利用离子交换树脂从钼酸盐溶液中分离钨和钒
来源期刊:中国有色金属学报(英文版)2017年第12期
论文作者:朱先正 霍广生 倪捷 宋琼
文章页码:2727 - 2732
关键词:钨;钒;分离;离子交换;钼酸盐
Key words:tungsten; vanadium; removal; ion exchange; molybdate
摘 要:利用葡氨基螯合树脂D403从钼酸盐溶液中分离钨和钒。静态实验表明,在弱碱性条件(pH=9.25)下,接触时间为4 h时,钨和钒优先被树脂吸附。当Mo/V (Mo/W)摩尔比超过40时,分离系数αV Mo 与αW Mo 分别达到45和18。解吸结果显示,使用1 mol/L NaOH溶液在1 h内即解吸树脂上的钨和钒。等温吸附过程结果表明,D403对3种金属离子的吸附均属于Freundlich模型。动态离子交换实验结果进一步证实了D403树脂在钼酸盐溶液中除钨和钒的实用性,除钨率和除钒率分别高达90%和99.4%。
Abstract: The removal of tungsten (W) and vanadium (V) from molybdate solutions was studied using the poly hydroxyl chelating resin D403 in batch and column experiments. The batch experiments indicated that tungsten and vanadium could be preferentially adsorbed by the D403 resin for 4 h in molybdate solution at a pH of approximately 9.25. Separation factors, and , were above 45 and 18, respectively, when the molar ratios of Mo/V and Mo/W in the solution exceeded 40. Elution tests illustrated that vanadium and tungsten could be easily eluted from the resin with 1 mol/L sodium hydroxide solution in only 1 h. To further explore the sorption mechanism of the resin, the experimental equilibrium isotherm data of the three metals fitted well with the Freundlich model. The column experiments confirmed the adaptability of the D403 resin in the production of sodium molybdate with a removal rate of tungsten surpassing 90% and that of vanadium of 99.4%.
Trans. Nonferrous Met. Soc. China 27(2017) 2727-2732
Xian-zheng ZHU, Guang-sheng HUO, Jie NI, Qiong SONG
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 10 August 2016; accepted 17 December 2016
Abstract: The removal of tungsten (W) and vanadium (V) from molybdate solutions was studied using the poly hydroxyl chelating resin D403 in batch and column experiments. The batch experiments indicated that tungsten and vanadium could be preferentially adsorbed by the D403 resin for 4 h in molybdate solution at a pH of approximately 9.25. Separation factors, 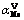 and
and  , were above 45 and 18, respectively, when the molar ratios of Mo/V and Mo/W in the solution exceeded 40. Elution tests illustrated that vanadium and tungsten could be easily eluted from the resin with 1 mol/L sodium hydroxide solution in only 1 h. To further explore the sorption mechanism of the resin, the experimental equilibrium isotherm data of the three metals fitted well with the Freundlich model. The column experiments confirmed the adaptability of the D403 resin in the production of sodium molybdate with a removal rate of tungsten surpassing 90% and that of vanadium of 99.4%.
, were above 45 and 18, respectively, when the molar ratios of Mo/V and Mo/W in the solution exceeded 40. Elution tests illustrated that vanadium and tungsten could be easily eluted from the resin with 1 mol/L sodium hydroxide solution in only 1 h. To further explore the sorption mechanism of the resin, the experimental equilibrium isotherm data of the three metals fitted well with the Freundlich model. The column experiments confirmed the adaptability of the D403 resin in the production of sodium molybdate with a removal rate of tungsten surpassing 90% and that of vanadium of 99.4%.
Key words: tungsten; vanadium; removal; ion exchange; molybdate
1 Introduction
Molybdenum has strategic and industrial importance with extensive applications in many fields [1]. Due to the rapid consumption of conventional molybdenum sources, the recovery of molybdenum from low-grade and complex ores has become increasingly essential. Raw molybdenum resources, such as Ni-Mo ore from Lower Cambrian Shale, contain molybdenum and a certain amount of valuable metals, such as tungsten and vanadium [2,3]. The recovery of these valuable metals from secondary sources has become increasingly more desirable. These sources, for example, spent residue catalysts, alloys or steel scraps [4,5], usually contain certain amounts of vanadium, tungsten and molybdenum. However, impurities in molybdate extracts must be strictly restricted [6]. Hence, it is extremely important to separate tungsten and vanadium from molybdate solutions so that these molybdenum resources can be effectively utilized.
