

王 钟1,刘家岐1,刘 琛1,袁 双1, 3, 4,王 强2
(1. 东北大学 冶金学院,沈阳110819;
2. 东北大学 材料电磁过程研究教育部重点实验室,沈阳 110819;
3. 东北大学 低碳钢铁前沿技术研究院,沈阳 110819;
4. 东北大学 辽宁省低碳钢铁技术工程研究中心,沈阳 110819)
近年来,随着社会的发展,传统化石能源消耗急剧增加,环境问题日益加剧,可再生清洁能源的开发迫在眉睫。电化学水分解法是公认为将电能转化为氢燃料的一种实用策略。因此,为了实现大规模制氢,开发低成本、资源丰富、高效且稳定的电催化剂至关重要。过渡金属磷化物具有价格低廉、资源丰富、化学稳定性等特点,被广泛应用于电催化析氢领域。本文总结了过渡金属磷化物用于电催化析氢的制备方法,且详细概述了从形貌设计、界面调控、材料复合3个方面,提高电解水析氢反应性能,并对其今后发展趋势以及面临的机遇和挑战进行展望。
文章编号:1004-0609(2021)-11-3344-18 中图分类号: 文献标志码:A
引文格式:王 钟, 刘家岐, 刘 琛, 等. 过渡金属磷化物用于电解水析氢反应的研究进展[J]. 中国有色金属学报, 2021, 31(11): 3344-3361. DOI: 10.11817/j.ysxb.1004.0609.2021-40198
WANG Zhong, LIU Jia-qi, LIU Chen, et al. Recent progress of transition metal phosphides in hydrogen evolution reaction of electrolyzed water[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(11): 3344-3361. DOI: 10.11817/j.ysxb.1004.0609.2021-40198
我国虽然能源种类众多,但是化石燃料储量较少。就目前而言,我国煤炭与石油的使用占据能耗总量的80%以上,且化石燃料中普遍存在氮、硫等元素,这类元素在一定的条件下会和氧发生反应,产生NO2、SO2、SO3等酸性气体,对环境造成严重的污染。当遇到水时,这些酸性气体会和水发生反应生成对应的酸,渗进土壤,破环土地的酸碱度,造成农作物大量减产[1-2]。因此,人类开发可再生绿色清洁的能源刻不容缓[3-6]。氢气作为理想燃料,在所有化学燃料中具有最高的质量能量密度,是替代化石燃料清洁能源的最佳载体[7-8]。现阶段制取氢气的3种主要方法(如图1):电解水制氢、化石能源制氢、生物能制氢[9-11]。其中电解水制氢具有纯度高、成本低、安全且环保等优势,但制氢过程的能耗问题也不可忽略[12]。为了减少供电能耗,提高催化效率,需要更高效的催化剂来制取氢气,以便能在低电位下提供较大的电流密度。目前,贵金属铂是电催化制氢公认的催化材料,但是其价格昂贵且储存量低,刺激了人们对其他高效非贵金属催化材料的探索[13]。当前,对于非贵金属催化材料的研究集中在过渡金属碳化物、过渡金属硫化物、过渡金属氮化物、过渡金属氧化物和过渡金属磷化物(TMPs)[14-18]。很多金属在化反应制取氢气过程中,由于表面与吸附的氢两者间作用太强,从而导致氢难以脱附,表现出较差的析氢性能。然而,过渡金属中磷元素的存在可以有效地降低吸附氢的自由能,并且TMPs能够在更宽的pH区间内保持结构稳定,因而TMPs成为非贵金属催化剂材料研究热点[19]。
本文对最近年来过渡金属磷化物电解水制取氢气进行综合分析。本文侧重点主要集中在过渡金属磷化物的制备、提高HER催化性能的方法以及结构-组成与催化性能之间存在的构效关系。最后,总结了过渡金属磷化物作为析氢反应催化剂所面临的挑战,并且提出了其未来的发展方向。
1 过渡金属磷化物制备方法
过渡金属磷化物可以分为负载型和非负载型,其中单金属磷化物最为常见,但由于双金属之间的协同作用,所以双金属磷化物逐渐成为热门研究材料[20]。由于,过渡金属磷化物中的磷源可以为红磷、白磷、三正辛基膦、三苯基膦、亚磷酸盐、次磷酸盐等[21-24],所以其制备方法多样化。早期,科研人员深入研究了多种TMPs合成方法:元素化合法、固态置换反应法、磷化氢反应法、有机金属分解法、熔融盐电解法等(见表1),但该类制备方法需要高温、高压的外界环境,并且原材料价格昂贵,因此限制了他们的实际应用。下面将详细讲述几种常用的TMPs制备方法:液相反应法、气固反应法、热解还原法、电沉积法(见表2)。
液相反应法是在惰性气体保护下,将原材料(过渡金属盐溶液、金属化合物、磷源)充分混合进行化学反应。该化学反应中的磷源通常采用三辛基膦(TOP)、三苯基膦(TPP)、三苯基膦(PPh3)、三辛基氧化膦(TOPO)等有机膦作为磷源,高温下这些化合物中的C—P键会发生明显的断裂,从而与金属盐溶液中的金属源发生化学反应[25-27]。TMPs的尺寸、结构、形貌取决于反应温度、磷化物种类和原材料的配比。由于反应需要有机膦作为磷源及高温条件,所以反应体系易腐蚀、易燃烧,因此,需要在实验过程中持续通入惰性气体。WANG等[28]采用不同的磷化方式获得了3种Ni2P/SiO2催化剂,当预先还原Ni/SiO2时,即使在443 K的温度下,高度分散的纳米镍颗粒也很容易被PPh3磷化,结果表明在这种情况下的纳米镍颗粒完全被磷化,最终,合成了细小且均匀的Ni2P纳米颗粒(6 nm)。PAN等[29]将TOP和乙酰丙酮作为磷源和镍源,在油胺溶液中制备了碳纳米管负载的Ni2P纳米颗粒(见图2(a)),极大提高了比表面积。
气固反应法的磷源以次磷酸钠(NaH2PO2)、次磷酸铵(NH4H2PO2)、红磷(P)为主,当加热温度升高至250 ℃以上时,次磷酸钠、次磷酸铵便会分解产生磷化氢(PH3),对于红磷而言,当加热温度超过400 ℃时会产生磷蒸汽。在管式炉内,将磷源置于上风处,高温分解后产生的磷化氢或磷蒸汽与下风处的金属源反应生成TMPs。其中,次磷酸盐热分解法工艺简单,无需高温、高压、程序升温等复杂步骤,因此被公认为最具有前景的TMPs制备方法。LI等[30]通过液相法将FeOx生长在碳纳米管上(FeOx/CNT),然后在350 ℃下被氢气还原为Fe/CNT,接着在氩气保护下与NaH2PO2磷化,最后,生成了核壳纳米粒子(Fe@FeP/CNT)。作者对该材料做了稳定性测试(见图2(b)),发现金属与磷化物之间的电子相互作用,可以将氢原子的结合强度提高到最佳水平。图2(c)展示了一个典型案例[31],首先葡萄糖分子、钴离子和铁离子在水热条件下反应生成过渡金属氧化物前驱体,其次在空气中煅烧2 h,得到CoFe2O4空心球(见图2(d)),然后将NaH2PO2和CoFe2O4空心球分别置于管式炉上游和下游,加热至400 ℃并保温2 h,最终成功制备了双金属CoFeP空心球。
