Trans. Nonferrous Met. Soc. China 31(2021) 3310-3327
Progress in Ti3O5: Synthesis, properties and applications
Peng-fei ZHAO1,2*,Guang-shi LI1,2*,Wen-li LI1,2, Peng CHENG1,2, Zhong-ya PANG1,2, Xiao-lu XIONG1,2, Xing-li ZOU1,2, Qian XU1,2, Xiong-gang LU1,2,3
1. School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China;
2. State Key Laboratory of Advanced Special Steel & Shanghai Key Laboratory of Advanced Ferrometallurgy, Shanghai University, Shanghai 200444, China;
3. School of Materials Science, Shanghai Dianji University, Shanghai 201306, China
Received 18October 2021; accepted 18November 2021
Abstract:
he crystal structure, physical, chemical and phase transition properties of trititanium pentoxide (Ti3O5) have aroused a broad range of research effort since the 1950s. Different crystalline forms (α, β, γ, δ and λ) of Ti3O5 exhibit various properties. Particularly, reversible phase transitions between λ- and β-Ti3O5 have been attracting increasing research interest, which brings new potential applications of Ti3O5 materials in the field of energy and data storage.More recently, Ti3O5 materials have shown excellent performance in trace detection, microwave absorption and virus adsorption, which has expanded its application fields. Here, the essential properties of different crystal forms of Ti3O5 are described in detail. An intensive overview of Ti3O5 preparation methods and applications is comprehensively summarized.
Key words:
Ti3O5; phase transition; pressure induction; data storage; catalyst support;
1 Introduction
Aside from stoichiometric titanium dioxide (TiO2), which has been studied extensively and used in numerous fields ranging from alloys to photo-catalytic materials, titanium binary oxides have a series of suboxides. These titanium suboxides comply with the generic formula of TinO2n-1 and include NaCl-type TiO, corundum-type Ti2O3 and “MagnEli phases” TinO2n-1(n≥3) [1-12]. However, whether trititanium pentoxide (Ti3O5) belongs to this series remains controversial because the typical shear planes existing in MagnEli phases are not found in the crystal structure [13-15].
Since the 1950s, many scholars have conducted considerable research on Ti3O5. Yet, the focus is mostly on structure, physical and chemical properties, and five different Ti3O5 crystal structures (α, β, γ, δ and λ) have been identified [16]. These phases are closely related structurally and exhibit different properties in terms of conductivity, magnetism and electronic structure. In addition, there are some intriguing phase-transition characteristics between them under different external conditions. At room temperature, bulk Ti3O5 is kept in the form of a stable β phase with a monoclinic structure. However, this phase transforms into the λ phase at 460 K with an isostructural phase transition and further transforms into the α phase at 514 K, undergoing a structural transition from monoclinic to orthorhombic. γ-Ti3O5 can be obtained through slow cooling of α-Ti3O5 at high temperatures, transforming into δ-Ti3O5 at approximately 237 K.
Recently, the research on Ti3O5 has mainly focused on its preparation methods and applications as functional materials, as shown in Fig. 1. In 2003, the first applications of Ti3O5 for oxygen sensors were reported. The features of metal-like and variable atom ratio of oxygen to titanium confer α-Ti3O5 potential applications in oxygen sensors. TiO2 is a widely studied photo-catalytic material, and titanium suboxides, such as Ti2O3, Ti4O7 and Ti8O15, also exhibit good photo-activity due to the existence of oxygen vacancies. Therefore, the photo-catalytic activity and performance of Ti3O5 have also been studied. Furthermore, Ti3O5 holds the advantages of good chemical stability, non-toxicity and good acid and alkali resistance through which it can be recommended for catalyst support applications. In addition, the discovery of the photo-induced phase transition between λ and β phases has raised a new discussion and piqued research interest in science, introducing new applications into optical memory. This intriguing reversible phase transition could also be triggered by other external stimulation, such as pressure, temperature and current, which further broadened the application of Ti3O5 in energy storage, THz sensors and smart windows [17]. Recently, the excellent performance of Ti3O5 in trace detection, microwave absorption and virus adsorption has provided broad prospects for its futureapplication.
Although there are intensive experimental and computational studies, a comprehensive summary of the structure, physical properties, preparation and application of Ti3O5 is lacking. A deep understanding of the basic physicochemical properties of Ti3O5might aid in developing applications in new areas. As a result, we initially discussed synthetic approaches of Ti3O5. Especially, we systematically discussed crystal and chemical structure properties of different crystal forms of Ti3O5, including reversible phase transition phenomena between them. Through this discussion, we summarized a comprehensive overview of the application of Ti3O5 in gas sensors, energy storage, optical storage media, catalysts and other new application fields. Finally, the focus for the future direction of Ti3O5was provided.
2 Synthetic approaches for trititanium pentoxide (Ti3O5)
Numerous methods have been reported forpreparing Ti3O5 since it was identified.Typically, TiO2 is selected as the raw material for preparing Ti3O5 by using reduction methods, and metal titanium, carbon, carbonaceous organic material or reducing atmosphere (H2, CO) can be used as reducing agents for preparing Ti3O5 using reduction methods. Reducing agents, which have a higher oxygen affinity, are oxidized to their corresponding oxides, reducing TiO2 to Ti3O5, simultaneously. In addition, some methods such as electro-chemical reduction and pulsed laser deposition (PLD) are also used to meet the need to obtain different forms or properties of Ti3O5.
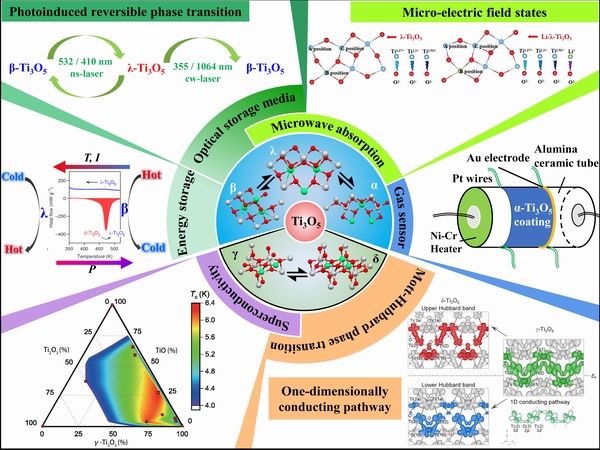
Fig. 1 Schematic of application of different crystal forms of trititanium pentoxide (Ti3O5) in various fields
2.1 Metallothermic reduction
Metal titanium is the most commonly used reductant at the start of the preparation of Ti3O5. The key to preparing a high purity target product lies in the appropriate ratio of titanium to TiO2. The reduction processes work at temperatures above 1000 °C under an inert atmosphere for several days using an electric arc furnace or electric resistance furnace, as shown in Fig. 2. The redox reaction is given as follows:
Ti(s)+5TiO2(s)=2Ti3O5(s) (1)
For instance, in the experiments of 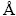 SBRINK and MAGNELI[18], TiO2 and Ti were used as raw materials and mixed with a stoichiometric ratio to prepare Ti3O5 using an electric arc furnace under an argon atmosphere. After two-week annealing at 1150 °C, high-quality β-Ti3O5 was obtained. To prepareγ-Ti3O5 films, KUROKAWA et al [19] firstly synthesized Ti2O3by sintering mixed Ti and TiO2 pellets at 1000 °C for 12 h. Secondly, γ-Ti3O5 films grew on α-Al2O3 (0001) substrates using Ti2O3 as a target by the PLD method. Using the same method, FAN et al [20] used titanium metal as a target to directly grow γ-Ti3O5 on α-Al2O3 (0001) substrates by tuning the oxygen pressure. In contrast to other reported targets, titanium has the advantage of easily preparingTi3O5 films because this is an exothermic process and the oxygen content is easy to control. Titanium is an ideal reductant because noimpurities are introduced in this process. However, this method is limited by high costs and prolonged treatment time.
SBRINK and MAGNELI[18], TiO2 and Ti were used as raw materials and mixed with a stoichiometric ratio to prepare Ti3O5 using an electric arc furnace under an argon atmosphere. After two-week annealing at 1150 °C, high-quality β-Ti3O5 was obtained. To prepareγ-Ti3O5 films, KUROKAWA et al [19] firstly synthesized Ti2O3by sintering mixed Ti and TiO2 pellets at 1000 °C for 12 h. Secondly, γ-Ti3O5 films grew on α-Al2O3 (0001) substrates using Ti2O3 as a target by the PLD method. Using the same method, FAN et al [20] used titanium metal as a target to directly grow γ-Ti3O5 on α-Al2O3 (0001) substrates by tuning the oxygen pressure. In contrast to other reported targets, titanium has the advantage of easily preparingTi3O5 films because this is an exothermic process and the oxygen content is easy to control. Titanium is an ideal reductant because noimpurities are introduced in this process. However, this method is limited by high costs and prolonged treatment time.
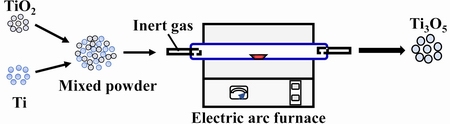
Fig. 2 Schematic illustration of metallothermic reduction process
In addition, KITADA et al [21] selected Zr as a reductant and TiO2 as a titanium source using the sol–gel method to prepare macroporous titanium suboxides (TinO2n-1; n=2, 3, 4, 6). Firstly, porous TiO2 precursor monolith was fabricated using ethyl acetoacetate as a chelating agent and NH4NO3 as mineral salt. Then, several suboxides were obtained under different reduction degrees depending on the amount of the reductant. A single phase of Ti3O5 can be prepared under a 6:1.05 molarratio of TiO2 to Zr at 1150 °C. The as-prepared Ti3O5had a macroporous structure with low bulk densities (1.78 g/cm3) and large porosities (58%).