Due to their similar chemical properties in solution, it is difficult to isolate tungsten, vanadium and molybdenum from each other [7,8]. Many researchers have proposed various methods for the separation of vanadium and molybdenum in aqueous solution, such as precipitation [9], adsorption with activated carbon [10], ion exchange [11,12] and solvent extraction [13-15]. In terms of separation of tungsten from molybdate solution, methods like extraction [8] and ion exchange [16,17] have also been carried out. However, few studies have considered the simultaneous removal of vanadium and tungsten from molybdate aqueous solutions.
In previous studies, a poly-hydroxyl chelating resin, D403, containing a meglumine function group (i.e., —CH2N(CH3)CH2(CHOH)4CH2OH, which contains a ternary amino group and a poly hydroxyl group) has been found to selectively remove tungsten from molybdate solutions in alkaline systems [16]. Furthermore, because vanadium predominantly occurs as polymeric ions ( ) [18] in weak basic aqueous solutions (approximately pH 9.0) and because molybdenum exists as monomeric ions due to its lower polymerizing tendency [19], poly-vanadiate ions have higher electrovalence than monomeric molybdenum ions. Thus, vanadium is preferentially adsorbed and removed from weak alkaline molybdate aqueous solutions with certain adsorbents, i.e., those that contain amine functional groups, such as AG1-X8 [20], D296 [18] (quaternary amino group), and D314 [21] (tertiary amino group). Thus, the D403 resin, which contains a ternary amino group, could be utilized to remove vanadium from molybdate solutions. Compared with the other separation methods mentioned, use of D403 resin could simultaneously remove tungsten and vanadium from molybdate solutions without using other chemical reagents.
) [18] in weak basic aqueous solutions (approximately pH 9.0) and because molybdenum exists as monomeric ions due to its lower polymerizing tendency [19], poly-vanadiate ions have higher electrovalence than monomeric molybdenum ions. Thus, vanadium is preferentially adsorbed and removed from weak alkaline molybdate aqueous solutions with certain adsorbents, i.e., those that contain amine functional groups, such as AG1-X8 [20], D296 [18] (quaternary amino group), and D314 [21] (tertiary amino group). Thus, the D403 resin, which contains a ternary amino group, could be utilized to remove vanadium from molybdate solutions. Compared with the other separation methods mentioned, use of D403 resin could simultaneously remove tungsten and vanadium from molybdate solutions without using other chemical reagents.
In this work, a poly-hydroxyl chelating resin is used to remove tungsten and vanadium in molybdate aqueous solutions. Batch experiments and column experiments were carried out to investigate the adsorption and elution behaviors of tungsten and vanadium and to further understand the sorption mechanism on the resin. Equilibrium isotherm experiments were also explored.
2 Experimental
2.1 Materials
A macro poly-hydroxyl D403 chelating resin, purchased from Jiangsu Suqing Industrial Co., Ltd., was used in this study. Prior to experiments, the resin was alternately pre-treated with 5% NaOH and 5% HCl aqueous solutions, and washed to near-neutral pH. All chemicals used in this work were of analytical grade, and all concentrations of tungsten (W), vanadium (V) and molybdenum (Mo) were detected using an inductively coupled plasma-atomic emission spectrometer (ICP-AES, Thermo Electron Corporation, USA).
2.2 Batch experiments
The experiments were carried out by varying adsorption conditions, such as pH value, contact time and Mo/V or Mo/W molar ratios. The ternary mixed solution was prepared in the laboratory. For each experiment, the testing solution was adjusted to a specific pH value with hydrochloride acid before the adsorption process. These solutions were set to stand for 1 h. Then, 10 mL D403 resin and 50 mL prepared solution were mixed. Samples were agitated for 4 h at ambient temperature (stirring speed of 300 r/min), and the loaded resin was separated by filtration at the end of sorption.
The effects of the eluent concentration and contact time were investigated in desorption experiments, which were conducted by mixing 10 mL loaded resin and 60 mL eluent solutions in a water bath for certain duration. The prepared saturated loading resin was washed with distilled water before desorption.