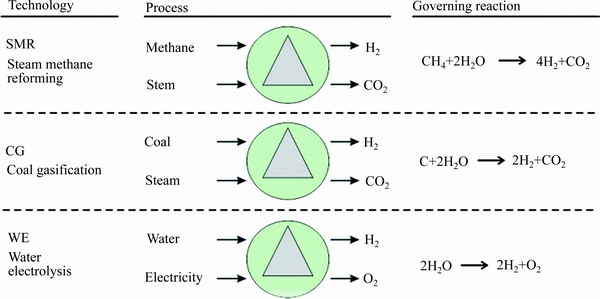
图1 制取氢气方法示意图[9]
Fig. 1 Schematic diagram of hydrogen production[9]
表1 早期过渡金属磷化物制备方法
Table 1 Preparation method of early transition metal phosphide
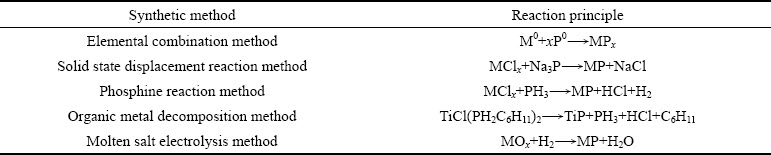
表2 常见过渡金属磷化物制备方法概述
Table 2 Overview of preparation methods of common transition metal phosphides
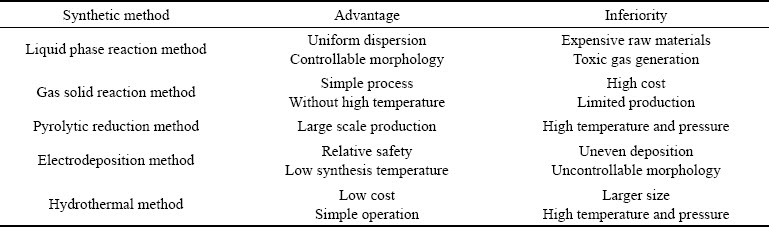
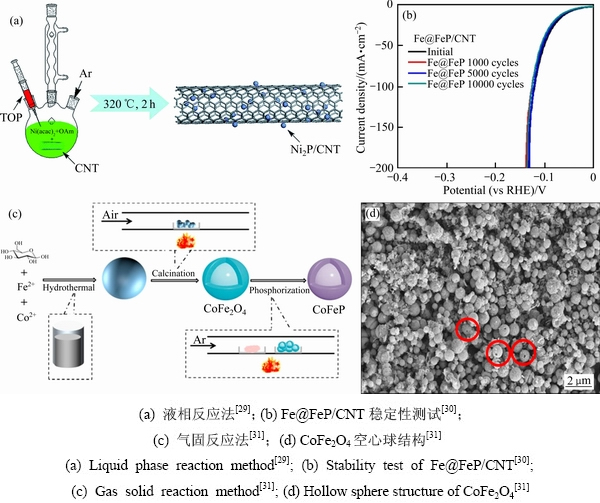
图2 液相反应法和气固反应法的制备流程图及形貌与性能测试
Fig. 2 Preparation flow chart, morphology and performance test of liquid phase reaction method and gas solid reaction method
热解还原法被应用于大规模TMPs的制备,该方法多以磷酸盐、亚磷酸盐、多金属氧酸盐、植酸等作为磷源,金属盐作为金属源,在高温(500~1000 ℃)及还原气氛(Ar/H2)条件下P—O键断裂与金属离子结合生成TMPs。MA等[32]采用该方法规模化制备了氮掺杂碳壳包覆的纳米晶体(CoMoP@C),由于碳壳拥有较强的质子吸收能力,因此,极大地提高析氢性能。结果表明,当测试溶液的PH范围在0~1时,CoMoP@C的析氢性能接近商业Pt/C,当pH范围在2~14时,CoMoP@C的析氢性能优于商业Pt/C。LU等[33]以MIL-88A为前驱体,植酸为磷源,合成了镍掺杂FeP/C空心纳米棒。WANG等[34]通过简单的室温搅拌制备了高度均匀的ZIF-67纳米立方体(见图3(a)),然后将植酸溶液加入含有一定量ZIF-67和聚乙烯吡咯烷酮(PVP)的乙醇溶液中,在程序升温还原条件下,磷酸基中的P—O键被破坏,生成CoP/C纳米颗粒(见图3(b))。
电沉积法的磷源一般采用次磷酸钠,基底可用铜箔、钛箔、碳布、泡沫镍等,反应机理是溶液中金属离子和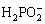 生成TMPs吸附在基底表面。该制备方法具有低成本和低能耗的特点,但也存在难以控制TMPs的细致结构。CHENG等[35]探讨了电沉积方式(直流、脉冲)、沉积时间、电流和电压大小对TMPs的影响。PEI等[36]采用一步式恒流密度电沉积法在钛片上沉积了高活性和廉价的Co-Ni-P薄层(见图3(c))。CAO等[37]以0.2 mol/L NiCl2×6H2O溶液、0.2 mol/L NaH2PO4×H2O溶液、0.25 mol/L NH4Cl溶液和5种不同含量的CuCl2×2H2O溶液(0 mol/L、0.01 mol/L、0.02 mol/L、0.03 mol/L、0.04 mol/L)制备电解质,并在10 mA/cm2的恒定电流密度下持续20 min,发现铜离子的浓度是形成空心树枝状结构(见图3(d))的关键因素。YANG等[38]在碳布上电沉积了Ni-P纳米颗粒,该颗粒填充CC表面上未被Ni2P纳米片占据的位置。实验发现,Ni-P纳米颗粒的电沉积时间对Ni-P/Ni2P/CC复合材料的催化性能有显着影响,当电沉积的时间较短(60 s)时,可能无法提供足够的活性位点,然而,沉积时间的较长(180 s)时,则可能会导致Ni2P的一些活性位点被覆盖。因此,合适的沉积时间对材料的催化活性尤为重要。
生成TMPs吸附在基底表面。该制备方法具有低成本和低能耗的特点,但也存在难以控制TMPs的细致结构。CHENG等[35]探讨了电沉积方式(直流、脉冲)、沉积时间、电流和电压大小对TMPs的影响。PEI等[36]采用一步式恒流密度电沉积法在钛片上沉积了高活性和廉价的Co-Ni-P薄层(见图3(c))。CAO等[37]以0.2 mol/L NiCl2×6H2O溶液、0.2 mol/L NaH2PO4×H2O溶液、0.25 mol/L NH4Cl溶液和5种不同含量的CuCl2×2H2O溶液(0 mol/L、0.01 mol/L、0.02 mol/L、0.03 mol/L、0.04 mol/L)制备电解质,并在10 mA/cm2的恒定电流密度下持续20 min,发现铜离子的浓度是形成空心树枝状结构(见图3(d))的关键因素。YANG等[38]在碳布上电沉积了Ni-P纳米颗粒,该颗粒填充CC表面上未被Ni2P纳米片占据的位置。实验发现,Ni-P纳米颗粒的电沉积时间对Ni-P/Ni2P/CC复合材料的催化性能有显着影响,当电沉积的时间较短(60 s)时,可能无法提供足够的活性位点,然而,沉积时间的较长(180 s)时,则可能会导致Ni2P的一些活性位点被覆盖。因此,合适的沉积时间对材料的催化活性尤为重要。
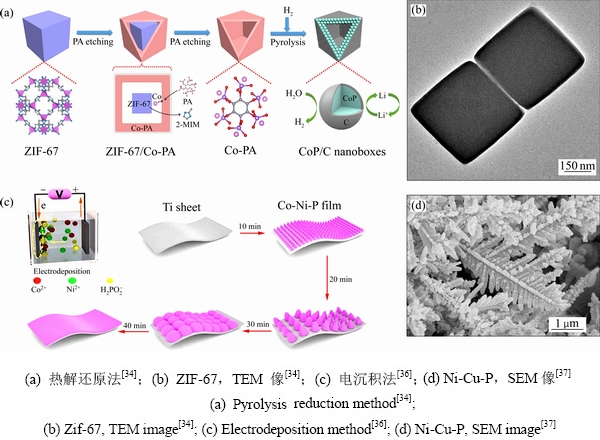
图3 热解还原法和电沉积法的制备流程图及形貌
Fig. 3 Preparation process and morphology of pyrolytic reduction method and electrodeposition method
近年来,随着能源结构的调整,吸引大量研究人员探索新的过渡金属磷化物制备方法,例如水热法、溶剂热法、微乳液法、超临界二氧化碳法和室温固相合成法等[39-43]。在众多的方法里,其中溶剂热法和水热法被普遍使用。水热法制备流程为首先使用密闭反应釜作为反应设备,以水溶液或者其他溶剂作为反应介质,然后,在高温高压下进行的反应。该方法与传统方法相比,溶剂热/水热制备流程比较简单,产生的磷化物具有更大的比表面积、更稳定的结构、更多的活性位点[44-46]。