2.2 Carbothermal reduction
To develop an efficient, economical and rapid method for preparing nano-λ-Ti3O5, CHAI et al [22] proposed a carbothermal reduction method. The redox reaction is given as follows:
3TiO2(s)+C(s)=Ti3O5(s)+CO(g) (2)
The key to preparing a high purity target product lies in the appropriate ratio of carbon to TiO2. In addition, the roasting temperature, holding time and argon gas flow rate were also studied in detail. λ-Ti3O5 was synthesized at 1050 °Cunder 4.5 wt.% carbon black content for 3 h in an inert atmosphere. The resulting powder had a specific surface area of 12.82 m2/g with an average particle size of (3.0±1.0) μm. After that, using the same reduction approach, they reported the effect of coating layers, such as Al2O3 or SiO2, on the formation of nano-λ-Ti3O5. In 2020, a new approach using commercial polyethylene glycol 600 as a carbon source and tetra-n-butyl titanate as a titanium source was reported by CAI et al [23]. Spherical shape powder with an average particle size of 40 nm was obtained at 1070 °C for 2 h. Subsequently, YANG et al [24] were inspired by the preparation of TinO2n-1 using polyvinyl alcohol as a carbon source and proposed using phenol resin, which had highly reduced properties, as reducing agents to prepare recorded purity β-Ti3O5compacts. The β-Ti3O5 of the final product was as high as 98.06% according to X-ray diffraction (XRD) refinement results.
The carbothermal reduction has unique advantages such as high yield and economic and environmental friendliness; however, it is difficult to avoid carbon residue within the as-prepared Ti3O5.
2.3 Hydrogen reduction
In 1968, IWASAKI et al [25] used TiO2 as a titanium source and H2 as a reductant to synthesize Ti3O5. A D-type phase, hereafter called λ-Ti3O5, was obtained at 1250 °C for 3 h in a hydrogen gas stream. The redox reaction is given as follows:
3TiO2(s)+H2(g)=Ti3O5(s)+H2O(g) (3)
To prepare α-Ti3O5 thin films, ZHENG [26] used Ti(OC4H9) as a titanium resource to obtain Ti4+ containing sol. Firstly, TiO2 thin films were fabricated by a dip-coating method on Al2O3 substrates. Secondly, after reducing these TiO2 thin films with hydrogen at 1200 °C for 4 h, α-Ti3O5 thin films were formed. To prepare nano-λ-Ti3O5, OHKOSHI et al [27] selected TiO2 as raw material and reduced it under hydrogen stream (0.3 dm3/min) at 1200 °C for 2 h. The prepared crystals were composed of nano-crystals in (25±15) nm with a flake form. In addition, a combination of reverse-micelle and sol–gelmethod was also used to synthesize nano-λ-Ti3O5. The process is illustrated in Fig. 3 [28], and the prepared sample has a cubic shape with (21±11) nm. Using this approach, NASU et al [28] synthesized different grain sizes of λ-Ti3O5 by controlling the sintering temperature; however, the hydrogen flow rate did not influence it.
In the experimentsof ALIPOUR MOGHADAMESFAHANI et al[29], to obtain catalyst support that contains Ti3O5, TiO2 was selected as a titanium source and a gaseous mixture containing hydrogen (H2:N210:90, vol.%) as a reducing agent.
The problems of carbon residue and other impurities can be solved well; however, the potential explosion hazard restricts its applications.
2.4 Carbon monoxide reduction
In addition to hydrogen reduction, crystal growth under CO reducing atmosphere is also a practical method for preparing Ti3O5 in the early years. For instance, BARTHOLOMEW and WHITE [30] successfully grew Ti3O5 from Na2B4O7-B2O3 flux by controlling oxygen fugacity under the CO atmosphere, which originated from graphite. The Ti3O5 single crystal was obtained under a certain oxygen fugacity at 1300 °C.
2.5 Electro-chemical reduction
Since the electro-chemical de-oxidization of TiO2 in molten calcium chloride to directly prepare titanium was proposed by CHEN et al [31] many scholars have dedicated effort to the in-depth study of this process [31,32]. DRING et al [33] investigated cathodic de-oxygenation of TiO2 at 900 °C under certain potentials lower than those of the formation of calcium. The reduction from TiO2 to Ti3O5 was detected at a potential of 300 mV, which was negative for the TiO2 open-circuit on the TiO2 working electrode. Further studies on pre-dominance diagrams were also conducted by them to better understand the electro-chemical de-oxidization of TiO2 [34]. On this basis, the kinetic parameters related to different reduction processes were calculated by KAR and EVANS[35]using a coupled electro-chemical and diffusion model. These studies have demonstrated the feasibility and availability of the proposedapproach; however, they mainly focused on electro-chemical de-oxidization processes of TiO2 rather than the preparation of Ti3O5. After that, ERTEKIN et al [36] developed a one-stepelectro-deposition method for synthesizing TinO2n-1 films by an electro-deposition method using an acetonitrile solution as a supporting electrolyte. TiOSO4 and H2O2 play a major role in this process,and both γ-Ti3O5andλ-Ti3O5can be obtained fromindium–tin–oxidecoatedglass substrates at certain potentials and temperatures. In their follow-up work, β-Ti3O5 was also detected on the substrates using the same methods [37]. These studies provided a new idea for preparing Ti3O5 thin films with the advantage of without requiring additional heating. However, the obtained products are a complex mixture of titanium sub-oxides or a mixture of Ti3O5 with different crystals. Therefore, further optimization regarding deposition conditions is needed.
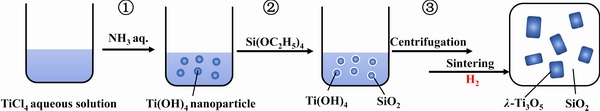
Fig. 3 Schematic of λ-Ti3O5nano-crystal synthesis process[28]. Reproduced with permission. Copyright 2014, Institute of Physics Publishing
2.6 Chemical vapor transport
In addition, a cheaper, efficient and easy method called chemical vapour transport was applied to the crystal growth of Ti2O3 [38]. After that, a series of titanium suboxide crystals were fabricated using this method. MERCIER et al [39] selected TiO2 as the starting material and used hydrogen to reduce it to the desired suboxide. Then, they selected TeCl4 or NH4Cl as a transport agent for the single-phase growth of Ti3O5. STROBEL and PAGE [40] used TeCl4 or Cl2 as a transport agent to prepare TinO2n-1 with n=2-9. Most of the samples were twinned crystals because of the particular sensitivity of Ti3O5 to oxygen. In experimentsof HONG[41], commercial TiO2 and titanium were used as raw materials, and they were mixed at a suitable stoichiometric ratio. The mixed powders were annealed in an arc furnace under an argon atmosphere to obtain Ti2O3 and β-Ti3O5, which were mixed in stoichiometric proportions. Then, the obtained mixtures were pressed into pellets, which were used to prepare single crystals of γ-Ti3O5 using TiCl4 as a transport agent. Notably, this is the first report on the preparation of single crystals of γ-Ti3O5. After that, the crystal structure and physical properties of γ-Ti3O5 were studied systematically by HONG and  SBRINK [42].
SBRINK [42].
3 Structures and properties
3.1 Phase structures
Ti3O5 is a polycrystalline compound with variable crystallographic structures (β, λ, α, γand δ). The schematic representation and lattice parameters of the different crystal structures are shown in Fig. 4 and Table 1 (β phase: ICSD card No. 75194; λ phase: ICSD card No. 75193;α phase: ICSD card No. 50984; γ phase: ICSD card No. 35148; δ phase: CCDC card No. 1004604).
β-Ti3O5, originally known as the LM (low-temperature) structure, is a stable phase at room temperature with a monoclinic symmetry and space group C2/m. Figure 4(a) shows that there are three independent Ti atomic sites, labelled Ti(1), Ti(2) and Ti(3), respectively and each of them is octahedrally coordinated to six oxygen atoms, forming a distorted TiO6 structure. The crystal could be viewed as comprising TiO6 by sharing corners on the b-axis and edges in the ac plane. The average valences for Ti(1), Ti(2) and Ti(3) are 3.0, 3.7 and 3.3, respectively [43].λ-Ti3O5, originally known as the HM (high-temperature) structure, is a meta-stable phase with monoclinic symmetry and space group C2/m. As shown in Fig. 4(b), distorted TiO6 shares six edges with its neighbours. This specialised junction makes Ti atoms with the same atomic environment, and the average valence is 3.3.α-Ti3O5, hereafter called the HO (high temperature orthorhombic) structure, with orthorhombic symmetry and space group Cmcmis a high-temperature phase [44]. Figure 4(c) shows that the crystal could be viewed as comprising TiO6 by sharing corners on an axis and sharing six edges with its neighbours. There are two independent Ti atomic sites, labelled Ti(1) and Ti(2), respectively and their average valence is 3.3.γ-Ti3O5, which is another stable phase at room temperature with monoclinic symmetry and space group I2/c. It was reported by  SBRINK et al [45], and the structure is shown in Fig. 4(d). There are two independent Ti atomic sites, labelled Ti(1) and Ti(2), respectively and the crystal structure could be viewed as comprising two characteristic chains. One chain is made of regular Ti(1)O6octahedra joined by shared corners, whereas the other one has distorted Ti(2)O6octahedra joined by shared edges and a common face. The valence states for Ti(1) and Ti(2) are 3.36 and 3.30, respectively. In δ-Ti3O5(Fig. 4(e)), the Ti(1) site of γ-Ti3O5 becomes two different sites, labelling Ti(1a) and Ti(1b), respectively, thereby forming three independent Ti atomic sites, with corresponding valence states of 3.66, 3.16 and 3.2, respectively [46].
SBRINK et al [45], and the structure is shown in Fig. 4(d). There are two independent Ti atomic sites, labelled Ti(1) and Ti(2), respectively and the crystal structure could be viewed as comprising two characteristic chains. One chain is made of regular Ti(1)O6octahedra joined by shared corners, whereas the other one has distorted Ti(2)O6octahedra joined by shared edges and a common face. The valence states for Ti(1) and Ti(2) are 3.36 and 3.30, respectively. In δ-Ti3O5(Fig. 4(e)), the Ti(1) site of γ-Ti3O5 becomes two different sites, labelling Ti(1a) and Ti(1b), respectively, thereby forming three independent Ti atomic sites, with corresponding valence states of 3.66, 3.16 and 3.2, respectively [46].