2.3 Column experiments
For the column-loading experiments, prepared solutions were directly passed through glass column (13 mm in diameter and 1010 mm in height) at a flow rate of approximately 48 mL/h (0.7VB h-1). The bed volume (VB) of the resin was 66.5 mL. An auto sampler was used to collect a number of 20 mL effluent samples, which were subsequently analyzed. After the adsorption process, the saturated loading resin was drip washed with distilled water until tungsten was not detected in the effluent. Subsequently, the eluent was analyzed. First, the effluent was obtained at a flow rate of approximately 93 mL/h (1.4VB h-1), and an auto sampler was used to collect a number of 25 mL effluent samples.
2.4 Adsorption isotherm experiments
In the adsorption isotherm experiments, 10 mL D403 wet resin was mixed with a series of volume solutions containing different concentrated compounds in a water-bath shaker for 4 h at room temperature. The pH values of all the solutions were adjusted to be 9.25 with hydrochloric acid.
Like other chemical processes, the ion exchange equilibrium was characterized by corresponding equilibrium isotherms. In this case, the adsorption equilibrium between W, Mo and V is simulated with Henry model [22], Langmuir model [23] and Freundlich model [24]. These three models are as follows:
Henry model
Qe=KHCe (1)
Freundlich model
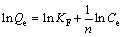 (2)
(2)
Langmuir model
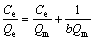 (3)
(3)
where Qe is the equilibrium adsorption capacity of the wet resin (mg/mL); Ce is concentration of the equilibrium solution after the exchange process (g/L); Qm is the maximum adsorption capacity of the resin (mg/mL); KH, KF, b and n are the corresponding constant parameters of these models.
The adsorption ratio (φ, %), the separation factor for W over Mo ( ), the separation factor for V over Mo (
), the separation factor for V over Mo ( ), the capacity of the resin (Qe, mg/mL), the elution ratio (f, %) and the removal ratio in each column experiment (ω, %) are calculated as follows:
), the capacity of the resin (Qe, mg/mL), the elution ratio (f, %) and the removal ratio in each column experiment (ω, %) are calculated as follows:
 (4)
(4)
 (5)
(5)
 (6)
(6)
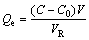 (7)
(7)
 (8)
(8)
 (9)
(9)
where C0 and C are the initial and final concentrations of the metal in solution, g/L; Cd is the concentration of metals in the eluent, g/L; V,  and Vd are the volumes of the feeds, resin and eluents, respectively, mL;
and Vd are the volumes of the feeds, resin and eluents, respectively, mL; 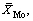

 and XMo, XW, XV are the molar fractions of Mo, W and V in the resin phase and in aqueous phase, respectively; R0 and R1 are the molar ratios in the initial solution to those in the total eluents in the column- loading tests.
and XMo, XW, XV are the molar fractions of Mo, W and V in the resin phase and in aqueous phase, respectively; R0 and R1 are the molar ratios in the initial solution to those in the total eluents in the column- loading tests.
3 Results and discussion
3.1 Batch sorption experiments
3.1.1 Effect of pH value
To investigate the influence of pH value on the separation of Mo, W and V, experiments were conducted using a ternary mixed solution at various pH values. The results are presented in Fig. 1.

Fig. 1 Effect of pH values on removal of W and V in molybdate solutions
The adsorption rates for Mo decreased from 63% to 13% as the pH increased from 5.25 to 9.25 and subsequently, remained steady at lower adsorption rates. Comparatively, both W and V followed nearly opposite to Mo. The rate for W approached 74.4% when the pH was approximately 8.25. The rate for V reached 71.2% when the pH value was approximately 9.2, whereas that of Mo was below approximately 30%. Overall, W, V and Mo were adsorbed on the resin when the solution pH was less than 6.5; however, when the pH exceeded 8.5, the resin showed preferential adsorption for W and V, which indicated that the sorption rates were correlated with the existing ion forms.