2 过渡金属磷化物析氢性能调控
由于过渡金属磷化物制备方式的多样化,因此,科研人员制备了多种纳米结构。第一类结构包括纳米团簇、纳米线、纳米管、纳米片、纳米阵列等[47-50],此类结构的合成需要通过粘结剂将活性物质修饰在导电界面上,从而导致活性位点被覆盖,造成堆积现象[51-52]。第二类为自支撑结构,为了避免活性物质集聚,将活性物质直接负载在自支撑电极上如铜箔、钛箔、碳布、泡沫镍等[53-57]。TMPs形貌结构、导电性以及活性位点是影响HER性能的关键因素[58],因此,本文从形貌设计、界面调控、材料复合三个途径作为研究重点,分析具有独特结构、良好导电性、多活性位点的过渡金属磷化物。
2.1 形貌设计
设计特定结构,可以增加比表面积,从而暴露更多的活性位点[59-62],因此,优化催化剂纳米结构来构建高效的形貌是实现析氢活性和稳定性的重要因素[63]。CHANG等[64]用油包水微乳液法制备了单晶镍Ni12P5空心球结构。该材料具有独特的空心结构、较高的比表面积(222.5 m2/g)以及丰富的孔隙率,从而提供了有效的扩散通道和更多的催化活性中心,增强了催化析氢活性。在酸性溶液中,当电流密为100 mA/cm2时,过电位277 mV,并且Tafel斜率仅为46 mV/dec(见图4(a)),与此同时,空心球结构具有良好的稳定性,因此在12 h电位测试中,Ni12P5空心球催化剂仍能表现出较高活性(见图4(b))。2018年,该团队[65]首次在碳纸上制备出了海胆状三元钴磷硫化物(CoPS/CP),这种独特的海胆状结构有利于电子传导,加速界面反应。在0.5 mol/L H2SO4溶液中,CoPS/CP的起始点位为4 mV,Tafel斜率为42.6 mV/dec。通过DFT理论计算得知CoPS/CP的氢吸附自由能(△GH*)为-0.12 eV(见图4(c)),因此,海胆状结构为高活性且稳定的催化材料提供了选择。类似地,YANG等[66]通过低温磷化法合成出海胆状的CoP纳米晶,该催化剂在酸性溶液中表现出优异的HER活性,产生电流密度100 mA/cm2时,过电位180 mV(见图4(d))。CHEN等[67]以三苯基膦为磷源,控制特有的磷化过程,合成了棒状和花状的Co2P,该形状具有较大的孔隙率以及比表面积(见图4(e)~(h))。总而言之,科研人员制备了多种不同的纳米结构,其目的是为了获得更大的比表面积,从而增加该材料活性位点的数量,以此来促进催化剂整体的析氢活性。
研究者发现对TMPs空间维度的调控,可以改变电荷分布和诱导局域电场[68-70]。零维(0D)纳米颗粒结合了低密度和高承载能力的特点,因此该维度材料受到了广泛关注。WANG等[71]提出了在磁场下制备具有可控壳厚度的0D Co2P纳米颗粒(见图5(a)~(d)),并且讨论了磁场进行壳调谐的机理:磁场可以通过加速溶解过程并减慢柯肯德尔效应过程来减小粒径、调节壳的厚度。这项工作为尺寸可调的过渡金属磷化物空心颗粒的合成提供了一种合理的方法。一维(1D)纳米材料具有高效电子传输能力,被广泛应用于析氢反应。LIN等[72]采用简单原位磷化法,在不同温度下合成了1D多孔的CoMoP纳米管(见图5(e)),结果表明,磷化温度600 ℃时CoMoP纳米管展现出最佳催化性能,在0.5 mol/L H2SO4溶液中,当电流密度达10 mA/cm2时,过电位为220 mV,Tafel斜率为136 mV/dec。两维(2D)材料有较大的比表面积,这将有利于活性物质与电解液之间发生反应。TANG等[73]报道了电沉积法在泡沫镍上制备2D Ni-P/NF镍磷合金薄膜(见图5(f)~(g)),通过对其性能测试,得出Ni-P/NF是一种高效催化剂的结论。在1 mol/L KOH溶液中电流密度达到10 mA/cm2时,过电位为80 mV,并且Ni-P/NF在碱性介质中具有很强的耐久性。尽管科研人员对零维/一维/二维材料进行了大量研究,但其仍受制备繁琐和易自聚集限制,因此,具有更高比表面积的三维结构(3D)受到了广泛关注。CHANG等[74]在碳纸上生长3D S-Ni5P4 NPA/CP纳米板阵列(见图5(h)~(j))。与磷化镍和硫化镍相比,3D S-Ni5P4 NPA/CP纳米板阵列有较大的比表面,有利于硫和磷相互调节电子性能。电流密度为10 mA/cm2和100 mA/cm2,过电位分别为56 mV及104 mV,Tafel斜率仅为43.6 mV/dec。PI等[75]在碳维布上制备了自立多孔的WP2/CC纳米片阵列,由于其独特的三维结构,因此,有显著的催化性能以和较高的稳定性。
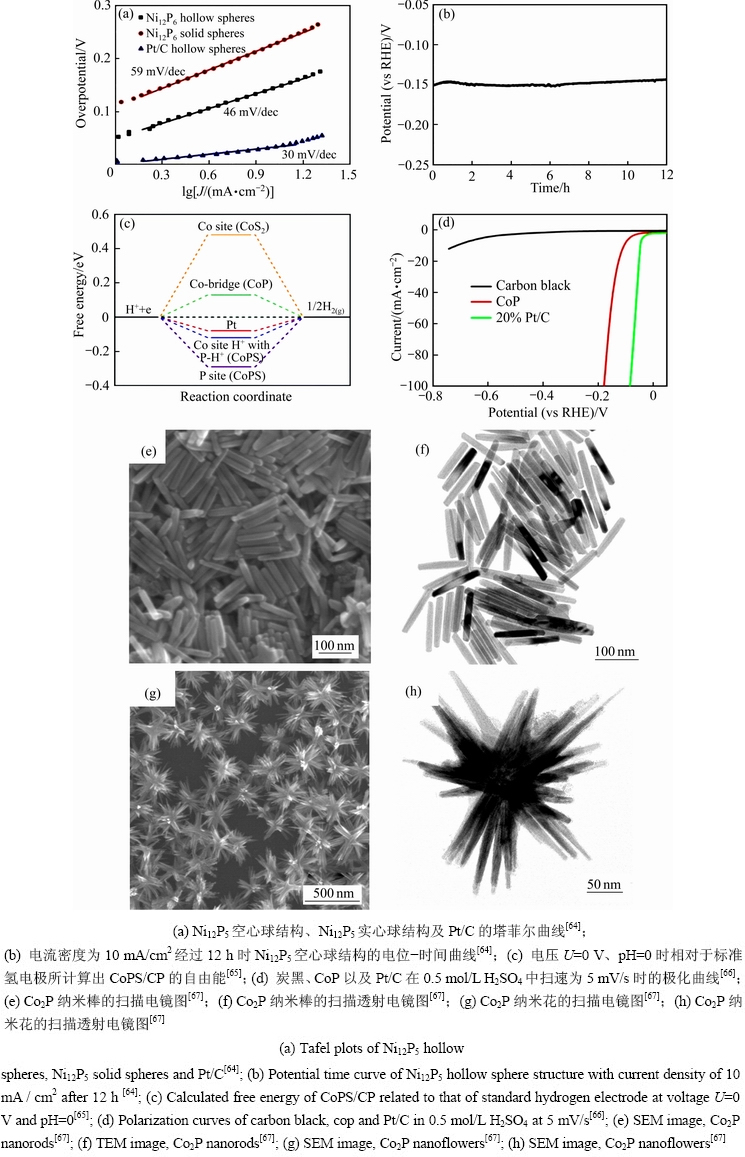
图4 特定空间结构的纳米材料形貌及催化性能
Fig. 4 Morphology and catalytic properties of nano materials with specific spatial structure
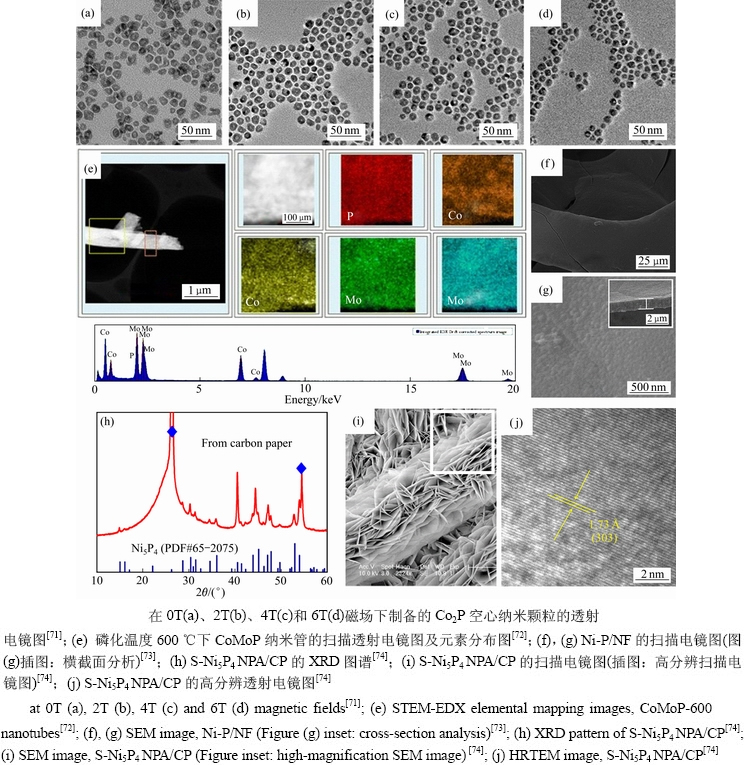
图5 不同空间尺寸的纳米材料形貌及测试
Fig. 5 Morphologies and measurements of nano materials with different space sizes: TEM images of Co2P hollow NPs prepared
2.2 界面调控