3.2 Phase transition properties
Phase transitions between Ti3O5 phases have been identified in numerous studies. These phases experience different transition processes during warming and cooling with corresponding changes in lattice parameters, magnetic state and electric resistivity. During the heating process, the room-temperature stable β phase transforms into a meta-stableλ phase at approximately 460 K with the first-order phase transition and an abrupt reduction of resistivity from semiconductors to metals [47,48]. The lattice parameters are also changed, especially the expansion of the c-axis, increasing the volume. As the temperature is increased to 514 K, the λ phase further transforms into the α phase with the second-order phase transition [49]. However, there is no sudden change in the magnetic state and resistivity. The meta-stable λphases can be stabilized at room temperature by introducing impurity elements such as Fe, V, Mg, Li and Al, and with an increase in impurity content, the transition temperature is decreased accordingly [50-55]. In addition, the meta-stable λ phase could also be stabilized at room temperature in nano-scale, exhibiting a reversible phase transition between λ and β by an external stimulus such as laser light, pressure, temperature and current [56].
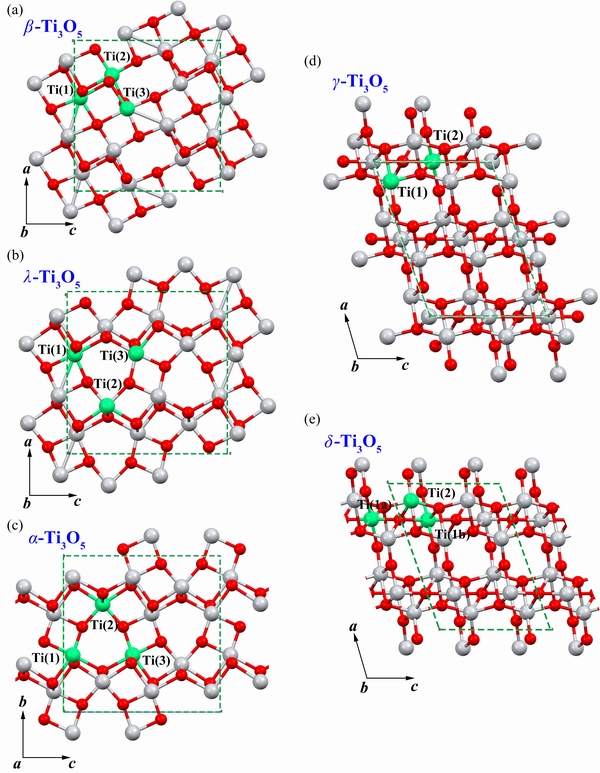
Fig. 4 Schematics of crystal structures of Ti3O5 (β, λ, α, γ and δ)
Table 1 Lattice parameters of different Ti3O5 phases

During the cooling process, the room-temperature stable γ phase transforms into the δ phase at approximately 237 K, which undergoes a Mott–Hubbard metal-insulator phase transition due to the breaking of a one-dimensionally conducting pathway, as shown in Fig. 5 [46]. From the electrical conductivity and optical measurement results, the γ phase has metallic conductor properties, whereas the δ phase exhibits semiconductor properties, as shown in Fig. 5(b). However, different results were obtained in terms of phase-transition temperature (225 K) in the thin film of the γ phase [57]. The most likely reason would be internal stress or strain caused by lattice-constant mismatches and different expansion coefficients between the substrate and product.
4 Applications of trititanium pentoxide (Ti3O5)
4.1 Gas sensor
Ti3O5is considered the most promising gas sensor substitute in high-temperature solid-state gas sensors. ZHENG [58] used α-Ti3O5 in oxygen sensing for the first time. α-Ti3O5 thin films were synthesized by hydrogen reduction TiO2 thin films, which were obtained on Al2O3 substrates usingTi(OC4H9)4 as a precursor. The α-Ti3O5 thin films exhibited an impressive low resistivity-temperature coefficient with better high-temperature stability and reproducibility. However, their oxygen sensitivity needs to be further improved. After that, significant improvements in oxygen sensitivity and response rate were achieved by 5 at.%Ce and 1 at.% W doping, which offer more effective active sites on the surface of materials [26]. Compared with pure α-Ti3O5, W doping reduced the resistivity-temperature coefficient, whereas Ce doping increased the structure stable temperature to 700 °C.ZHANG et al [59] synthesized Ti3O5 sub-micron rods by a sintering method using H3TiO5nano-fibers as a precursor. However, the sensor characteristics are not satisfactory.
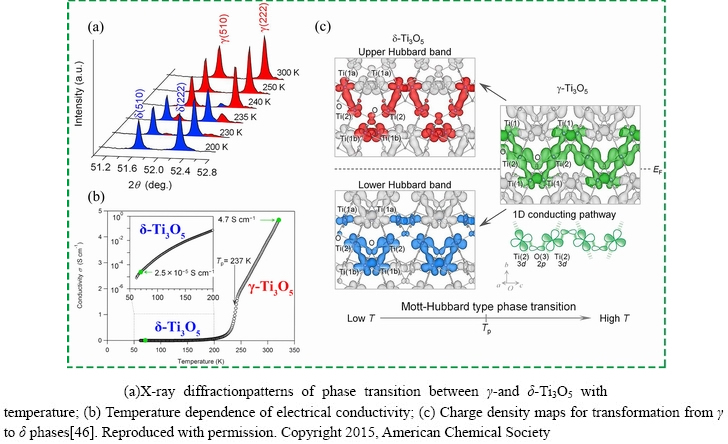
Fig. 5 Phase transition mechanism
LI et al [60] prepared β-Ti3O5 by carbothermal reduction of TiO2, demonstrating certain oxygen sensitivity by experiment and density functional theory (DFT) calculations. However, its performance is not good based on the response and recovery time in 20% O2+80% N2 and 20% H2+80% N2 atmospheres. Therefore, further optimization is required.
4.2 Energy storage material
λ-Ti3O5 is a meta-stable phase and frequently appears at high temperatures.OHKOSHIet al[27,56]found that λ-Ti3O5 could exist at room temperature with nano-scale ((25±15) nm or (21±11) nm) due to thermodynamic local energy minimum. The as-prepared nano-λ-Ti3O5 was a metallic conductor and showed Pauli paramagnet. In addition, they found a reversible phase transition phenomenon between λ and β phases. It can be induced by laser light, which confers enormous potential in optical storage media. Subsequently, TOKORO et al [61] reported other induced reversible phase-transition factors, such as pressure, temperature and current, and specifically, λ-Ti3O5 could transform into β-Ti3O5 under external pressure with heat release. Vice versa, β-Ti3O5 could absorb heat from the external environment, transforming it into λ-Ti3O5 under external stimuli such as heat, current and light. This feature gives Ti3O5 enormous potential in heat storage. The absorbed energy during the phase transition from βto λ was (230±20) kJ/L with an induced temperature, and (240±40) kJ/L energy was released from λto β with an induced pressure.
Because λ-Ti3O5 can only exist at room temperature with nano-scale, WEI et al [62] studied the effect of coating layers on the stability of meta-stable phaseλ-Ti3O5. In their experiment, inorganic layers, such as Al2O3 or SiO2, were coated on the nano-rutile TiO2 particles to prepare λ-Ti3O5. Results indicated that the Al2O3-SiO2 dual-coated TiO2 sample emerged as λ-Ti3O5. However, a similar phenomenon was not found in the uncoated or SiO2-coated TiO2 sample. Therefore, the introduction of Al3+ from the coating layer played an important role in the formation and stabilization of λ-Ti3O5. After that, they proposed a doping strategy to investigate the stabilizing effect of Al3+ on λ-Ti3O5 [63]. From the d-spacing of the (110) plane, the value of the Al3+ doped sample (0.35 nm) was lower than the typical value (0.37 nm), indicating that Al3+ was doped in the substitutional mode. The as-prepared samples could exist at room temperature with a micro-crystal scale (2 μm) under 6.0 at.% Al doping. Furthermore, the effect of Al3+ doping on phase transition was extensively investigated. The results indicated that Al3+ doping not only reduced the transition temperature (β→λ) but also promoted the transition from β to λ. In 2019, WANG et al [64], inspired by the reference to the MgO-TiO2 equilibrium phase diagram, proposed an Mg doping strategy to stabilize λ-Ti3O5 at room temperature. Similar results concerning phase-transition properties were obtained. Note that the stabilization effects of Mg doping were more efficient than those of Al doping in terms of requiring less amount of Al3+. A possible reason was the larger ionic radius and lower valence of Mg2+.
In 2019, OHKOSHIet al [65] prepared a heat storage ceramic and used it to recycle waste heat from automobiles. The grain size of as-prepared λ-Ti3O5 was 10 times larger than that of previous samples of hydrogen reduction, called block-type λ-Ti3O5. From pressure-induced results, the phase transition from λ to β required a relatively low external pressure (50% transforms under 7 MPa). Temperature-induced results showed that the endothermic peak was at approximately 471 K (198 °C) with 237 kJ/L. After that, inspiredby the aforementioned metal doping strategy, NAKAMURA et al [66] calculated the formation energy of λ-Ti3O5 with 54 different doping elements, and the results indicated that only six elements (Sc, Zr, Nb, Hf, Ta and W) promoted the formation of the λ phase with lower formation energy. Subsequently, bulk Ti3O5was prepared using TiO2 as a titanium source and Ti as a reductant with different doping elements by an arc-melting technique, as shown in Fig. 6 [66]. However, only Sc doping formed the λ phase according to the XRD result. Sc doping content materials were synthesized, and the chemical formulas were Sc0.09Ti2.91O5, Sc0.105Ti2.895O5 and Sc0.108Ti2.92O5. Temperature-induced results showed that the corresponding endothermic peaks were at about67, 45 and 38 °C, respectively.Compared with previously reported storage ceramics,the capacity of energy storage (75 kJ/L) has been decreased by more than two-thirds. However, the critical transition temperature from β to λ decreased substantially, thereby providing a wider range of applications, as shown in Fig. 6(d). It shows great potential for use in power plants through the absorption of thermal energy from hot water. However, the relatively high pressure of the transformation from λ to β will be a challenge in practical applications.
