HCl solution (scan size: 49.5 μm ×50 μm)
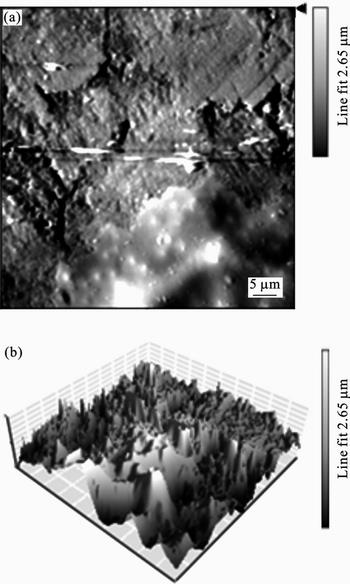
Fig. 16 2D (a) and 3D (b) AFM images of mild steel exposed to 1 mol/L HCl solution in presence of 2.0×10–3 mol/L 2-HBP (scan size: 49.5 μm ×50 μm)
4 Conclusions
1) This synthetic Schiff base acts as excellent inhibitor for mild steel corrosion in 1 mol/L HCl solution, especially in high concentration. Inhibition efficiency of this compound increases with increasing their concentrations due to the formation of a film on the steel surface.
2) Corrosion current density is increased by increasing the temperature, but the increasing rate in lower in the presence of Schiff base compound.
3) Polarization measurements demonstrate that 2-HBP acts mostly as mixed type Inhibitor.
4) Impedance measurements indicate that with increasing inhibitor concentration, the polarization resistance (Rct) increases, while the double layer capacitance (Cdl) decreases.
5) The adsorption of this compound on the mild steel surface obeys the Langmuir adsorption isotherm. The thermodynamic adsorption parameters show that the inhibitor is absorbed by a spontaneous exothermic process and both physisorption and chemisorption process can be suggested for this compound.
6) The high resolution AFM micrographs show a smoother surface for inhibited metal samples rather than uninhibited samples due to the formation of film on the inhibited surface.
References
[1] AHAMAD I, PRASAD R, QURAISHI M A. Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions [J]. Corros Sci, 2010, 52: 933–942.
[2] ISSAADI S, DOUADI T, ZOUAOUI A, CHAFAA S, KHAN M A, BOUET G. Novel thiophene symmetrical Schiff base compounds as corrosion inhibitor for mild steel in acidic media [J]. Corros Sci, 2011, 53: 1484–1488.
[3] SOLMAZ R. Investigation of the inhibition effect of 5-((E)-4-phenylbuta-1, 3-dienylideneamino)-1, 3, 4-thiadiazole-2-thiol Schiff base on mild steel corrosion in hydrochloric acid [J]. Corros Sci, 2010, 52: 3321–3330.
[4] AHAMAD I. Experimental and quantum chemical characterization of the adsorption of some Schiff base compounds of phthaloylthiocarbohydrazide on the mild steel in acid solutions [J]. Mater Chem Phys, 2010, 124: 1155–1165.
[5] LOWMUNKHONG P, UNGTHARARAK D, SUTTHIVAIYAKIT P. Tryptamine as a corrosion inhibitor of mild steel in hydrochloric acid solution [J]. Corros Sci, 2010, 52: 30–36.
[6] EMREGU K C, DUZGUN E, ATAKOL O. The application of some polydentate Schiff base compounds containing aminicnitrogens as corrosion inhibitors for mild steel in acidic media [J]. Corros Sci, 2006, 48: 3243–3260.
[7] HOSSEINI S M A, AZIMI A. The inhibition effect of the new schiff base, namely 2, 20-[bis-N (4-choloro benzaldimin)]-1, 10-dithio against mild steel corrosion [J]. Mater Corros, 2008, 59: 41–45
[8] SOLMAZ R, ALTUNBAS E. KARDAS G. Adsorption and corrosion inhibition effect of 2-((5-mercapto-1, 3, 4-thiadiazol-2-ylimino) methyl) phenol Schiff base on mild steel [J]. Mater Chem Phys, 2011, 125: 796–801.
[9] BADIEA AM, MOHANA K N. Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in presence of 2-hydrazino-4, 7-dimethylbenzothiazole in industrial water medium [J]. Corros Sci, 2009, 51: 2231–2241.
[10] STANLY JACOB K, PARAMESWARAN G. Corrosion inhibition of mild steel in hydrochloric acid solution by Schiff base furointhiosemicarbazone [J]. Corros Sci, 2010, 52: 224–228
[11] HASANOV R. Electrochemical and quantum chemical studies of some Schiff bases on the corrosion of steel in H2SO4 solution [J]. Appl Surf Sci, 2007, 253: 3913–3921
[12] PRABHU R A, VENKATESHA T V, SHANBHAG A V, KULKARNI G M, KALKHAMBKAR R G. Inhibition effects of some Schiff bases on the corrosion of mild steel in hydrochloric acid solution [J]. Corros Sci, 2008, 50: 3356–3362.
[13] FAIRHURST S A, HUGHES D L, KLEINKES U, LEIGH G J, SANDERS J R, WEISNER J. Non-planar co-ordination of the Schiff- basedianion N, N′–2, 2 dimethyltrimethylenebis [salicylideneiminate (2–)] to vanadium [J]. J Chem Soc Dalton Trans, 1995(3): 321–326.
[14] GEERLINGS P, PROFT F D, LANGENAEKER W. Conceptual density functional theory [J]. Chem Rev, 2003, 103: 1793–1873.
[15] LEE C, YANG W, PARR R G. Development of the colle- salvetticonelation energy formula into a functional of the electron density [J]. Phys Rev, 1988, 37: 785–789.