引入空位缺陷是调控TMPs催化性能的有效办法,空位缺陷的存在改变了局域的原子结构,从而引起电子结构的变化,增加活性位点,降低了HER的反应能垒[76-78]。KWONG等[79]首次报道了Fe空位缺陷来调节磷化铁(FeP)的电子结构,其中Mg作为“牺牲掺杂剂”引入FeP中,通过化学浸出产生了Vc-FeP薄膜。根据计算表明,原始FeP最活跃的位点具有△GH*=0.16 eV。添加Mg会使△GH*降低至0.05 eV,这表明氢相互作用更强。对于Vc-FeP,氢相互作用进一步提高到△GH*=0.02 eV(见图6(a))。与FeP和Mg-FeP相比,Vc-FeP中铁空位在磷化物空位中产生了相对富P的环境。其中P带有部分负电荷,促进质子捕获,保持较好的析氢活性(见图6(b))。CHENG等[80]对NiAl氢氧化物(NiAl-LDH)进行蚀刻和磷化,形成独特多孔结构的纳米片(NiAlδP)(见图6(c))。从图6(d)中可以清晰观察到大量的原子空位,该空位缺陷有利于电解质的接触渗透和电子转移。目前,采用阳离子空位缺陷调控磷化物的电催化性能已经有较为完善的研究,而TMPs中阴离子空位缺陷对其电催化性能的影响研究还比较有限。DUAN等[81]在泡沫镍表面上制备了磷空位缺陷的(v-Ni12P5)(见图6(e)~(f))。DFT计算表明,v-Ni12P5内具有大量电子积累,从而在磷化镍晶体中引起了显着的电子再分布(见图6(g)~(h)),极大地提高了析氢催化活性。实验结果与预期一样,在1 mol/L KOH溶液中,产生10 mA/cm2电流密度,需要的过电位为27.7 mV,并且Tafel斜率也较小,仅为30.88 mV/dec。总的来说,无论是引入阳离子空位还是阴离子空位,都可以有效的提高催化活性。由于磷空位可以削弱M(过渡金属)3d和P 2p轨道的杂化,丰富磷空位附近M和磷原子的电子密度,并促进氢原子(H*)的解吸过程[82-85],因此,对磷空位的探索逐渐成为TMPs空位研究的热点。
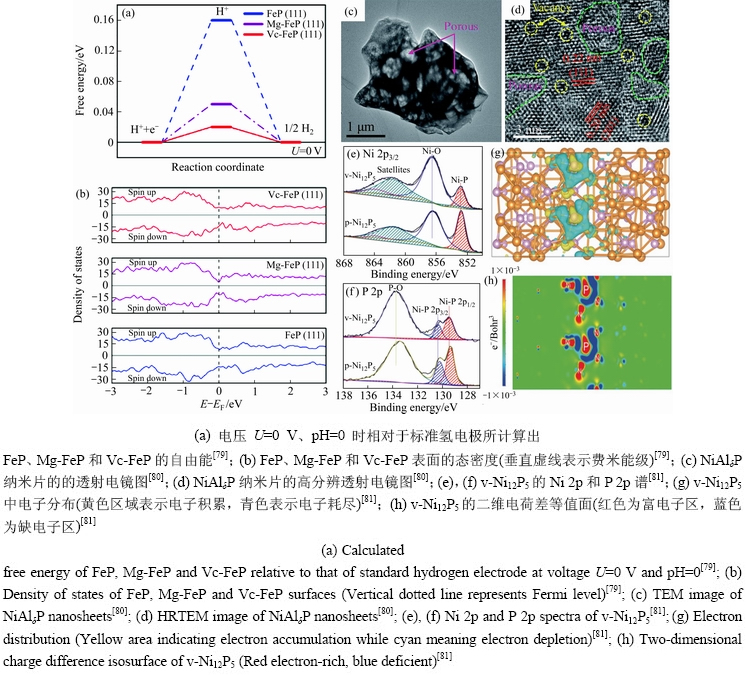
图6 阴离子和阳离子空位缺陷的纳米材料形貌及理论计算
Fig. 6 Morphologies and theoretical calculation of nano materials with anionic and cationic vacancy defects
异质原子掺杂是从原子尺度上调控TMPs析氢的方法,异质原子的添加破坏了金属磷化物晶格的周期性,引起了局域电子结构的改变,从而优化电荷转移[86-89]。根据原子种类可分为金属掺杂、非金属掺杂、金属-非金属掺杂[90-93]。PAN等[94]合成了Fe、Ni、Mn掺杂的CoP空心多面体(见图7(a)),通过X射线吸收近边结构谱可知,金属原子的掺杂改变了CoP的电子结构,电荷由掺杂的金属原子向Co原子转移(见图7(b)和7(c))。由H吸附自由能可得,原子的掺杂改变了△GH*,其中Ni原子的掺杂最小(-0.03 eV)。因此,Ni原子掺杂CoP的过电位最小。LIU等[95]在钛片上生长了Mn掺杂CoP纳米片阵列(见图7(d)),由于锰的掺杂,削弱了Co和H原子之间的相互作用,因此,增强了吸附态氢原子的脱附。在0.5 mol/L H2SO4中产生10 mA/cm2电流密度时,需要的过电位仅为49 mV,Tafel斜率为55 mV/dec;在1 mol/L KOH中,产生相同的电流密度,需要的过电位为76 mV,Tafel斜率为82 mV/dec。ZHANG等[96]将O原子引入MoP、CoP中,增强了它们的固有电导率,拉长了Mo—P和Co—P键,促进电荷的转移,从而获得了优异的HER活性。ZHANG等[97]提出了将N原子同时掺杂在磷化物和载体上,该制备方法利用次磷酸铵分解产生的氨和磷化氢气体与前驱体进行反应,得到N掺杂碳纳米管负载氮掺杂磷化钼催化剂(N-MoP/N-CNT)。N的掺杂调谐了电子结构并且与碳纳米管产生耦合效应,促进了析氢活性(图7(e)~(f))。当电流密度为10 mA/cm2时,过电势为(103±5) mV,低于MoP纳米颗粒的过电势(243 mV)。单一金属和非金属原子掺杂体系无法很好平衡H2O的吸附和解离,而多种元素之间的协同作用能进一步地优化催化剂对HER中间物种的吸附能,降低HER过电位。XU等[98]设计出了一种Cu和O共掺杂的方法,制备出了CoP纳米线阵列(见图7(g)),金属-非金属的掺杂创造了大量的晶格缺陷,增加了活性位点,O和Cu共掺杂CoP纳米线阵列的催化活性比未掺杂的CoP纳米线阵列提高了近10倍(见图7(h))。对上述文献探讨可知,异质原子掺杂可有效调整电子结构、增强润湿性、降低动能势垒和引入额外的活性位点。近年来,通过掺杂一系列金属原子,它们不仅可以增强电导率,还可以通过调节电子结构来优化氢吸附能。
2.3 材料复合
过渡金属磷化物与导电材料复合,可以提高材料的导电性,且基底与活性组分之间存在协同作用,增强了过渡金属磷化物颗粒的稳定性,削弱团聚现象[99-101]。TANG等[102]在钛箔上制备出了Fe-CoP/Ti纳米阵列。对结果分析,钛箔的存在提高活性物间的导电性。令人惊讶的是,这种Fe-CoP/Ti对NaBH4的水解脱氢也具有很高的活性。相比于其他基底,碳布(CC)具有价格低廉,高导电性以及良好的的柔韧性等特点。LIANG等[103]通过低温磷酸化,在CC上开发了自支撑的FeP纳米棒阵列(见图8(a)和(b)),在酸性溶液中FeP NAs/CC表现出高催化活性,仅需58 mV的过电势即可提供10 mA/cm2的电流密度,塔菲尔斜率为45 mV/dec。同时,TIAN等[104]通在CC上直接生长FeP纳米颗粒(FeP/CC)。在大电流密度下(100 mA/cm2),需要的过电位仅为108 mV(见图8(c))。泡沫镍具有较大的比表面积,可以加速离子的扩散,促进电子转移率。TONG[105]通过在氮掺杂碳包覆的泡沫镍上,生长出三维纳米片(NiCoP/NF@NC)。毫无疑问,在碱性溶液中,当电流密度为10 mA/cm2时,过电位仅为31.8 mV,塔菲尔斜率62.3 mV/dec。石墨烯充当底物,不仅可以避免颗粒聚集,而且还可以促进电子转移。WU等[106]通过高温磷化制备出了氧化石墨烯负载的磷化钼纳米颗粒(MoP-RGO)(见图8(d))。结果表明,900℃下制备的MoP-RGO展现出稳定的析氢性能(见图8(e)~(f))。RIYAJUDDIN等[107]将泡沫镍、石墨烯和碳纳米管三者复合作为导电基底,与Ni2P-CuP2异质结构合成出超亲水双金属磷化物(见图8(g))。该基底改善了导电性,加速了电子传输,并且提供了有利的亲水性和憎水性表面,在电极-电解质界面形成低欧姆电阻路径,促进其更快地去除气泡。在酸性介质中的电流密度为10 mA/cm2时显示12 mV的超低电势(见图8(h))。此外,在500 mA/cm2的高电流密度下可持续至少10 d(见图8(i))。在性能测试过程中,通过黏合剂将催化材料与导电载体结合,然而,此种方法,易造成催化材料从电极上脱落,不利于电子转移,严重制约了催化活性和稳定性。因此,直接在导电基材上生长的TMPs被认为是潜在的电催化剂候选者。