The monomeric ions of tungsten and molybdenum, i.e., 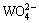 and
and 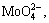 respectively, begin to transform into polymeric ions at pH level below 7.5 and 6.5, respectively [25]. Comparatively, vanadium has a stronger tendency for polymerization, especially because the primary form of vanadium is polymeric ion at pH level below 9.0 [18,19,26]. Because of the greater ionic radius and higher electrovalence, polymeric vanadium ions show a better affinity toward anion exchange resins, which typically contain amino functional groups, such as AG1-X8 [20], D296 [18] (quaternary amino group), and D314 [21] (tertiary amino group), and chelating resins, such as DDAS, CUW and CW-2 [27]. Hence, vanadium can easily be removed from molybdate solutions by controlling the dominant forms of metal ions.
respectively, begin to transform into polymeric ions at pH level below 7.5 and 6.5, respectively [25]. Comparatively, vanadium has a stronger tendency for polymerization, especially because the primary form of vanadium is polymeric ion at pH level below 9.0 [18,19,26]. Because of the greater ionic radius and higher electrovalence, polymeric vanadium ions show a better affinity toward anion exchange resins, which typically contain amino functional groups, such as AG1-X8 [20], D296 [18] (quaternary amino group), and D314 [21] (tertiary amino group), and chelating resins, such as DDAS, CUW and CW-2 [27]. Hence, vanadium can easily be removed from molybdate solutions by controlling the dominant forms of metal ions.
When the pH value surpassed 7.0, W and Mo existed as monomeric ions in aqueous solution. However, the resin showed a higher affinity toward W than Mo, as indicated in Fig. 1. The D403 resin contained poly- hydroxyl and tertiary amino groups. In previous studies, D403 resins preferentially adsorbed 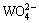 over
over  under weakly basic conditions (pH>8). Given their similar properties, and given the results of comparative experiments with D301 resins (having only tertiary amino groups), the selectivity for
under weakly basic conditions (pH>8). Given their similar properties, and given the results of comparative experiments with D301 resins (having only tertiary amino groups), the selectivity for 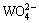 in D403 could be due to the presence of hydroxyl groups [16].
in D403 could be due to the presence of hydroxyl groups [16].
As a result, the separation factors for W and V over Mo were similar and remained low at approximately 0.5 under pH 6.5. As the solution acidity decreased, these values rapidly increased, and reached a maximum as the pH approached 9.25. The separation factor of W over Mo reached 12.27 and that of V over Mo approached 15.48. Under these conditions, the simultaneous removal of W and V from alkaline molybdate solutions is feasible. The optimal pH value for the separation of W and V from molybdate solutions is approximately 9.25.
3.1.2 Effect of contact time
The influence of the contact time on the removal of W and V from the molybdate solutions was explored. The results shown in Fig. 2 indicated that the adsorption of Mo rapidly reached equilibrium within 1 h at which the adsorption ratio was stable at approximately 27%. The adsorption of W and V slowly increased over time. Consequently, the separation factors continuously increased with increasing contact time and finally reached a maximum of 43 for  and 15 for
and 15 for  after 4 h. However,
after 4 h. However,  continued to increase at a significantly faster rate than
continued to increase at a significantly faster rate than  . An appropriate contact time of 4 h was used for subsequent experiments.
. An appropriate contact time of 4 h was used for subsequent experiments.
3.1.3 Effects of Mo/W and Mo/V molar ratios
To investigate the influence of the Mo/W (and Mo/V) molar ratios on the removal of W and V from the molybdate solutions, a series of mixed solutions (50 mL) containing 30 g/L Mo and various concentrations of W and V were prepared, with the W/V molar ratio keeping constant at 1:1. The results shown in Fig. 3 indicated that the adsorption rate of W and V obviously increased whereas that of Mo remained approximately invariant with increasing Mo/V (and Mo/W) molar ratios. In each experiment, the volume of the resin was invariant (10 mL). The adsorption rates of W and V decreased with increasing concentrations, and the adsorption rate of Mo showed a decreasing tendency due to the competitive sorption of W and V.