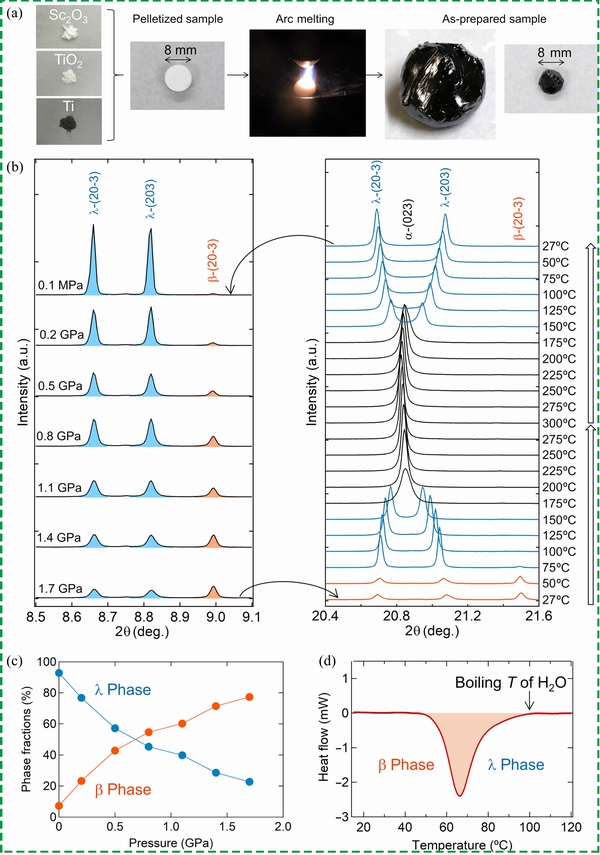
Fig. 6 (a) Schematic of synthetic process of bulk λ-Sc0.09Ti2.91O5; (b) Synchrotron X-ray diffraction patterns of pressure and temperature-induced phase-transition process; (c) Phase fractions at different pressures; (d) Endothermic performance from β to λ[66]. Reproduced with permission. Copyright 2020, Science
SUN et al [67] reported Ti3O5nano-film combined with carbon nano-tubes (CNTs)/Ni obtained bythe PLD method under vacuum and showed improved performance as super-capacitors due to the core–shell nano-structure of Ti3O5@CNTs/Ni, as shown in Fig. 7. In contrast to those regular TiO2-based super-capacitors, Ti3O5@CNTs/Ni showed an excellent specific capacitance of 445.7 F/g at a rate capacity of 1 A/g. The DFT calculation results indicated that the formation of Ti3O5 led to a substantial reduction in the bandgap compared with TiO2.
The above observations strongly confirm that Ti3O5 holds promise for energy storage applications.
4.3 Optical storage media
In 2010, OHKOSHI et al [27] synthesized nano-crystalline λ-Ti3O5 and showedphoto-induced reversible properties between λ and β phases, as shown in Fig. 8. It was the firstly reported that Ti3O5 possessed photo-inducedphase transition at room temperature. λ-Ti3O5 showed light absorption across a broad range of wavelengths, so the reversible phenomenon was observed under different incident nano-second-pulsed laser light (355, 532 and 1064 nm), which conferredTi3O5 enormous potential in optical storage media. In addition, λ-Ti3O5 could also be obtained with continuous-wave laser irradiation from β-Ti3O5 to α-Ti3O5to λ-Ti3O5, as shown in Fig. 8(d). These features make Ti3O5 ideal for use as high-density optical memory devices, and the memory density is several hundred times greater than that of conventional devices.

Fig. 7 (a) Schematic of material synthesis process; (b) Transmission electron microscopy images of core–shell nano-structure[67]. Reproduced with permission. Copyright 2021, Elsevier

Fig. 8 (a) Reversible phase transition between λ and β induced by 532 nm pulsed laser light with different laser-power densities; (b) Variation in amount of λ-Ti3O5; (c) X-ray diffraction patterns of reversible phase transition; (d) Schematic of reversible phase transition induced by pulsed laser or continuous-wave (cw) laser [27]. Reproduced with permission. Copyright 2010, Nature
To intrinsically understand the origin of the photo-induced performance of Ti3O5, LIU et al[68] investigated the differences among the optical properties of materials using the DFT method. Results indicated that λ-Ti3O5 and β-Ti3O5 exhibited high variance across the visible spectrum in terms of absorption and reflectivity properties. The hard X-ray photo-electron spectroscopy results indicated that some satellites can be found in both the O and Ti spectra. These phenomena could arise not because of charge-transfer excitations but valence plasmon excitations [69]. To gain more insight into the mechanism, a time-resolved diffusion reflection technique was used to study the dynamic process of this phase transition. There is a threshold for the photo-induced phase transition from β to λ, and it begins within a few hundred femto-seconds with a non-thermal process [70]. The already formed λ-phase domains increased with a prolonged irradiation time, and a permanent phase transition was achieved when the particles were sufficiently large. Furthermore, the phasetransition time induced by nano-second-pulsed or continuous-wave laser was estimated by a combination of single-shot time-resolved reflectivity measurements and Raman spectroscopy technologies. The reversible phase transition under nano-second pulse occurred at nano-second time scale (λ→β: 900 ns; β→λ: 20 ns); however, the λ to β transition under continuous-wave laser occurred at milli-second scale [71]. The aforementioned studies obtained several important findings, which provide novel insights into the photo-induced reversible properties of λ and β phases. However, all results were observed through indirect methods. After that, TASCA et al[72] directly observed the photo-induced process in structure with the excitation of a pulsed laser using a time-resolved powder XRD technique. A phase transition from β to λ with a time faster than the experimental time resolution (10 μs) occurred, followed by a relaxation time of 20 μs. In 2021, MARIETTE et al[73] performed a more precise and complete characterization of this process using femto-second powder XRD, as shown in Fig. 9. They suggested that the photo-induced phase transition between λ and β phases was initiated by the propagation of elastic deformations rather than the initial nucleation and growth process. The stress caused by structural changes led to a strain wave that traveled at the speed of sound, eventually leading to the photo-induced phase transition.

Fig. 9 (a) Experimental setup of time-resolved powder X-ray diffraction (XRD); (b) Relative intensive change of XRD patterns at different time scales; (c) Rietveld refinement results of XRD patterns for laser off and t=7.5 ps[73]. Reproduced with permission. Copyright 2021, Nature
4.4 Catalyst support
In recent years, the application of titanium sub-oxides, such as Ti4O7, Ti6O11 and Ti8O15, in chemical catalysts has been extensively studied due to their excellent performance in electrical conductivity, corrosion resistance and high-temperature stability [74-79]. Similarly, Ti3O5 also exhibits tremendous potential for use in electrode and catalyst support materials owing to its better electro-chemical activities and stability towards strong acids and bases [80].
Some researchers have discovered the potential application of Ti3O5 when they introduce Ti3O5 as catalyst support for fuel cells for cathodic oxygen reduction [81-84]. ALIPOUR MOGHADAM ESFAHANI et al[85]synthesized Ti3O5-Mo carbon-free support for platinum-based catalyst proton exchange membrane fuel cells (PEMFCs), as shown in Fig. 10. Significant highcatalyst activity of 73 mA/mg (Pt)ata current density of 1.1 mA/cm2and 0.9 V was observed for Mo-doped Ti3O5, followed by that of commercial Pt/C catalyst support [86,87]. The enhanced catalyst activity was due to the combined effect of oxygen vacancies and Ti3+ defects in Ti3O5. Stability and durability were also significantly improved compared to commercial Pt/C from the results of potential cycling and ultraviolet-visible (UV-Vis) measurements of electrolytes. After that, they introduced Mo and Si into Ti3O5 (Ti3O5Mo0.2Si0.4), showing improved performance compared with previous results (1.57 mA/cm2 at 0.9 V) [29,88]. Recently, ALIPOUR MOGHADAM ESFAHANIet al[89] have introduced N-functional groups into Ti3O5-Mo to improve catalyst activity and stability. To ensure catalyst activity, they further investigated the durability of Pt/Ti3O5Mo0.2Si0.4 using multiple accelerated stress tests and found that the support not only stabilizes the catalyst but also ensures the effectiveness of active sites [90].
In addition to its application in PEMFCs, Pt/Ti3O5Mo0.2Si0.4 could be extended to direct methanol fuel cells (DMFCs). Excellent activities were found in methanol oxidation reactions, which were the major reaction in anodes of DMFCs [91]. A significantly higher current density of 58.92 mA/cm2 was observed for Pt/Ti3O5Mo0.2Si0.4, followed by those of Pt/C catalysts. In addition, the activation energy for related reactions considerably decreased, simultaneously showing higher exchangecurrent densities.
SHI et al [92] prepared Ti4O7/λ-Ti3O5 dual-phase nano-fibers using the hydro-thermal reaction method and used them in the oxygen reduction reaction. Ti4O7 and λ-Ti3O5 in this structure exhibit mutual synergies in catalytic activity compared with the single-phase, providing a new idea for developing electrocatalysts. This material showed good performance in terms of methanol tolerance and cyclic stability. However, electrocatalytic performance should be further improved.
4.5 Photocatalysis
Titanium suboxides, such as Ti2O3, Ti3O5, Ti4O7 and Ti8O15, exhibit certain photo-activities due to existence of oxygen vacancies [13,93-96]. STEM et al [97] synthesized micro-scale Ti3O5 on silicon substrates using carbon-doped TiO2 thin films as a precursor, and the schematic illustration of prepared micro-scale meshes is shown in Fig. 11. Under visible light, it showed better absorbance and photo-luminescence emission performance due to defects within Ti3O5 introduced by doped carbon. Using the same method, they also prepared Ti3O5 thin film containing 75 wt% λ-Ti3O5, 25 wt% TiO2(rutile) and trace TiO2-xCx and it is expected to be useful in solar cells and photo-catalysis [98].
QI et al [99] reported Ti3O5 as a catalyst for photo-degradation. Ti3O5nano-rods were obtained by treating Ti5Si3 powders in O2 flow at 800 °Cfor 90 min. Under the UV-Vis condition, the Ti3O5nano-rods showed a good degradation effect towards methylene blue solutions, and the degradation rate could reach up to 80.0% [99].