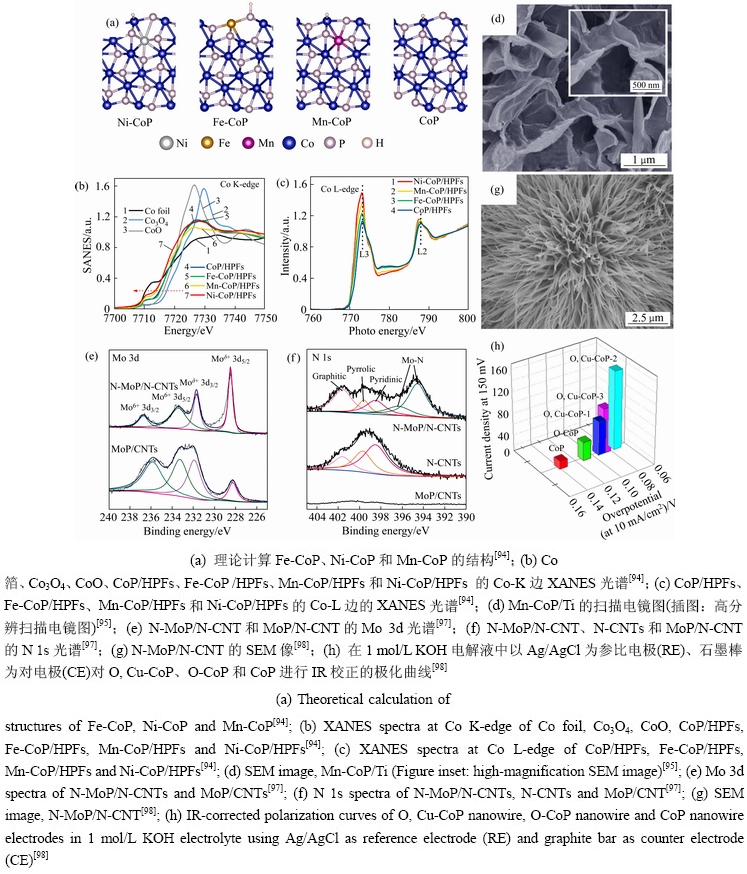
图7 金属与非金属掺杂的纳米材料形貌及催化性能测试
Fig. 7 Morphologies and catalytic properties of metal and non-metal doped nano materials
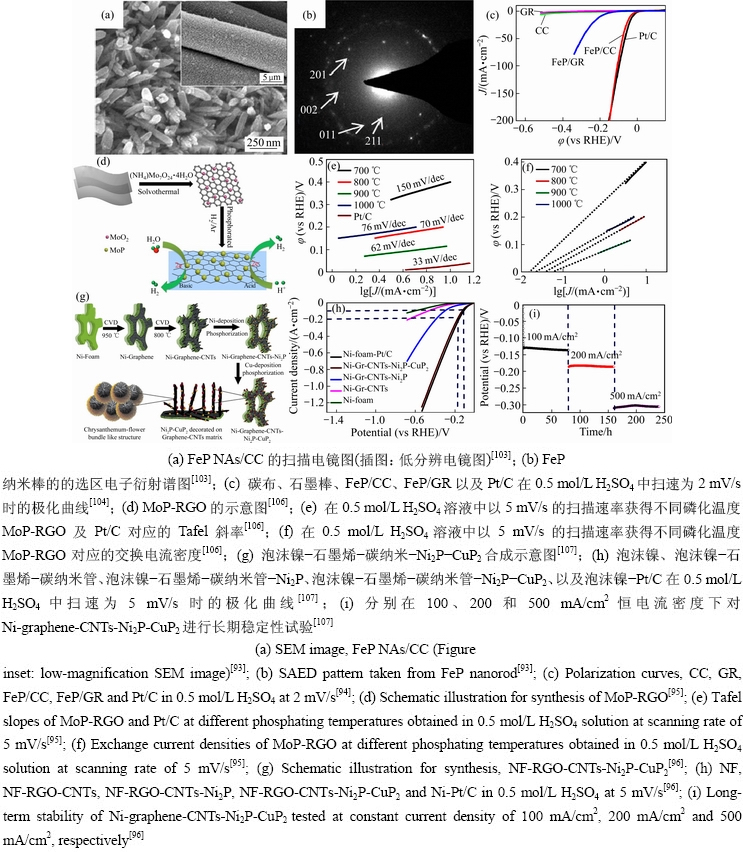
图8 导电材料复合的纳米材料形貌及催化性能测试
Fig. 8 Morphologies and catalytic properties of conductive composite nano materials
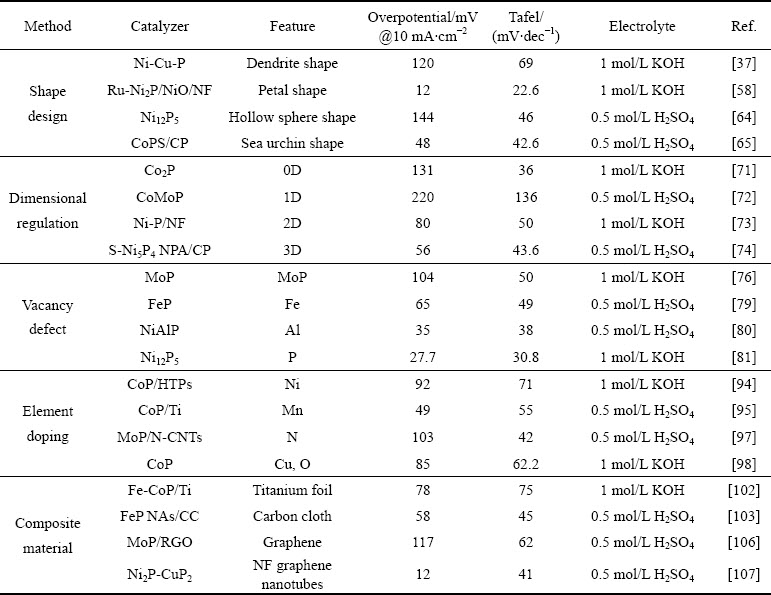
表3总结了不同过渡金属磷化物的析氢性能,从表中清楚看出,磷化物在析氢催化剂中展现出优异性能。为了提高催化活性,可以从如下几个角度深入:首先,引入贵金属来降低催化剂的过电位。例如,Ru-Ni2P/NiO/NF的析氢过电位仅为12 mV。其次,可以考虑将制备材料在载体上原位生长,这样优势在于,一方面可以提高导电性,另一方面,有效避免粘结剂带来的负面效应。最后,鉴于不同金属之间的协同效应,可以考虑双金属或者多金属体系。
3 结论与展望
为了聚焦“碳达峰、碳中和”绿色低碳的发展目标,这就需要持续提升能源利用效率,加快能源消费方式转变。然而,电解水制氢恰好可以有效缓解能源转换这一棘手问题。过渡金属磷化物凭借其独特的催化活性,被科研人员广泛研究。目前,科研工作者主要从形貌设计(结构、维度、高活性表面暴露)、界面调控(空位缺陷、异质原子掺杂)、材料复合等手段提高HER催化性能,虽然收到了一定的成效,但电解水制氢的市场化,仍然任重而道远。未来可考虑从如下几个方面进一步研究。
1) 关注原子级反应机理。当前,电解水析氢的最大困境在于人们无法从原子级上掌握电解水的反应过程。为了阐明机理,研究人员可以采用理论计算和实验分析相结合的方法,之所以加入理论计算,原因在于密度泛函理论可以鉴别中间产物和预测实际活性位点。
2) 探索全解水性能。研究表明,过渡金属磷化物在酸性条件下比碱性显示出更稳定的析氢性能。
表3 典型报道过渡金属磷化物的析氢性能
Table 3 HER performance of typical reported transition metal phosphide
然而,在实际的工业化应用中,所需要的催化剂应具备全pH(0~14)条件下都能展现出稳定性能。因此,社会迫切需要发展全pH中稳定且具有良好催化活性的TMPs。
3) 设计特定空位缺陷。随着对缺陷的日益关注,缺陷工程已成为电催化剂设计的常用策略。研究人员应重视如何引入特定缺陷以及多种缺陷的协同效应对提高析氢反应的促进作用。
4) 考虑TMPs在外界场下的制备。随着磁场、热场、声场、光场等理论体系的完善,学者们可以探究在外界场的环境下,所制备过渡金属磷物析氢性能的变化。
随着科学的进步与发展,相信在不远的将来,过渡金属磷化物在电解水析氢反应中存在的难点问题会逐步得到改善。遥想未来,氢能必将全方位地应用在动力设备上以及高效的提高人类生活水平中。
REFERENCES
[1] Vesborg P C K, Seger B, Chorkendorff I. Recent development in hydrogen evolution reaction catalysts and their practical implementation[J]. The Journal of Physical Chemistry Letters, 2015, 6(6): 951-957.
[2] Chua C K, Pumera M. Susceptibility of FeS2 hydrogen evolution performance to sulfide poisoning[J]. Electrochemistry Communications, 2015, 58: 29-32.
[3] 梁叔全, 程一兵, 方国赵, 等. 能源光电转换与大规模储能二次电池关键材料的研究进展[J]. 中国有色金属学报, 2019, 29(9): 2064-2114.LIANG Shu-quan, CHENG Yi-bing, FANG Guo-zhao, et al. Research progress of key materials for energy photoelectric conversion and large-scale energy storage secondary batteries[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(9): 2064-2114.
[4] 贺亚维, 吴世照, 李玉儒, 等. Pt-WC/Mnt三元纳米复合材料的制备及其电催化性能[J]. 中国有色金属学报, 2020, 30(2): 392-400.HE Ya-wei, WU Shi-zhao, LI Yu-ru, et al. Preparation of Pt-WC/Mnt nano-composite and its electrocatalytic activity[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(2): 392-400.
[5] GAO H, YUE H H, QI F, et al. Few-layered ReS2 nanosheets grown on graphene as electrocatalyst for hydrogen evolution reaction[J]. Rare Metals, 2018, 37(12): 1014-1020.
[6] XIE W F, LI Z H, SHAO M F, et al. Layered double hydroxide-based core-shell nanoarrays for efficient electrochemical water splitting[J]. Frontiers of Chemical Science and Engineering, 2018, 12(3): 537-554.