Fig. 2 Effect of contact time on co-removal of W and V from molybdate solutions

Fig. 3 Effects of Mo/W and Mo/V molar ratios on removal of W and V from molybdate solutions
The adsorption rates of W and V approached 84% and 94% as the Mo/W and Mo/V molar ratios exceeded 40, respectively. The separation factors also increased to high levels:  to approximately 45 and
to approximately 45 and  to approximately 18. Thus, this macro chelating resin could be utilized to effectively separate tungsten and vanadium from high Mo/W or Mo/V molar ratio solutions.
to approximately 18. Thus, this macro chelating resin could be utilized to effectively separate tungsten and vanadium from high Mo/W or Mo/V molar ratio solutions.
3.2 Batch elution experiments
Elution experiments were conducted using mixed saturated loading resins and eluents at ambient temperature over various durations. The results showed that tungsten and vanadium can be desorbed from the resin using a 1 mol/L NaOH solution over 1 h period. The desorption rates approached 99%.
3.3 Equilibrium adsorption isotherms
The experimental data from the equilibrium adsorption isotherms are shown in Fig. 4. The equilibrium adsorption capacities of W, V and Mo increased with increasing metal concentrations. The adsorption isotherm value for W and V was obviously higher than that for Mo, which indicated a stronger affinity of D403 toward W and V over Mo.

Fig. 4 Ternary-component equilibrium adsorption of W, V and Mo with D403 resin at pH 9.25 and 25 °C
To better understand the adsorption of these metals in solution, Henry, Freundlich and Langmuir models were used to fit the isotherm data. All three metals followed the Freundlich model well (Fig. 5). And the equilibrium equations are described in Table 1.

Fig. 5 Freundlich isotherm plot of W, V and Mo on D403 resin
The corresponding isotherm constants KF and n represent the sorption capacity and sorption intensity, respectively [25], i.e., higher values of KF and n indicate stronger D403 resin affinity. The D403 resin showed the highest adsorption capacity for tungsten at KF of 11.894 and the strongest affinity toward vanadium at n value of 1.678.
Table 1 Fitted results of isotherm data

3.4 Column experiments
3.4.1 Loading profiles in sodium molybdate system
Loading tests with sodium molybdate system were conducted. The results are shown in Fig. 6. The resin D403 showed greater affinities toward tungsten and vanadium than molybdenum. As shown in the loading profiles, the breakthrough volume of molybdenum was 80 mL. The adsorption of molybdenum quickly approached equilibrium at approximately 180 mL and was saturated when the C/C0 ratio reached 1. Effluents with residual traces of tungsten and vanadium were obtained in solutions with Mo/W and Mo/V molar ratios of 912 and 16500, respectively. The corresponding removal ratios of tungsten and vanadium were 90% and 99.4%, respectively. Therefore, the D403 resin could be utilized to effectively and simultaneously remove tungsten and vanadium from molybdate solutions.

Fig. 6 Loading profiles for sodium molybdate solutions
3.4.2 Elution profiles in sodium system
The elution-column experiments were investigated using 1 mol/L sodium hydroxide solutions. The results are shown in Fig. 7. The elution tendency of these metals was similar in solutions, and the concentrations of tungsten, vanadium and molybdenum in effluents reached peaks at approximately 60-80 mL. The average molar ratios in the effluent of W/Mo and V/Mo approached 0.152 and 0.136, respectively, compared with initial ratios of 0.01.

Fig. 7 Elution profiles for sodium molybdate solutions
For the effluent solutions, amino anion exchangers could be utilized to separate vanadium at pH level below approximately 9.0 [18]. Then, the adsorption of tungsten using a weakly basic anion exchange resin (D301) at pH 7-7.5 could be performed [25]. Through this method, a pure molybdate solution was obtained. Tungsten and vanadium could then be obtained by resin elution.
4 Conclusions
1) A poly-hydroxyl chelating resin containing meglumine groups was tested. The results showed that vanadium and tungsten could be simultaneously and effectively removed from molybdate solutions.
2) A series of batch experiments showed that the D403 resin performed well in alkaline solutions at pH of approximately 9.25. When the molar ratios of Mo/V and Mo/W in the solutions exceeded 40, the separation factors,  and
and  exceeded 45 and 18, respectively. This implied that the D403 resin had a better affinity for vanadium and tungsten than molybdenum. The desorption tests illustrated that vanadium and tungsten were easily eluted from the resin using 1 mol/L sodium hydroxide solution over 1 h.
exceeded 45 and 18, respectively. This implied that the D403 resin had a better affinity for vanadium and tungsten than molybdenum. The desorption tests illustrated that vanadium and tungsten were easily eluted from the resin using 1 mol/L sodium hydroxide solution over 1 h.