4.6 Superconductivity
Excellent superconductivity performance was found by YOSHIMATSU et al [100] inγ-Ti3O5 thin films, with the highest super-conducting transition temperature of 7.1 K among simple oxides. These thin films were prepared usingPLD method on differentsubstrates, and their properties were considerably influenced by the atmosphere around the substrates. The superconductivity properties were attributed to bipolaronic superconductivity, which has much to do with oxygen non-stoichiometry and epitaxial stabilization. After that, they investigated in more depth the mechanism of superconductivity performance of γ-Ti3O5 by regulating the structure phase transformation [19]. A super-conducting phase diagram containing TiO, Ti2O3 and γ-Ti3O5was created on the basis of the experimental results to clarify the superconducting state arising from γ-Ti3O5.

Fig. 10 (a) Schematic of material synthesis process; (b) Transmission electron microscopy images of Ti3O5–Mo support[85]. Reproduced with permission. Copyright 2017, Elsevier
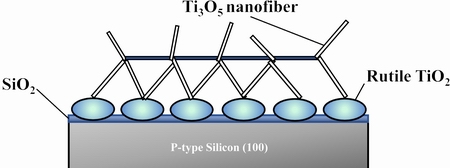
Fig. 11 Schematic of Ti3O5nano-fiber on silicon substrate[97].Reproduced with permission. Copyright 2011, Elsevier
FAN et al [20] grew a series of Ti3O5 thin films on α-Al2O3 substrates using the PLD method by controlling oxygen pressure (from 4×10-4 to 1×10-3Pa). γ-Ti3O5 was prepared when the oxygen concentration reached 1×10-3 Pa. However, no superconductivity phenomenon was found, as shown in Fig. 12[20]. Thus, further experiments need to be conducted to investigate the detailed mechanisms.
4.7 Other application
Excellent microwave absorption performance was observed inλ-Ti3O5and Li-doped λ-Ti3O5 due to the multivalent characteristic of Ti ions [101]. Three Ti ions with different valence states formed different micro-electric fields in the materials, which will improve the microwave absorption performance in a broad frequency range. Thus, the as-prepared λ-Ti3O5 showed a higher efficient absorption bandwidth than most of the other oxide-based microwave absorbing materials. In addition, Li-doped λ-Ti3O5showed the highest EAB/d values (EAB is the effective absorption bandwith; d is the sample thickness) due to the formation of the Li–O micro-electric field.
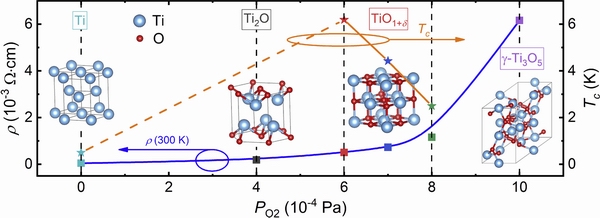
Fig. 12 Super-conducting properties of Ti bulk and trititanium pentoxide (Ti3O5) films at different oxygen pressures. Squares: 300 K; Stars: Tc[20]. Reproduced with permission. Copyright 2019, Elsevier

Fig. 13 (a) Schematic of material synthesis process; (b) Scanning electron microscopy images of hierarchical micro-spheres of γ-Ti3O5[102].Reproduced with permission. Copyright 2019, American Chemical Society
LI et al [102]synthesized γ-Ti3O5 by hydrogen reduction of hierarchical micro-spheres of TiO2, as shown in Fig. 13, and used it as the substrate of surface-enhanced Raman scattering (SERS). The as-prepared substrate exhibited a lower limit of detection (10-10 mol/L) for Rhodamine 6G and excellent stability performance under conditions of high-temperature oxidation and concentrated alkali and acid with a high specific area of 405.8 m2/g. In addition, it still showed good SERS activity after several cycles of use. Compared with TiO2 substrates, γ-Ti3O5 had improved detection sensitivity as high as 10000-fold.
DING et al [103] synthesized Ti3O5/Ti4O7nano-fibers by hydro-thermal method and used them as adsorbents to capture SARS-CoV-2b (severe acute respiratory syndrome coronavirus 2) for further detection or to scavenge it from the environment. These dual-phase adsorbents exhibited a high affinity for proteins or phospholipids. Compared with Ti6O11, they showed improved adsorption and efficient performance with lower virus concentrations after adsorption. This study provided a new way for practical applications of Ti3O5.
5 Summary and perspective
Over a couple of decades, a considerable research effort has gone into the crystal structure, preparation methods, physical and chemical properties and applications of Ti3O5. There has been an exhaustive understanding of different Ti3O5 crystalline forms (α, β, γ, δ and λ) in terms of physical and chemical properties, and the change in physical properties that have resulted from phase transitions between these forms. Such properties confer Ti3O5 potential interest for use in gas sensors, photo-catalysis, catalyst support, superconductivity, etc. Especially, the unique pressure–heat, pressure–light and pressure–current reversible phase transitions between λ- and β-Ti3O5 with different external stimulations have been piquing increasing research interest. These also offer new applications of Ti3O5in the field of energy and data storage. In addition, several new methods have also been proposed for preparing Ti3O5 with high quality and different grain sizes.In practice, different grain sizes have a significant impact on the room temperature stability, phase transition and heat storage performance of λ-Ti3O5. However, further work to understand the phase-transition mechanism between λ and β phases is required. More recently, Ti3O5 has shown amazing application potential in the fields of trace detection, microwave absorption and virus adsorption, which further broadens the application range of Ti3O5. Therefore, Ti3O5 is expected to be a multi-purpose material in the future.
Nowadays, there are sufficient research and understanding of the basic physicochemical properties for different crystal forms of Ti3O5. Thus, the future research focus would be how to develop new applications of Ti3O5in the field of energy utilization and conversion.
Acknowledgments
The authors would like to thank the financial supports from the National Natural Science Foundation of China (Nos. 52004157, U1860203, 52022054, 51974181), the Shanghai Sailing Program, China (No. 21YF1412900), the Shanghai Rising-Star Program, China (No. 19QA1403600), the Shanghai Engineering Research Center of Green Remanufacture of Metal Parts, China (No. 19DZ2252900), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, China (No.TP2019041), the “Shuguang Program” supported by the Shanghai Education Development Foundation and the Shanghai Municipal Education Commission, China (No. 21SG42), the Independent Research and Development Project of State Key Laboratory of Advanced Special Steel, Shanghai Key Laboratory of Advanced Ferrometallurgy, Shanghai University, China (No. SKLASS 2020-Z10), and the Science and Technology Commission of Shanghai Municipality, China (No. 19DZ2270200).
References
[1] SHAYAN M, EGHBALI B, NIROUMAND B. Fabrication of AA2024-TiO2 nanocomposites through stir casting process [J]. Transactions of Nonferrous Metals Society of China, 2020, 30: 2891-2903.
[2] YUMAK N, ASLANTA? K. A review on heat treatment efficiency in metastable β titanium alloys: The role of treatment process and parameters [J]. Journal of Materials Research and Technology, 2020, 9: 15360-15380.
[3] ZHENG Li, SHI Qian, LIU Xuan-yong. Induced antibacterial capability of TiO2 coatings in visible light via nitrogen ion implantation [J]. Transactions of Nonferrous Metals Society of China, 2020, 30: 171-180.
[4] DU Yu-xuan, YANG Xin-liang, LI Zu-shu, HAO Fang, MAO You-chuan, LI Shao-qiang, LIU Xiang-hong, FENG Yong, YAN Zhi-ming. Shear localization behavior in hat-shaped specimen of near-α Ti-6Al-2Zr-1Mo-1V titanium alloy loaded at high strain rate [J]. Transactions of Nonferrous Metals Society of China, 2021, 31: 1641-1655.
[5] SHI Xiao-hui, CAO Zu-han, FAN Zhi-yuan, ECKERT J, QIAO Jun-wei. Static coarsening behavior of equiaxedα phase in Ti-8Al-1Mo-1V alloy [J]. Transactions of Nonferrous Metals Society of China, 2021, 31: 1628-1640.
[6] ZOU Cheng-xiong, LI Jin-shan, WANG William-yi, ZHANG Ying, LIN De-ye, YUAN Rui-hao, WANG Xiao-dan, TANG Bin, WANG Jun, GAO Xing-yu. Integrating data mining and machine learning to discover high-strength ductile titanium alloys [J]. ActaMaterialia, 2021, 202: 211-221.
[7] GAO Yang, YIN Zhi-bin, JI Qian, JIANG Jia-bing, TAO Zheng-zheng, ZHAO Xiao-long, SUN Si-jia, WU Ai-guo, ZENG Le-yong. Black titanium dioxide@ manganese dioxide for glutathione-responsive MR imaging and enhanced photothermal therapy [J]. Journal of Materials Chemistry B, 2021, 9: 314-321.
[8] SU Yue, ZHANG Wei, CHEN Shan-ming, YAO Dan-wen, XU Ji-lian, CHEN Xiao-bo, LIU Lei, XU Huai-liang. Engineering black titanium dioxide by femtosecond laser filament [J]. Applied Surface Science, 2020, 520: 146298.
[9] LIU Xing-xin, YU Xiao-yan, SHA Li, WANG Yu-qian, ZHOU Zhuo, ZHANG Shu-ting. The preparation of black titanium oxide nanoarray via coking fluorinated wastewater and application on coking wastewater treatment [J]. Chemosphere, 2021, 270: 128609.
[10] YU Min, SAUNDERS T, GRASSO S, MAHAJAN A, ZHANG Hang-feng, REECE M J. MagnEli phase titanium suboxides by flash spark plasma sintering [J]. ScriptaMaterialia, 2018, 146: 241-245.
[11] CANCAREVIC M, ZINKEVICH M, ALDINGER F. Thermodynamic description of the Ti–O system using the associate model for the liquid phase [J]. Calphad, 2007, 31: 330-342.
[12] OKAMOTO H. O-Ti (oxygen-titanium) [J]. Journal of Phase Equilibria and Diffusion, 2011, 32: 473-474.
[13] DOMASCHKE M, ZHOU Xue-mei, WERGEN L, ROMEIS S, MIEHLICH ME, MEYER K, PEUKERT W, SCHMUKI P. MagnEli-phases in anatase strongly promote cocatalyst-free photocatalytic hydrogen evolution [J]. ACS Catalysis, 2019, 9: 3627-3632.