[7] ZHENG L L, XIAO X Y, LI Y, et al. Enhanced photocatalytic activity of TiO2 nanoparticles using WS2/g-C3N4 hybrid as co-catalyst[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1117-1126.
[8] DING W L, CAO Y H, LIU H, et al. In situ growth of NiSe@Co0.85Se heterointerface structure with electronic modulation on nickel foam for overall water splitting[J]. Rare Metals, 2021, 40(6): 1373-1382.
[9] Zou X X, Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting[J]. Chemical Society Reviews, 2015, 44(15): 5148-5180.
[10] Trancik J E. Renewable energy: Back the renewables boom[J]. Nature, 2014, 507(7492): 300-302.
[11] LIU Y R, HU W H, LI X, et al. Facile one-pot synthesis of CoS2-MoS2/CNTs as efficient electrocatalyst for hydrogen evolution reaction[J]. Applied Surface Science, 2016, 384: 51-57.
[12] ZHOU B W, LI J W, ZHANG X, et al. Engineering P-doped Ni3S2-NiS hybrid nanorod arrays for efficient overall water electrolysis[J]. Journal of Alloys and Compounds, 2021, 862: 158391.
[13] WANG J, XU F, JIN H Y, et al. Non-noble metal-based carbon composites in hydrogen evolution reaction: Fundamentals to applications[J]. Advanced Materials, 2017, 29(14): 1605838.
[14] YANG C F, ZHAO R, XIANG H, et al. Ni-activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions[J]. Advanced Energy Materials, 2020, 10(37): 2002260.
[15] Zheng L, Zhang W, Gao B, et al. One-pot synthesis of few-layered molybdenum disulfide anchored on N, S-codoped carbon for enhanced hydrogen generation[J]. Materials Today Energy, 2021, 19: 100600.
[16] JIN H Y, GU Q F, CHEN B, et al. Molten salt-directed catalytic synthesis of 2D layered transition-metal nitrides for efficient hydrogen evolution[J]. Chem, 2020, 6(9): 2382-2394.
[17] Kim M, Lee B, JU H, et al. Reducing the barrier energy of self-reconstruction for anchored cobalt nanoparticles as highly active oxygen evolution electrocatalyst[J]. Advanced Materials, 2019, 31(32): 1901977.
[18] Zhao X, Xue Z, Chen W, et al. Eutectic synthesis of high-entropy metal phosphides for electrocatalytic water splitting[J]. ChemSusChem, 2020, 13(8): 2038-2042.
[19] SHI Y M, ZHANG B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction[J]. Chemical Society Reviews, 2016, 45(6): 1529-1541.
[20] Yun G N, Ahn S J, Takagaki A, et al. Hydrodeoxygenation of γ-valerolactone on bimetallic NiMo phosphide catalysts[J]. Journal of Catalysis, 2017, 353: 141-151.
[21] Xuan Y, Quan H, Shen Z, et al. Band-gap and charge transfer engineering in red phosphorus-based composites for enhanced visible-light-driven H2 evolution[J]. Chemistry—A European Journal, 2020, 26(10): 2285-2292.
[22] ZHANG S J, SONG L M, WU X Q, et al. Synthesis of high-dispersed NiCoP/SiO2 and hydrodesulfurization performance[J]. Vacuum, 2014, 108: 45-48.
[23] McEnaney J M, Crompton J C, Callejas J F, et al. Amorphous molybdenum phosphide nanoparticles for electrocatalytic hydrogen evolution[J]. Chemistry of Material, 2014, 26(16): 4826-4831.
[24] Popczun E J, McKone J R, Read C G, et al. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction[J]. Journal of the American Chemical Society, 2013, 135(25):9267-9270.
[25] Zhang H, Ha D H, Hovden R, et al. Controlled synthesis of uniform cobalt phosphide hyperbranched nanocrystals using tri-n-octylphosphine oxide as a phosphorus source[J]. Nano Letters, 2011, 11(1): 188-197.
[26] Zheng X, Yuan S, Tian Z, et al. One-pot synthesis of hollow nickel phosphide nanoparticles with tunable void sizes using triphenylphosphine[J]. Materials Letters, 2009, 63(27): 2283-2285.
[27] Zheng X, Yuan S, Tian Z, et al. Nickel/nickel phosphide core-shell structured nanoparticles: Synthesis, chemical, and magnetic architecture[J]. Chemistry of Materials, 2009, 21(20): 4839-4845.
[28] Wang J, Chen H, Fu Y, et al. Highly active Ni2P/SiO2 catalysts phosphorized by triphenylphosphine in liquid phase for the hydrotreating reactions[J]. Applied Catalysis B: Environmental, 2014, 160/161: 344-355.
[29] PAN Y, HU W H, LIU D P, et al. Carbon nanotubes decorated with nickel phosphide nanoparticles as efficient nanohybrid electrocatalysts for the hydrogen evolution reaction[J]. Journal of Materials Chemistry A, 2015, 3(24): 13087-13094.
[30] Li X, Liu W, Zhang M, et al. Strong metal-phosphide interactions in core-shell geometry for enhanced electrocatalysis[J]. Nano Letters, 2017, 17(3): 2057-2063.
[31] Du Y, Qu H, Liu Y, et al. Bimetallic CoFeP hollow microspheres as highly efficient bifunctional electrocatalysts for overall water splitting in alkaline media[J]. Applied Surface Science, 2019, 465: 816-823.
[32] Ma Y Y, Wu C X, Feng X J, et al. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C[J]. Energy & Environmental Science, 2017, 10(3): 788-798.
[33] Lu X F, Yu L, Lou X W D. Highly crystalline Ni-doped FeP/carbon hollow nanorods as all-pH efficient and durable hydrogen evolving electrocatalysts[J]. Science Advances, 2019, 5(2): eaav6009.
[34] Wang X, Na Z, Yin D, et al. Phytic acid-assisted formation of hierarchical porous CoP/C nanoboxes for enhanced lithium storage and hydrogen generation[J]. ACS Nano, 2018, 12(12): 12238-12246.
[35] Cheng J, Li T, ULLAH S, et al. Giant magnetocaloric effect in nanostructured Fe-Co-P amorphous alloys enabled through pulse electrodeposition[J]. Nanotechnology, 2020, 31(38): 385704.
[36] Pei Y, Yang Y, Zhang F, et al. Controlled electrodeposition synthesis of Co-Ni-P film as a flexible and inexpensive electrode for efficient overall water splitting[J]. ACS Applied Materials & Interfaces, 2017, 9(37): 31887-31896.
[37] Cao M, Xue Z, Niu J J, et al. Facile electrodeposition of Ni-Cu-P dendrite nanotube films with enhanced hydrogen evolution reaction activity and durability[J]. ACS Applied Materials & Interfaces, 2018, 10(41): 35224-35233.
[38] YANG F, YANG S, NIU Q, et al. Fabrication of a 3D self-supporting Ni-P/Ni2P/CC composite and its robust hydrogen evolution reaction properties in alkaline solution[J]. New Journal of Chemistry, 2020, 44(20): 8183-8190.
[39] Liu Y, Wang L, Feng H, et al. Microemulsion-assisted self-assembly and synthesis of size-controlled porphyrin nanocrystals with enhanced photocatalytic hydrogen evolution[J]. Nano Letters, 2019, 19(4): 2614-2619.
[40] Adesuji E T, Khalil L, Videa M, et al. From nano to macro: Hierarchical platinum superstructures synthesized using bicontinuous microemulsion for hydrogen evolution reaction[J]. Electrochimica Acta, 2020, 354: 136608.
[41] Jiang Y, Wang D, Pan Z, et al. Microemulsion-mediated hydrothermal synthesis of flower-like MoS2 nanomaterials with enhanced catalytic activities for anthracene hydrogenation[J]. Frontiers of Chemical Science and Engineering, 2018, 12(1): 32-42.
[42] MOTOS-PEREZ B, UZIO D, AYMONIER C, et al. Preparation of nickel phosphide hydrodesulfurization catalysts assisted by supercritical carbon dioxide[J]. Chem Cat Chem, 2015, 7(21): 3441-3444.
[43] Tian S, Li X, Wang A , et al. Facile preparation of Ni2P with a sulfur-containing surface layer by low-temperature reduction of Ni2P2S6[J]. Angewandte Chemie International Edition, 2016, 55(12): 4030-4034.
[44] Zou X X, Wu Y Y, Liu Y P, et al. In situ generation of bifunctional, efficient Fe-based catalysts from mackinawite iron sulfide for water splitting[J]. Chem, 2018, 4(5): 1139-1152.
[45] Liu J L, ZHENG Y, Wang Z Y, et al. Free-standing single- crystalline NiFe-hydroxide nanoflake arrays: A self-activated and robust electrocatalyst for oxygen evolution[J]. Chemical Communications, 2018, 54(5): 463-466.
[46] Long J Y, Gong Y, Lin J H. Metal-organic framework derived Co9S8@CoS@CoO@C nanoparticles as efficient electro-and photo-catalysts for the oxygen evolution reaction[J]. Journal of Materials Chemistry A, 2017, 5(21): 10495-10509.