3) The isotherm simulation results showed that all three metals fitted well with the Freundlich model. The sorption quantity KF of W was the highest at 11.894, and the sorption intensity n of V was the highest at 1.678. This further conformed that the D403 resin had the strongest affinity towards V in alkaline solutions.
4) The column experiments further confirmed the separation ability of the D403 resin in a sodium molybdate system with the removal ratio of tungsten surpassing 90% and that of vanadium of 99.4%.
References
[1] PARK K H, REDDY B R, MOHAPATRA D, NAM C W. Hydrometallurgical processing and recovery of molybdenum trioxide from spent catalyst [J]. International Journal of Mineral Processing, 2006, 80(2-4): 261-265.
[2] ZHAO Zhong-wei, LI Jiang-tao, CAO Cai-fang, HUO Guang-sheng, ZHANG Gang, LI Hong-gui. Recovery and purification of molybdenum from Ni-Mo ore by direct air oxidation in alkaline solution [J]. Hydrometallurgy, 2010, 103(1-4): 68-73.
[3] JIA Shuai-guang, CHEN Xing-yu, LIU Xu-heng, LI Yue-xing. The research status and development trends of Ni-Mo ore [J]. China Tungsten Industry, 2012, 27(6): 0008-0012. (in Chinese)
[4] AMIRI F, YAGHMAEI S, MOUSAVI S M. Bioleaching of tungsten-rich spent hydrocracking catalyst using Penicillium simplicissimum [J]. Bioresource Technology, 2011, 102: 1567-1573.
[5] LIAO Shi-ming, BO Tan-lun. Extractive metallurgy of vanadium [M]. Beijing: Metallurgical Industrial Press, 1985: 4-19. (in Chinese)
[6] XIANG Tie-gen, YANG Bo-hua. Extractive metallurgy of molybdenum [M]. Changsha: Central South University Press, 2002: 35-188. (in Chinese)
[7] LI Xin-gbin, WEI Chang, WU Jun, LI Min-ting, DENG Zhi-gan, LI Cun-xiong, XU Hong-sheng. Co-extraction and selective stripping of vanadium (IV) and molybdenum (VI) from sulphuric acid solution using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester [J]. Separation and Purification Technology, 2012, 86: 64-69.
[8] NING Peng-ge, CAO Hong-bin, ZHANG Yi. Selective extraction and deep removal of tungsten from sodium molybdate solution by primary amine N1923 [J]. Separation and Purification Technology, 2009, 70(1): 27-33.
[9] PARK K H, MOHAPATRA D, REDDY B R. Selective recovery of molybdenum from spent HDS catalyst using oxidative soda ash leach/carbon adsorption method [J]. Journal of Hazardous Materials, 2006, 138: 311-316.
[10] MUKHERJEE T K, BIDAYE A C, GUPTA C K. Recovery of molybdenum from spent acid of lamp making industries [J]. Hydrometallurgy, 1988, 20: 147-154.
[11] LI Qing-gang, ZENG Li, XIAO Lian-sheng, YANG Ya-nan, ZHANG Qi-xiu. Completely removing vanadium from ammonium molybdate solution using chelating ion exchange resins [J]. Hydrometallurgy, 2009, 98(3-4): 287-290.
[12] HENRY P, van LIERDE A. Selective separation of vanadium from molybdenum by electrochemical ion exchange. [J]. Hydrometallurgy, 1998, 48: 73-81.
[13] ZENG Li, CHENG Chu-Yong. Recovery of molybdenum and vanadium from synthetic sulphuric acid leach solutions of spent hydrodesulphurisation catalysts using solvent extraction [J]. Hydrometallurgy, 2010, 101(3-4): 141-147.
[14] ZHU Zhao-wu, TULPATOWICZ Karol, PRANOLO Yoko, CHENG Chu-Yong. Solvent extraction of molybdenum and vanadium from sulphate solutions with Cyphos IL 101 [J]. Hydrometallurgy, 2015, 154: 72-77.