[14] MALIK H, SARKAR S, MOHANTY S, CARLSON K. Modelling and synthesis of MagnEli phases in ordered titanium oxide nanotubes with preserved morphology [J]. Scientific Reports, 2020, 10: 8050.
[15] van LANDUYT J, AMELINCKX S. On the generation mechanism for shear planes in shear structures [J]. Journal of Solid State Chemistry, 1973, 6: 222-229.
[16] ANDERSSON S, COLLEN B, KUYLENSTIERNA U, MAGNELI A. Phase analysis studies on the titanium-oxygen system [J]. ActaChemicaScandinavica, 1957, 11: 1641-1652.
[17] SHI Qi-wu, CHAI Guo-qing, HUANG Wan-xia, SHI Yan-li, HUANG Bo, WEI Dan, QI Jian-qi, SU Fu-hai, XU Wen, LU Tie-cheng. Fabrication of nanocrystallineλ-Ti3O5 with tunable terahertz wave transmission properties across a temperature induced phase transition [J]. Journal of Materials Chemistry C, 2016, 4: 10279-10285.
[18]  SBRINK S, MAGNELI A. Note on the crystal structure of trititanium pentoxide [J]. ActaChemicaScandinavica, 1957, 11: 1606-1607.
SBRINK S, MAGNELI A. Note on the crystal structure of trititanium pentoxide [J]. ActaChemicaScandinavica, 1957, 11: 1606-1607.
[19] KUROKAWA H, YOSHIMATSU K, SAKATA O, OHTOMO A. Effects of phase fraction on superconductivity of low-valence eutectic titanate films [J]. Journal of Applied Physics, 2017, 122: 055302.
[20] FAN Yun-jie, ZHANG Chao, LIU Xiang, LIN Yue, GAO Guan-yin, MA Chao, YIN Yue-wei, LI Xiao-guang. Structure and transport properties of titanium oxide (Ti2O, TiO1+δ, and Ti3O5) thin films [J]. Journal of Alloys and Compounds, 2019, 786: 607-613.
[21] KITADA A, HASEGAWA G, KOBAYASHI Y, KANAMORI K, NAKANISHI K, KAGEYAMA H. Selective preparation of macroporous monoliths of conductive titanium oxides TinO2n-1 (n=2, 3, 4, 6) [J]. Journal of the American Chemical Society, 2012, 134: 10894-10898.
[22] CHAI Guo-qing, HUANG Wan-xia, SHI Qi-wu, ZHENG Shu-ping, WEI Dan. Preparation and characterization of λ-Ti3O5 by carbothermal reduction of TiO2 [J]. Journal of Alloys and Compounds, 2015, 621: 404-410.
[23] CAI Yu, SHI Qi-wu, WANG Ming-zhe, LV Xiang, CHENG Ye, HUANG Wan-xia. Synthesis of nanoscale lambda-Ti3O5 via a PEG assisted sol-gel method [J]. Journal of Alloys and Compounds, 2020, 848: 156585.
[24] YANG Shun-shun, ZHANG Le, MA Yue-long, SUN Bing-heng, SHAN Yin-shuang, SHI Ze-di, ZHOU Tian-yuan, WANG Yun, SELIM FA, LI Yan-bin. A novel carbon thermal reduction approach to prepare recorded purity β-Ti3O5 compacts from titanium dioxide and phenolic resin [J]. Journal of Alloys and Compounds, 2021, 853: 157360.
[25] IWASAKI H, BRIGHT NFH, ROWLAND JF. The polymorphism of the oxide Ti3O5 [J]. Journal of the Less Common Metals, 1969, 17: 99-110.
[26] ZHENG Liao-ying. The oxygen sensing properties and mechanisms of M-doped α-Ti3O5 thin films (M= Ce, W ions) [J]. Sensors and Actuators B: Chemical, 2003, 94: 294-297.
[27] OHKOSHI S I, TSUNOBUCHI Y, MATSUDA T, HASHIMOTO K, NAMAI A, HAKOE F, TOKORO H. Synthesis of a metal oxide with a room-temperature photoreversible phase transition [J]. Nature chemistry, 2010, 2: 539-545.
[28] NASU T, TOKORO H, TANAKA K, HAKOE F, NAMAI A, OHKOSHI S. Sol-gel synthesis of nanosizedλ-Ti3O5 crystals [J]. IOP Conference Series: Materials Science and Engineering, 2014, 54: 012008.
[29] ALIPOUR MOGHADAMESFAHANI R, RIVERA GAVIDIA L M, GARC?A G, PASTOR E, SPECCHIA S. Highly active platinum supported on Mo-doped titanium nanotubes suboxide (Pt/TNTS-Mo) electrocatalyst for oxygen reduction reaction in PEMFC [J]. Renewable Energy, 2018, 120: 209-219.
[30] BARTHOLOMEW RF, WHITE WB. Growth of the intermediate oxides of titanium from borate fluxes under controlled oxygen fugacities [J]. Journal of Crystal Growth, 1970, 6: 249-252.
[31] CHEN G Z, FRAY D J, FARTHING T W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride [J]. Nature, 2000, 407: 361-364.
[32] WANG Bin, LIU Kui-ren, CHEN Jian-she. Reaction mechanism of preparation of titanium by electro-deoxidation in molten salt [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 2327-2331.
[33] DRING K, DASHWOOD R, INMAN D. Voltammetry of titanium dioxide in molten calcium chloride at 900 °C [J]. Journal of the Electrochemical Society, 2005, 152: E104.
[34] DRING K, DASHWOOD R, INMAN D. Predominance diagrams for electrochemical reduction of titanium oxides in molten CaCl2 [J]. Journal of the Electrochemical Society, 2005, 152: D184.
[35] KAR P, EVANS J W. Determination of kinetic parameters by modeling of voltammograms for electrochemical reduction of titanium dioxide [J]. Electrochemistry communications, 2006, 8: 1397-1403.
[36] ERTEKIN Z, TAMER U, PEKMEZ K. Cathodic electrochemical deposition of MagnEli phases TinO2n-1 thin films at different temperatures in acetonitrile solution [J]. ElectrochimicaActa, 2015, 163: 77-81.
[37] ERTEKIN Z, PEKMEZ N ?, PEKMEZ K. One-step electrochemical deposition of thin film titanium suboxide in basic titanyl sulfate solution at room temperature [J]. Journal of Solid State Electrochemistry, 2020, 24: 975-986.
[38] PESHEV P, IVANOVA M. Growth of Ti2O3 single crystals by a chemical transport reaction [J]. Physica Status Solidi (a), 1975, 28: K1-K4.
[39] MERCIER J, SINCE JJ, FOURCAUDOT G, DUMAS J, DEVENYI J. Growth and characterization of titanium suboxide crystals [J]. Journal of Crystal Growth, 1977, 42: 583-587.
[40] STROBEL P, PAGE Y. Crystal growth of TinO2n-1 oxides (n=2 to 9) [J]. Journal of Materials Science, 1982, 17: 2424-2430.
[41] HONG SH. Crystal growth of some intermediate titanium oxide phasesγ-Ti3O5, β-Ti3O5, Ti4O7, and Ti2O3 by chemical transport reactions [J]. ActaChemScand, 1982, 207-217.
[42] HONG SH,  SBRINK S. The structure of γ-Ti3O5 at 297K [J]. ActaCrystallographica(Section B): Structural Crystallography and Crystal Chemistry, 1982, 38: 2570-2576.
SBRINK S. The structure of γ-Ti3O5 at 297K [J]. ActaCrystallographica(Section B): Structural Crystallography and Crystal Chemistry, 1982, 38: 2570-2576.
[43]  SBRINK S, MAGNELI A. Crystal structure studies on trititanium pentoxide, Ti3O5 [J]. ActaCrystallographica, 1959, 12: 575-581.
SBRINK S, MAGNELI A. Crystal structure studies on trititanium pentoxide, Ti3O5 [J]. ActaCrystallographica, 1959, 12: 575-581.
[44] ONODA M, OGAWA Y, TAKI K. Phase transitions and the doping effect in Ti3O5 [J]. Journal of Physics: Condensed Matter, 1998, 10: 7003.
[45]  SBRINK G,
SBRINK G,  SBRINK S, MAGNELI A, OKINAKA H, KOSUGE K, KACHI S. Ti3O5 modification of V3O5-type structure [J]. ActaChemicaScandinavica, 1971, 25: 3889-3890.
SBRINK S, MAGNELI A, OKINAKA H, KOSUGE K, KACHI S. Ti3O5 modification of V3O5-type structure [J]. ActaChemicaScandinavica, 1971, 25: 3889-3890.
[46] TANAKA K, NASU T, MIYAMOTO Y, OZAKI N, TANAKA S, NAGATA T, HAKOE F, YOSHIKIYO M, NAKAGAWA K, UMETA Y, IMOTO K, TOKORO H, NAMAI A, OHKOSHI S I. Structural phase transition between γ-Ti3O5 and δ-Ti3O5 by breaking of a one-dimensionally conducting pathway [J]. Crystal Growth & Design, 2015, 15: 653-657.
[47] RAO CNR, RAMDAS S, LOEHMAN RE, HONIG JM. Semiconductor-metal transition in Ti3O5 [J]. Journal of Solid State Chemistry, 1971, 3: 83-88.
[48] KEYS LK. Magnetic studies of Ti3O5 [J]. Physics Letters A, 1967, 24: 628-630.
[49] ONODA M. Phase transitions of Ti3O5 [J]. Journal of Solid State Chemistry, 1998, 136: 67-73.
[50] GREY IE, WARD J. An X-ray and Mossbauer study of the FeTi2O5-Ti3O5 system [J]. Journal of Solid State Chemistry, 1973, 7: 300-307.
[51] KELLERMAN DG. Effect of doping on the phase transition in Ti3O5 [J]. Journal of Inorganic Materials, 1983, 19: 221.
[52] WECHSLER B A, NAVROTSKY A. Thermodynamics and structural chemistry of compounds in the system MgO-TiO2 [J]. Journal of Solid State Chemistry, 1984, 55: 165-180.