[47] Jiang P, Liu Q, Sun X. NiP2 nanosheet arrays supported on carbon cloth: An efficient 3D hydrogen evolution cathode in both acidic and alkaline solutions[J]. Nanoscale, 2014, 6(22): 13440-13445.
[48] Zhang Y, Wang Y, Wang T T, et al. Heterostructure of 2D CoP nanosheets/1D carbon nanotubes to significantly boost the alkaline hydrogen evolution[J]. Advanced Materials Interfaces, 2020, 7(2): 1901302.
[49] Zhang S C, Xiong T, Tang X F, et al. Engineering inner-porous cobalt phosphide nanowire based on controllable phosphating for efficient hydrogen evolution in both acidic and alkaline conditions[J]. Applied Surface Science, 2019, 481(15): 1524-1531.
[50] YU L, MISHRA I K, XIE Y L, et al. Ternary Ni2(1-x)Mo2xP nanowire arrays toward efficient and stable hydrogen evolution electrocatalysis under large-current-density[J]. Nano Energy, 2018, 53: 492-500.
[51] ZHENG H Y, HUANG X B, WU Z Y, et al. Controlled synthesis of 3D flower-like Ni2P composed of mesoporous nanoplates for overall water splitting[J]. Chemistry—An Asian Journal, 2017, 12(22): 2956-2961.
[52] Huang J Y, Chen M T, Zhang X W, et al. P-doped 3D graphene network supporting uniformly vertical MoS2 nanosheets for enhanced hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2020, 45(7): 4043-4053.
[53] Bach L G, Thi M L N, Son N T, et al. Mesoporous gold nanoparticles supported cobalt nanorods as a free-standing electrochemical sensor for sensitive hydrogen peroxide detection[J]. Journal of Electroanalytical Chemistry, 2019, 848: 113359.
[54] QAZI U Y, JAVAID R, TAHIR N, et al. Design of advanced self-supported electrode by surface modification of copper foam with transition metals for efficient hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2020, 45(58): 33396-33406.
[55] YOON T, KIM K S. One-step synthesis of CoS-doped β-Co(OH)2@Amorphous MoS2+x hybrid catalyst grown on nickel foam for high-performance electrochemical overall water splitting[J]. Advanced Functional Materials, 2016, 26(41): 7386-7393.
[56] Tian J Q, Liu Q, Asiri A M, et al. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0-14[J]. Journal of the American Chemical Society, 2014, 136(21): 7587-7590.
[57] Liang Y H, Liu Q, Luo Y L, et al. Zn0.76Co0.24S/CoS2 nanowires array for efficient electrochemical splitting of water[J]. Electrochimica Acta, 2016, 190: 360-364.
[58] Yang C, Gao M Y, Zhang Q B, et al. In-situ activation of self-supported 3D hierarchically porous Ni3S2 films grown on nanoporous copper as excellent pH-universal electrocatalysts for hydrogen evolution reaction[J]. Nano Energy, 2017, 36: 85-94.
[59] Chia X Y, Pumera M. Characteristics and performance of two-dimensional materials for electrocatalysis[J]. Nature Catalysis, 2018, 1(12): 909-921.
[60] 杨玉美, 石倩玉, 于雅娜, 等. ZIF-67衍生纳米磷化钴催化硼氢化钠水解制氢[J]. 中国有色金属学报, 2020, 30(8): 1982-1989.YANG Yu-mei, SHI Qian-yu, YU Ya-na, et al. ZIF-67 derived cobalt phosphides nanocatalysts for catalytic hydrolysis of sodium borohydride to generate hydrogen[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(8): 1982-1989.
[61] 曹晓兰, 隋 升, 李 冰, 等. 还原剂浓度对原位沉积铂纳米线催化层结构和性能的影响[J]. 中国有色金属学报, 2020, 30(3): 604-611.CAO Xiao-lan, SUI Sheng, LI Bing. Effects of reducing agent concentrations on structure and properties of in-situ deposited platinum nanowires catalytic layer[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(3): 604-611.
[62] ZHANG H M, WU X Y, CHEN C, et al. Spontaneous ruthenium doping in hierarchical flower-like Ni2P/NiO heterostructure nanosheets for superb alkaline hydrogen evolution[J]. Chemical Engineering Journal, 2021, 417: 128069.
[63] Wan S H, Qi J, Zhang W, et al. Hierarchical Co(OH)F superstructure built by low-dimensional substructures for electrocatalytic water oxidation[J]. Advanced Materials, 2017, 29(28): 1700286.
[64] Chang J F, Li S T, Li G Q, et al. Monocrystalline Ni12P5 hollow spheres with ultrahigh specific surface areas as advanced electrocatalysts for the hydrogen evolution reaction[J]. Journal of Materials Chemistry A, 2016, 4(25): 9755-9759.
[65] Chang J F, Ouyang Y X, Ge J J, et al. Cobalt phosphosulfide in the tetragonal phase: A highly active and durable catalyst for the hydrogen evolution reaction[J]. Journal of Materials Chemistry A, 2018, 6(26): 12353-12360.
[66] YANG H, ZHANG Y, HU F, et al. Urchin-like CoP nanocrystals as hydrogen evolution reaction and oxygen reduction reaction dual-electrocatalyst with superior stability[J]. Nano Letters, 2015, 15(11): 7616-7620.
[67] Chen X, Cheng M, CHEN D, et al. Shape-controlled synthesis of Co2P nanostructures and their application in supercapacitors[J]. ACS Applied Materials & Interfaces, 2016, 8(6): 3892-3900.
[68] Tan C, Cao X, Wu X J, et al. Recent advances in ultrathin two-dimensional nanomaterials[J]. Chemical Reviews, 2017, 117(9): 6225-6331.
[69] Liu K H, Zhong H X, Li S J, et al. Advanced catalysts for sustainable hydrogen generation and storage via hydrogen evolution and carbon dioxide/nitrogen reduction reactions[J]. Progress in Materials Science, 2018, 92: 64-111.
[70] WU C, WANG X Y, PEI W L, et al. Tailoring the shape and size of wet-chemical synthesized FePt nanoparticles by controlling nucleation and growth with a high magnetic field[J]. Nanoscale, 2019, 11(32): 15023-15028.
[71] Wang X Y, Liu C H, WU C, et al. Magnetic field assisted synthesis of Co2P hollow nanoparticles with controllable shell thickness for hydrogen evolution reaction[J]. Electrochimica Acta, 2020, 330: 135191.
[72] Lin Y, Liu M, Pan Y, et al. Porous Co-Mo phosphide nanotubes: An efficient electrocatalyst for hydrogen evolution[J]. Journal of Materials Science, 2017, 52(17): 10406-10417.
[73] Tang C, Asiri A M, Luo Y, et al. Electrodeposited Ni-P alloy nanoparticle films for efficiently catalyzing hydrogen- and oxygen-evolution reactions[J]. ChemNanoMat, 2015, 1(8): 558-561.
[74] Chang J F, Li K, Wu Z J, et al. Sulfur-doped nickel phosphide nanoplates arrays: A monolithic electrocatalyst for efficient hydrogen evolution reactions[J]. ACS Applied Materials & Interfaces, 2018, 10(31): 26303-26311.
[75] PI M Y, WANG X D, ZHANG D K, et al. A 3D porous WP2 nanosheets@carbon cloth flexible electrode for efficient electrocatalytic hydrogen evolution[J]. Frontiers of Chemical Science and Engineering, 2018, 12(3): 425-432.
[76] ZHANG X Y, WU Z Z, WANG D Z. Oxygen-incorporated defect-rich MoP for highly efficient hydrogen production in both acidic and alkaline media[J]. Electrochimica Acta, 2018, 281: 540-548.
[77] Gao W K, Chi J Q, Wang Z B, et al. Optimized bimetallic nickel-iron phosphides with rich defects as enhanced electrocatalysts for oxygen evolution reaction[J]. Journal of Colloid and Interface Science, 2019, 537: 11-19.
[78] LIN J H, YAN Y T, XU T X, et al. Rich P vacancies modulate Ni2P/Cu3P interfaced nanosheets for electrocatalytic alkaline water splitting[J]. Journal of Colloid and Interface Science, 2020, 564: 37-42.
[79] Kwong W L, GRACIA-ESPINO E, LEE C C, et al. Cationic vacancy defects in iron phosphide: A promising route toward efficient and stable hydrogen evolution by electrochemical water splitting[J]. Chem Sus Chem, 2017, 10(22): 4544-4551.
[80] Cheng W R, Zhang H, Zhao X, et al. A metal-vacancy- solid-solution NiAlP nanowall array bifunctional electrocatalyst for exceptional all-pH overall water splitting[J]. Journal of Materials Chemistry A, 2018, 6(20): 9420-9427.
[81] DAUN J J, CHEN S, ORTIZ-LEDON C A, et al. Phosphorus vacancies that boost electrocatalytic hydrogen evolution by two orders of magnitude[J]. Angewandte Chemie International Edition, 2020, 59(21): 8181-8186.
[82] WANG Z K, WANG S Y, MAL X, et al. Water-induced formation of Ni2P-Ni12P5 interfaces with superior electrocatalytic activity toward hydrogen evolution reaction[J]. Small, 2021, 17(6): 2006770.