[16] PETERSSON G A, AL-LAHAM M A. A complete basis set model chemistry. II: Open-shell systems and the total energys of the first-row atoms [J]. J Chem Phys, 1991, 94: 6081–6086.
[17] PETERSSON G A, BENNETT A, TENSFELDT T G, AL-LAHAM M A, SHIRLEY W A, MANTZARIS J. A complete basis set model chemistry. I: The total energies of closed-shell atoms and hydrides of the first-row elements [J]. J Chem Phys, 1988, 89: 2193–2219.
[18] MACDONALD J R. Note on the parameterization of the constant-phase admittance element [J]. Solid State Ion, 1984, 13: 147–149.
[19] DANAEE I, JAFARIAN M, FOROUZANDEH F, GOBAL F, MAHJANI M G. Kinetic interpretation of a negative time constant impedance of glucose electrooxidation [J]. J Phys Chem, 2008, 112: 15933–15940.
[20] NEGM N A, ELKHOLY Y M, ZAHRAN M K, TAWFIK S M. Corrosion inhibition efficiency and surface activity of benzothiazol-3-ium cationic Schiff base derivatives in hydrochloric acid [J]. Corros Sci, 2010, 52: 3523–3536.
[21] NEGM N A, GHUIBA F M, TAWFIK S M. Novelisoxazolium cationic Schiff base compounds as corrosion inhibitors for carbon steel in hydrochloric acid [J]. Corros Sci, 2011, 53: 566–3575.
[22] HEGAZY M A. A novel Schiff base-based cationic gemini surfactants: Synthesis and effect on corrosion inhibition of carbon steel in hydrochloric acid solution [J]. Corros Sci, 2009, 51: 2610–2618.
[23] MALLAIYA K, SUBRAMANIAM R, SRIKANDAN S S. Electrochemical characterization of the protective film formed by the unsymmetrical Schiff’s base on the mild steel surface in acid media [J]. Electrochim Acta, 2011, 56: 3857–3863.
[24] MIGAHED M A, NASSAR I F. Corrosion inhibition of tubing steel during acidization of oil and gas wells [J]. Electrochim Acta, 2008, 53: 2877–2882.
[25] KELES H. Electrochemical and thermodynamic studies to evaluate inhibition effect of2-[(4-phenoxy- phenylimino) methyl]-phenol in 1 M HCl on mild steel [J]. Mater Chem Phys, 2011, 130: 1317– 1324.
[26] EMREGUL K C, ATAKOL O. Corrosion inhibition of mild steel with Schiff base compounds in 1 M HCl [J]. Mater Chem Phys, 2003, 82:188–193.
[27] DANAEE I. Kinetics and mechanism of palladium electrodeposition on graphite electrode by impedance and noise measurements [J]. J. Electroanal Chem, 2011, 662: 415–420.
[28] DANAEE I, NOORI S. Kinetics of the hydrogen evolution reaction on NiMn graphite modified electrode [J]. Int J Hydrogen Energy, 2011, 36: 12102-12111.
[29] BADR G E. The role of some thiosemicarbazide derivatives as corrosion inhibitors for C-steel in acidic media [J]. Corros Sci, 2009, 51: 2529–2536.
[30] HEGAZY M A, AHMED H M, EL-TABEI A S. Investigation of the inhibitive effect of p-substituted4- (N, N, N-dimethyldodecylammonium bromide) benzylidene- benzene-2-yl-amineon corrosion of carbon steel pipelines in acidic medium [J]. Corros Sci, 2011, 53: 671–678.
[31] ALJOURANI J, RAEISSI K, GOLOZAR M A. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1 M HCl solution [J]. Corros Sci, 2009, 51: 1836–1843.
[32] OBOT I B, OBI-EGBEDI N O. Anti-corrosive properties of xanthone on mild steel corrosion in sulphuric acid: Experimental and theoretical investigations [J]. Current Appl Phys, 2011, 11: 382–392.
[33] HERRAG L, CHETOUANI A, ELKADIRI S, HAMMOUTI B, AOUNITI A. pyrazole derivatives as corrosion inhibitors for steel in hydrochloric acid [J]. Portugal Ectrochim Acta, 2008, 26: 211–220.
[34] BAYOL E, GURTENB T, GURTENA A A, ERBIL M. Interactions of some Schiff base compounds with mild steelsurface in hydrochloric acid solution [J]. Mater Chem Phys, 2008, 112: 624–630.
[35] KELES H, KELES M, DEHRI I, SERINDAG O. The inhibitive effect of 6-amino-m-cresol and its Schiff base on the corrosion of mild steel in 0.5 M HCl medium [J]. Mater Chem Phys, 2008, 112: 173–179.
[36] KELES H , KELES M. DEHRI I, SERINDAG O. Adsorption and inhibitive properties of aminobiphenyl and its Schiff base on mild steel corrosion in 0.5 M HCl medium [J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2008, 320: 138–145.
[37] GHAREBA S, OMANOVIC S. Interaction of 12-aminododecanoic acid with a carbon steel surface: Towards the development of ‘green’ corrosion inhibitors [J]. Corros Sci, 2010, 52: 2104–2113.
[38] LI X, MU G. Tween-40 as corrosion inhibitor for cold rolled steel in sulphuric acid: Weight loss study, electrochemical characterization and AFM [J]. Appl Surf Sci, 2005, 252: 1254–1265.
[39] ZHANG F, PAN J, CLAESSON P M. Electrochemical and AFM studies of mussel adhesive protein (Mefp-1) as corrosion inhibitor for carbon steel [J]. Electrochim Acta, 2011, 56: 1636–1645.
(Edited by HE Yun-bin)
Received date: 2012–04–16; Accepted date: 2012–07–13
Corresponding author: I. Danaee; Tel: +98–631–4429937; E-mail: danaee@put.ac.ir