[53] STEINER HJ, TURRILLAS X, STEELE B CH. Phase relationships and electrical properties of Ti3O5, CrTi2O5 and the pseudobrookite-type systems MgxTi3-xO5 and LixTi3-xO5 [J]. Journal of Materials Chemistry, 1992, 2: 1249-1256.
[54] GREY IE, LI C, MADSEN IC. Phase equilibria and structural studies on the solid solution MgTi2O5-Ti3O5 [J]. Journal of Solid State Chemistry, 1994, 113: 62-73.
[55] TAKAHAMA R, ISHII T, INDO D, ARIZONO M, TERAKURA C, TOKURA Y, TAKESHITA N, NODA M, KUWAHARA H, SAIKI T, KATSUFUJI T, KAJIMOTO R, OKUDA T. Structural, magnetic, transport, and thermoelectric properties of the pseudobrookite AlTi2O5-Ti3O5 system [J]. Physical Review Materials, 2020, 4: 074401.
[56] MAKIURA R, TAKABAYASHI Y, FITCH A N, TOKORO H, OHKOSHI S I, PRASSIDES K. Nanoscale effects on the stability of the λ-Ti3O5polymorph [J]. Chemistry—An Asian Journal, 2011, 6: 1886-1890.
[57] HUANG Bo, HUANG Wan-xia, SHI Qi-wu, ZHENG Shu-ping, SHEN Zu-jia. The preparation and phase transformation characteristics of γ-Ti3O5 thin film [J]. Journal of Materials Science: Materials in Electronics, 2017, 28: 7868-7873.
[58] ZHENG Liao-ying. The preparation and oxygen-sensing properties of α-Ti3O5 thin film [J]. Sensors and Actuators B: Chemical, 2003, 88: 115-119.
[59] ZHANG Xiao-yan, LIU Wan-ying, YU Hua, ZHONG Xiao-xi, WANG Li-jun, SINGH A, LIN Yuan-hua. Preparation and oxygen sensing properties of Ti3O5 submicron rods [J]. Micro & Nano Letters, 2016, 11: 811-813.
[60] LI Xiao-lei, LIU Ying, MA Shi-qing, YE Jin-wen, ZHANG Xiao-yan, WANG Guang-rui, QIU Yu-chong. The synthesis and gas sensitivity of the β-Ti3O5 powder: Experimental and DFT study [J]. Journal of Alloys and Compounds, 2015, 649: 939-948.
[61] TOKORO H, YOSHIKIYO M, IMOTO K, NAMAI A, NASU T, NAKAGAWA K, OZAKI N, HAKOE F, TANAKA K, CHIBA K J, MAKIURA R, PRASSIDES K, OHKOSHI S I. External stimulation-controllable heat-storage ceramics [J]. Nature Communications, 2015, 6: 7037.
[62] WEI Dan, HUANG Wan-xia, SHI Qi-wu, LU Tie-cheng, HUANG Bo. Effect of coating layers on nano-TiO2 particles on the preparation of nanocrystallineλ-Ti3O5 by carbonthermal reduction [J]. Journal of Materials Science: Materials in Electronics, 2016, 27: 4216-4222.
[63] SHEN Zu-jia, SHI Qi-wu, HUANG Wan-xia, HUANG Bo, WANG Ming-zhe, GAO Jun-zheng, SHI Yan-li, LU Tie-cheng. Stabilization of microcrystal λ-Ti3O5 at room temperature by aluminum-ion doping [J]. Applied Physics Letters, 2017, 111: 191902.
[64] WANG Ming-zhe, HUANG Wan-xia, SHEN Zu-jia, GAO Jun-zheng, SHI Yan-li, LU Tie-cheng, SHI Qi-wu. Phase evolution and formation of λ phase in Ti3O5 induced by magnesium doping [J]. Journal of Alloys and Compounds, 2019, 774: 1189-1194.
[65] OHKOSHI S I, TOKORO H, NAKAGAWA K, YOSHIKIYO M, JIA F, NAMAI A. Low-pressure-responsive heat-storage ceramics for automobiles [J]. Scientific Reports, 2019, 9: 13203.
[66] NAKAMURA Y, SAKAI Y, AZUMA M, OHKOSHI S I. Long-term heat-storage ceramics absorbing thermal energy from hot water [J]. Science Advances, 2020, 6: eaaz5264.
[67] SUN Peng, HU Xue-yan, WEI Guang-feng, WANG Rui-jing, WANG Qiang, WANG Huan-wen, WANG Xue-feng. Ti3O5nanofilm on carbon nanotubes by pulse laser deposition: Enhanced electrochemical performance [J]. Applied Surface Science, 2021, 548: 149269.
[68] LIU Rui, SHANG Jia-xiang, WANG Fu-he. Electronic, magnetic and optical properties of β-Ti3O5 and λ-Ti3O5: A density functional study [J]. Computational Materials Science, 2014, 81: 158-162.
[69] KOBAYASHI K, TAGUCHI M, KOBATA M, TANAKA K, TOKORO H, DAIMON H, OKANE T, YAMAGAMI H, IKENAGA E, OHKOSHI S I. Electronic structure and correlation in β-Ti3O5 and λ-Ti3O5 studied by hard X-ray photoelectron spectroscopy [J]. Physical Review B, 2017, 95: 085133.
[70] ASAHARA A, WATANABE H, TOKORO H, OHKOSHI S I, SUEMOTO T. Ultrafast dynamics of photoinduced semiconductor-to-metal transition in the optical switching nano-oxide Ti3O5 [J]. Physical Review B, 2014, 90: 014303.
[71] OULD-HAMOUDA A, TOKORO H, OHKOSHI SI, FREYSZ E. Single-shot time resolved study of the photo-reversible phase transition induced in flakes of Ti3O5 nanoparticles at room temperature [J]. Chemical Physics Letters, 2014, 608: 106-112.
[72] TASCA K R, ESPOSITO V, LANTZ G, BEAUD P, KUBLI M, SAVOINI M, GILES C, JOHNSON SL. Time-resolved X-ray powder diffraction study of photoinduced phase transitions in Ti3O5nanoparticles [J]. ChemPhysChem, 2017, 18: 1385-1392.
[73] MARIETTE C, LORENC M, CAILLEAU H, et al. Strain wave pathway to semiconductor-to-metal transition revealed by time-resolved X-ray powder diffraction [J]. Nature Communications, 2021, 12: 1239.
[74] WALSH FC, WILLS RGA. The continuing development ofMagnEli phase titanium sub-oxides and Ebonex? electrodes [J]. ElectrochimicaActa, 2010, 55: 6342-6351.
[75] LIN Hui, XIAO Run-lin, XIE Ru-zhen, YANG Li-hui, TANG Cai-ming, WANG Rong-rong, CHEN Jie, LV Si-hao, HUANG Qing-guo. Defect engineering on a Ti4O7electrode by Ce3+doping for the efficient electrooxidation of perfluorooctanesulfonate [J]. Environmental Science & Technology, 2021, 55: 2597-2607.
[76] LIU Meng-ting, JHULKI S, SUN Zi-fei, MAGASINSKI A, HENDRIX C, YUSHIN G. Atom-economic synthesis of MagnEli phase Ti4O7 microspheres for improved sulfur cathodes for Li-S batteries [J]. Nano Energy, 2021, 79: 105428.
[77] HE Chun-yong, CHANG Shi-yong, HUANG Xiang-dong, WANG Qing-quan, MEI Ao, SHEN Pei-kang. Direct synthesis of pure single-crystalline MagnEli phase Ti8O15 nanowires as conductive carbon-free materials for electrocatalysis [J]. Nanoscale, 2015, 7: 2856-2861.
[78] SHEN Pei-kang, HE Chun-yong, CHANG Shi-yong, HUANG Xiang-dong, TIAN Zhi-qun. MagnEli phase Ti8O15 nanowires as conductive carbon-free energy materials to enhance the electrochemical activity of palladium nanoparticles for direct ethanol oxidation [J]. Journal of Materials Chemistry A, 2015, 3: 14416-14423.
[79] LEE GW, PARK BH, NAZARIAN-SAMANI M, KIM YH, ROH KC, KIM KB. MagnEli phase titanium oxide as a novel anode material for potassium-ion batteries [J]. ACS Omega, 2019, 4: 5304-5309.
[80] WANG Li-jun, ZHANG Xiao-yan, LIU Wan-ying, XU Wen, SINGH A, LIN Yuan-hua. Electrochemical properties of Ti3O5 powders prepared by carbothermal reduction [J]. Journal of Materials Science: Materials in Electronics, 2017, 28: 6421-6425.
[81] IGNASZAK A, SONG Chao-jie, ZHU Wei-min, ZHANG Jiu-jun, BAUER A, BAKER R, NEBURCHILOV V, YE Si-yu, CAMPBELL S. Titanium carbide and its core-shelled derivative TiC@TiO2 as catalyst supports for proton exchange membrane fuel cells [J]. ElectrochimicaActa, 2012, 69: 397-405.
[82] NGUYEN TT, HO V T T, PAN CJ, LIU JY, CHOU HL, RICK J, SU WN, HWANG BJ. Synthesis of Ti0.7Mo0.3O2 supported-Pt nanodendrites and their catalytic activity and stability for oxygen reduction reaction [J]. Applied Catalysis B: Environmental, 2014, 154/155: 183-189.
[83] LORI O, ELBAZ L. Advances in ceramic supports for polymer electrolyte fuel cells [J]. Catalysts, 2015, 5: 1445-1464.
[84] ALIPOUR MOGHADAMESFAHANI R, MONTEVERDE VIDELA A H A, VANKOVA S, SPECCHIA S. Stable and methanol tolerant Pt/TiOx-C electrocatalysts for the oxygen reduction reaction [J]. International Journal of Hydrogen Energy, 2015, 40: 14529-14539.
[85] ALIPOUR MOGHADAM ESFAHANI R, VANKOVA SK, MONTEVERDE VIDELA AH A, SPECCHIA S. Innovative carbon-free low content Pt catalyst supported on Mo-doped titanium suboxide (Ti3O5-Mo) for stable and durable oxygen reduction reaction [J]. Applied Catalysis B: Environmental, 2017, 201: 419-429.