[83] LIN J H, YAN Y T, LIU T, et al. Optimize the electrocatalytic performances of NiCoP for water splitting by the synergic effect of S dopant and P vacancy[J]. International Journal of Hydrogen Energy, 2020, 45(32): 16161-16168.
[84] RAN Z, SHU C, HOU Z, et al. Modulating electronic structure of honeycomb-like Ni2P/Ni12P5 heterostructure with phosphorus vacancies for highly efficient lithium-oxygen batteries[J]. Chemical Engineering Journal, 2021, 413: 127404.
[85] WANG Y Q, ZHANG J T. Structural engineering of transition metal-based nanostructured electrocatalysts for efficient water splitting[J]. Frontiers of Chemical Science and Engineering, 2018, 12(4): 838-854.
[86] Wang Y, Kong B, Zhao D Y, et al. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting[J]. Nano Today, 2017, 15: 26-55.
[87] Huang X, Liu Z, Millet M M, et al. In situ atomic-scale observation of surface-tension-induced structural transformation of Ag-NiPx core-shell nanocrystals[J]. ACS Nano, 2018, 12(7): 7197-7205.
[88] Wang X Q, Chen Y F, Yu B, et al. Hierarchically porous W-doped CoP nanoflake arrays as highly efficient and stable electrocatalyst for pH-universal hydrogen evolution[J]. Small, 2019, 15(37): 1902613.
[89] 孙学良. 富磷型贵金属磷化物的绿色制备及电催化[J]. 物理化学学报, 2020, 37: 2011077.SUN Xue-liang. Green synthesis and electrocatalysis of P-rich noble metal phosphides[J]. Journal of the Physical Chemistry, 2020, 37: 2011077.
[90] Wang J, Li X Z, Wei B, et al. Activating basal planes of NiPS3 for hydrogen evolution by nonmetal heteroatom doping[J]. Advanced Functional Materials, 2020, 30(12): 1908708.
[91] LI Y J, WANG M, LIU S, et al. Preparation and properties of transition metal nitrides caged in N-doped hollow porous carbon sphere for oxygen reduction reaction[J]. Transactions of Nonferrous Metals Society of China, 2021, 31(5): 1427-1438.
[92] Xu K, Ding H, Zhang M X, et al. Regulating water-reduction kinetics in cobalt phosphide for enhancing HER catalytic activity in alkaline solution[J]. Advanced Materials, 2017, 29(28): 1606980.
[93] Fang Z, Peng L, Qian Y, et al. Dual tuning of Ni-Co-A (A=P, Se, O) nanosheets by anion substitution and holey engineering for efficient hydrogen evolution[J]. Journal of the American Chemical Society, 2018, 140(15): 5241-5247.
[94] PAN Y, SUN K A, LIN Y, et al. Electronic structure and d-band center control engineering over M-doped CoP (M= Ni, Mn, Fe) hollow polyhedron frames for boosting hydrogen production[J]. Nano Energy, 2019, 56: 411-419.
[95] Liu T T, Ma X, Liu D N, et al. Mn doping of CoP nanosheets array: An efficient electrocatalyst for hydrogen evolution reaction with enhanced activity at all pH values[J]. ACS Catalysis, 2017, 7(1): 98-102.
[96] Zhang G, Wang G C, Liu Y, et al. Highly active and stable catalysts of phytic acid-derivative transition metal phosphides for full water splitting[J]. Journal of the American Chemical Society, 2016, 138(44): 14686-14693.
[97] Zhang J T, SUI R, Xue Y R, et al. Direct synthesis of parallel doped N-MoP/N-CNT as highly active hydrogen evolution reaction catalyst[J]. Science China Materials, 2019, 62(5): 690-698.
[98] Xu K, Sun Y, Sun Y, et al. Yin-Yang harmony: Metal and nonmetal dual-doping boosts electrocatalytic activity for alkaline hydrogen evolution[J]. ACS Energy Letters, 2018, 3(11): 2750-2756.
[99] TIAN G Q, WEI S R, GUO Z T, et al. Hierarchical NiMoP2-Ni2P with amorphous interface as superior bifunctional electrocatalysts for overall water splitting[J]. Journal of Materials Science & Technology, 2021, 77: 108-116.
[100] Wu C, Cai J J, Zhu Y, et al. Hybrid reduced graphene oxide nanosheet supported Mn-Ni-Co ternary oxides for aqueous asymmetric supercapacitors[J]. ACS Applied Materials & Interfaces, 2017, 9(22): 19114-19123.
[101] Tian J Q, Liu Q, Cheng N Y, et al. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water[J]. Angewandte Chemie International Edition, 2014, 53(36): 9577-9581.
[102] Tang C, Zhang R, LU W B, et al. Fe-doped CoP nanoarray: A monolithic multifunctional catalyst for highly efficient hydrogen generation[J]. Advanced Materials, 2017, 29(2): 1602441.
[103] Liang Y H, Liu Q, Asiri A M, et al. Self-supported FeP nanorod arrays: A cost-effective 3D hydrogen evolution cathode with high catalytic activity[J]. ACS Catalysis, 2014, 4(11): 4065-4069.
[104] Tian J, Liu Q, Liang Y, et al. FeP nanoparticles film grown on carbon cloth: An ultrahighly active 3D hydrogen evolution cathode in both acidic and neutral solutions[J]. ACS Applied Materials & Interfaces, 2014, 6(23): 20579-20584.
[105] TONG M M, WANG L, YU P, et al. 3D Network nanostructured NiCoP nanosheets supported on N-doped carbon coated Ni foam as a highly active bifunctional electrocatalyst for hydrogen and oxygen evolution reactions[J]. Frontiers of Chemical Science and Engineering, 2018, 12(3): 417-424.
[106] Wu Z X, Wang J, Zhu J, et al. Highly efficient and stable MoP-RGO nanoparticles as electrocatalysts for hydrogen evolution[J]. Electrochimica Acta, 2017, 232: 254-261.
[107] Riyajuddin S, Azmi K, Pahuja M, et al. Super-hydrophilic hierarchical Ni-foam-graphene-carbon nanotubes-Ni2P-CuP2 nano-architecture as efficient electrocatalyst for overall water splitting[J]. ACS Nano, 2021, 15(3): 5586-5599.
WANG Zhong1, LIU Jia-qi1, LIU Chen1, YUAN Shuang1, 3, 4, WANG Qiang2
(1. School of Metallurgy, Northeastern University, Shenyang 110819, China;
2. Key Laboratory of Material Electromagnetic Process Research Ministry of Education, Northeastern University, Shenyang 110819, China;
3. Institute for Frontier Technologies of Low-Carbon Steelmaking, Northeastern University, Shenyang 110819, China
4. Liaoning Province Engineering Research Center for Technologies of Low-Carbon Steelmaking, Northeastern University, Shenyang 110819, China)
Abstract: In recent years, with the development of society, the consumption of traditional fossil energy has increased dramatically, and the environmental problems have become increasingly serious. Electrochemical water decomposition is a practical strategy for converting electric energy into hydrogen fuel. Therefore, in order to achieve large-scale hydrogen production, it is very important to develop low-cost, resource rich, efficient and stable electrocatalysts. Transition metal phosphides are widely used in the field of electrocatalytic dilute hydrogen because of their low price, good conductivity and chemical stability. In this paper, the preparation methods of transition metal phosphides for electrocatalytic hydrogen evolution were summarized, and the improvement of hydrogen evolution performance of electrolyzed water from three aspects of morphology design, interface control and material composite were summarized in detail. The future development trend, opportunities and challenges are also prospected.
Key words: transition metal phosphide; hydrogen evolution reaction; morphology design; interface control; material composite
Foundation item: Project(21701022) supported by the National Natural Science Foundation of China; Project (2018QNRC001) supported by the Young Elite Scientists Sponsorship Program by CAST; Project(XLYC1807214) supported by the Liaoning Revitalization Talents Program, China; Project(N2124007-1) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2021-05-06; Accepted date: 2021-08-19
Corresponding author: YUAN Shuang; Tel: +86-24-83681171; E-mail: yuans@smm.neu.edu.cn
(编辑 龙怀中)
基金项目:国家自然科学基金青年基金资助项目(21701022);中国科协青年人才托举工程资助项目(2018QNRC001);辽宁省兴辽英才计划青年拔尖人才资助项目(XLYC1807214);中央高校基本科研业务费资助项目(N2124007-1)
收稿日期:2021-05-06;修订日期:2021-08-19
通信作者:袁 双,副教授,博士;电话:024-83681171;E-mail:yuans@smm.neu.edu.cn
摘 要:近年来,随着社会的发展,传统化石能源消耗急剧增加,环境问题日益加剧,可再生清洁能源的开发迫在眉睫。电化学水分解法是公认为将电能转化为氢燃料的一种实用策略。因此,为了实现大规模制氢,开发低成本、资源丰富、高效且稳定的电催化剂至关重要。过渡金属磷化物具有价格低廉、资源丰富、化学稳定性等特点,被广泛应用于电催化析氢领域。本文总结了过渡金属磷化物用于电催化析氢的制备方法,且详细概述了从形貌设计、界面调控、材料复合3个方面,提高电解水析氢反应性能,并对其今后发展趋势以及面临的机遇和挑战进行展望。