[15] CHEN Yun, FENG Qi-ming, SHAO Yan-hai, ZHANG Guo-fan, OU Le-ming, LU Yi-ping. Investigations on the extraction of molybdenum and vanadium from ammonia leaching residue of spent catalyst [J]. International Journal of Mineral Processing, 2006, 79(1): 42-48.
[16] HUO Guang-sheng, PENG Chao, SONG Qiong, LU Xiao-ying. Tungsten removal from molybdate solutions using ion exchange [J]. Hydrometallurgy, 2014, 147-148: 217-222.
[17] LU Xiao-ying, HUO Guang-sheng, LIAO Chun-hua. Separation of macro amounts of tungsten and molybdenum by ion exchange with D309 resin [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(9): 3008-3013.
[18] HU Jian, WANG Xue-wen, XIAO Lian-sheng, SONG Song-ru, ZHANG Bao-qing. Removal of vanadium from molybdate solution by ion exchange [J]. Hydrometallurgy, 2009, 95: 203-206.
[19] ZHANG Jia-liang, ZHAO Zhong-wei. thermodynamic analysis for separation of tungsten and vanadium in W-V-H2O system [J]. The Chinese Journal of Nonferrous Metals, 2014, 24(6): 1656-1662. (in Chinese)
[20] NGUYEN Thi Hong, LEE Man Seung. Recovery of molybdenum and vanadium with high purity from sulfuric acid leach solution of spent hydrodesulfurization catalysts by ion exchange [J]. Hydrometallurgy, 2014, 147-148: 142-147.
[21] WANG Xue-wen, WANG Ming-yu, SHI Li-hua, HU Jian, QIAO Peng. Recovery of vanadium during ammonium molybdate production using ion exchange [J]. Hydrometallurgy, 2010, 104: 317-321.
[22] RENGARAJ S, JOO Cheol Kyun, KIM Younghun, YI Jongheop. Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H [J]. Journal of Hazardous Materials, 2003, 102(2-3): 257-275.
[23] LANGMUIR I. The adsorption of gases on plane surfaces of glass, mica and platinum [J]. Journal of American Chemical Society, 1918, 40(9): 1361-1403.
[24] FREUNDLICH H M F. Over the adsorption in solution [J]. J Phys Chem, 1906, 57: 385-470.
[25] ZHAO Zhong-wei, ZHANG Jia-liang, CHEN Xing-yu, LIU Xu-heng, LI Jiang-tao, ZHANG Wei-guang. Separation of tungsten and molybdenum using macroporous resin: Equilibrium adsorption for single and binary systems [J]. Hydrometallurgy, 2013, 140: 120-127.
[26] NGUYEN Thi Hong, LEE Man Seung. Separation of molybdenum and vanadium from acid solutions by ion exchange [J]. Hydrometallurgy, 2013, 136: 65-70.
[27] LI Qing-gang, ZHANG Qi-xiu, ZENG Li, XIAO Lian-sheng, YANG Ya-nan. Removal of vanadium from ammonium molybdate solution by ion exchange [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(3): 735-739.
朱先正,霍广生,倪 捷,宋 琼
中南大学 冶金与环境学院,长沙 410083
摘 要:利用葡氨基螯合树脂D403从钼酸盐溶液中分离钨和钒。静态实验表明,在弱碱性条件(pH=9.25)下,接触时间为4 h时,钨和钒优先被树脂吸附。当Mo/V (Mo/W)摩尔比超过40时,分离系数αV Mo 与αW Mo 分别达到45和18。解吸结果显示,使用1 mol/L NaOH溶液在1 h内即解吸树脂上的钨和钒。等温吸附过程结果表明,D403对3种金属离子的吸附均属于Freundlich模型。动态离子交换实验结果进一步证实了D403树脂在钼酸盐溶液中除钨和钒的实用性,除钨率和除钒率分别高达90%和99.4%。
关键词:钨;钒;分离;离子交换;钼酸盐
(Edited by Bing YANG)
Foundation item: Project (2014CB643405) supported by the National Basic Research Program of China
Corresponding author: Guang-sheng HUO; Tel: +86-18507312882; E-mail: gshuo@csu.edu.cn
DOI: 10.1016/S1003-6326(17)60301-7