[86] PARRONDO J, HAN T, NIANGAR E, WANG C, DALE N, ADJEMIAN K, RAMANI V. Platinum supported on titanium–ruthenium oxide is a remarkably stable electrocatayst for hydrogen fuel cell vehicles [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111: 45-50.
[87] WANG Gong-wei, HUANG Bing, XIAO Li, REN Zhan-dong, CHEN Hao, WANG De-li, ABRU?A H D, LU Jun-tao, ZHUANG Lin. Pt Skin on AuCu intermetallic substrate: A strategy to maximize Pt utilization for fuel cells [J]. Journal of the American Chemical Society, 2014, 136: 9643-9649.
[88] ALIPOUR MOGHADAM ESFAHANI R, EBRALIDZE II, SPECCHIA S, EASTON E B. A fuel cell catalyst support based on doped titanium suboxides with enhanced conductivity, durability and fuel cell performance [J]. Journal of Materials Chemistry A, 2018, 6: 14805-14815.
[89] ALIPOUR MOGHADAM ESFAHANI R, FRUEHWALD HM, LASCHUK N O, SULLIVAN MT, EGAN JG, EBRALIDZE II, ZENKINA OV, EASTON E B. A highly durable N-enriched titanium nanotube suboxide fuel cell catalyst support [J]. Applied Catalysis B: Environmental, 2020, 263: 118272.
[90] ALIPOUR MOGHADAM ESFAHANI R, EASTON E B. Exceptionally durable Pt/TOMS catalysts for fuel cells [J]. Applied Catalysis B: Environmental, 2020, 268: 118743.
[91] ALIPOUR MOGHADAM ESFAHANI R, MOGHADDAM R B, EASTON E B. High performance Pt/Ti3O5Mo0.2Si0.4electrocatalyst with outstanding methanol oxidation activity [J]. Catalysis Science & Technology, 2019, 9: 4118-4124.
[92] SHI Ru-yue, HUANG Ying, LI Miao-ran, ZHU Ying, HE Xue-xia, JIANG Rui-bin, LEI Zhi-bin, LIU Zong-huai, SUN Jie. Synthesis of Ti4O7/Ti3O5dual-phase nanofibers with coherent interface for oxygen reduction reaction electrocatalysts [J]. Materials, 2020, 13: 3142.
[93] SIREESHA P, SASIKUMAR R, CHEN S M, SU CC, RANGANATHAN P, RWEI SP. Carboxylic acid-functionalized multi-walled carbon nanotubes-polyindole/Ti2O3: A novel hybrid nanocomposite as highly efficient photo-anode for dye-sensitized solar cells (DSSCs) [J]. Applied Surface Science, 2017, 423: 147-153.
[94] TOYODA M, YANO T, TRYBA B, MOZIA S, TSUMURA T, INAGAKI M. Preparation of carbon-coated MagnEli phases TinO2n-1 and their photocatalytic activity under visible light [J]. Applied Catalysis B: Environmental, 2009, 88: 160-164.
[95] ZHAO Xin, ZHANG Xiao-jing, ZHAO Bo-lin, JIA Fei, HAN Dong-xue, FAN Ying-ying, NIU Li, IVASKA A. A direct oxygen vacancy essential Z-scheme C@Ti4O7/g-C3N4 heterojunctions for visible-light degradation towards environmental dye pollutants [J]. Applied Surface Science, 2020, 525: 146486.
[96] OU Gang, LI Zhi-wei, LI Dong-ke, CHENG Liang, LIU Zhuang, WU Hui. Photothermal therapy by using titanium oxide nanoparticles [J]. Nano Research, 2016, 9: 1236-1243.
[97] STEM N, CHINAGLIA EF, DOS SANTOS FILHO S. G. Microscale meshes of Ti3O5nano-and microfibers prepared via annealing of C-doped TiO2 thin films [J]. Materials Science and Engineering: B, 2011, 176: 1190-1196.
[98] STEM N, CHINAGLIA ED, DOS SANTOS FILHO S G. Ti3O5nano-and microfibers prepared via annealing of C-doped TiO2 thin films aiming at solar cell and photocatalysis applications [J]. ECS Transactions, 2011, 39: 347.
[99] QI Wen-qian, DU Jun, PENG Yi-chao, WANG Ya-lin, XU Yong-qiang, LI Xiu-yun, ZHANG Kai, GONG Cheng, LUO Mei, PENG Hai-long. Self-induced preparation of Ti3O5 nanorods by chemical vapor deposition [J]. Vacuum, 2017, 143: 380-385.
[100] YOSHIMATSU K, SAKATA O, OHTOMO A. Super- conductivity in Ti4O7 and γ-Ti3O5 films [J]. Scientific Reports, 2017, 7: 12544.
[101] FU Xian-kai, CHEN Wan-qi, HAO Xiao-wen, ZHANG Zhi-min, TANG Ruo-lan, YANG Bo, ZHAO Xiang, ZUO Liang. Preparing high purity λ-Ti3O5 and Li/λ-Ti3O5 as high-performance electromagnetic wave absorbers [J]. Journal of Materials Chemistry C, 2021, 9: 7976-7981.
[102] LI Ya-hui, BAI Hua, ZHAI Jun-feng, YI Wen-cai, LI Jun-fang, YANG Hai-feng, XI Guang-cheng. Alternative to noble metal substrates: Metallic and plasmonic Ti3O5 hierarchical microspheres for surface enhanced Raman spectroscopy [J]. Analytical Chemistry, 2019, 91: 4496-4503.
[103] DING Zhan-lin, WANG Hong, FENG Zhe, SUN Mei-qing. Synthesis of dual-phase Ti3O5/Ti4O7 nanofibers for efficient adsorption of SARS-CoV-2 [J]. Materials Letters, 2021, 300: 130167.
Ti3O5的合成、性能及应用研究进展
赵鹏飞1,2,李光石1,2,李文莉1,2,程鹏1,2,庞忠亚1, 2,熊晓璐1, 2,邹星礼1, 2,许茜1, 2,鲁雄刚1, 2, 3
1.上海大学 材料科学与工程学院,上海 200444;
2. 上海大学 省部共建高品质特殊钢冶金与制备国家重点实验室&上海市钢铁冶金新技术开发应用重点实验室,上海 200444;
3. 上海电机学院 材料学院,上海 201306
摘要:自20世纪50年代以来,人们对Ti3O5的晶体结构、物理、化学和相变性质进行了大量研究。不同晶体结构Ti3O5(α、β、γ、δ和λ)的性能各异,特别是λ与β相之间独特的可逆相变现象吸引了越来越多的研究兴趣,这也为Ti3O5在能源和数据存储领域开辟了新的应用。近年来,Ti3O5材料在痕量检测、微波吸收和病毒吸附等方面的优异表现,进一步拓宽了其应用领域。本文详细介绍不同晶体结构Ti3O5的基本性质,并对其制备方法和应用领域的研究进展进行了系统的综述。
关键词:Ti3O5;相变;压力诱导;数据存储;催化剂载体
(Edited by Wei-ping CHEN)
Corresponding author:Guang-shi LI, Tel: +86-21-66136518, E-mail: lgs@shu.edu.cn;
Xiao-lu XIONG, Tel: +86-21-66136518, E-mail: xlxiong@t.shu.edu.cn;
Xing-li ZOU, Tel: +86-21-66136518, E-mail: xlzou@shu.edu.cn
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
Abstract:The crystal structure, physical, chemical and phase transition properties of trititanium pentoxide (Ti3O5) have aroused a broad range of research effort since the 1950s. Different crystalline forms (α, β, γ, δ and λ) of Ti3O5 exhibit various properties. Particularly, reversible phase transitions between λ- and β-Ti3O5 have been attracting increasing research interest, which brings new potential applications of Ti3O5 materials in the field of energy and data storage.More recently, Ti3O5 materials have shown excellent performance in trace detection, microwave absorption and virus adsorption, which has expanded its application fields. Here, the essential properties of different crystal forms of Ti3O5 are described in detail. An intensive overview of Ti3O5 preparation methods and applications is comprehensively summarized.


 SBRINK and MAGNELI[18], TiO2 and Ti were used as raw materials and mixed with a stoichiometric ratio to prepare Ti3O5 using an electric arc furnace under an argon atmosphere. After two-week annealing at 1150 °C, high-quality β-Ti3O5 was obtained. To prepareγ-Ti3O5 films, KUROKAWA et al [19] firstly synthesized Ti2O3by sintering mixed Ti and TiO2 pellets at 1000 °C for 12 h. Secondly, γ-Ti3O5 films grew on α-Al2O3 (0001) substrates using Ti2O3 as a target by the PLD method. Using the same method, FAN et al [20] used titanium metal as a target to directly grow γ-Ti3O5 on α-Al2O3 (0001) substrates by tuning the oxygen pressure. In contrast to other reported targets, titanium has the advantage of easily preparingTi3O5 films because this is an exothermic process and the oxygen content is easy to control. Titanium is an ideal reductant because noimpurities are introduced in this process. However, this method is limited by high costs and prolonged treatment time.
SBRINK and MAGNELI[18], TiO2 and Ti were used as raw materials and mixed with a stoichiometric ratio to prepare Ti3O5 using an electric arc furnace under an argon atmosphere. After two-week annealing at 1150 °C, high-quality β-Ti3O5 was obtained. To prepareγ-Ti3O5 films, KUROKAWA et al [19] firstly synthesized Ti2O3by sintering mixed Ti and TiO2 pellets at 1000 °C for 12 h. Secondly, γ-Ti3O5 films grew on α-Al2O3 (0001) substrates using Ti2O3 as a target by the PLD method. Using the same method, FAN et al [20] used titanium metal as a target to directly grow γ-Ti3O5 on α-Al2O3 (0001) substrates by tuning the oxygen pressure. In contrast to other reported targets, titanium has the advantage of easily preparingTi3O5 films because this is an exothermic process and the oxygen content is easy to control. Titanium is an ideal reductant because noimpurities are introduced in this process. However, this method is limited by high costs and prolonged treatment time.












 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press