

崔蓝月1,薛 奎1,李硕琦1,邹玉红2,张 芬1,刘成宝1,曾荣昌1
(1. 山东科技大学 材料科学与工程学院,青岛 266590
2. 山东科技大学 化学与生物工程学院,青岛 266590)
近年来,生物可降解植入材料的应用发展迅速,其中镁及合金因其具有良好的生物相容性与生物可降解性,成为新一代具有广阔发展前景的生物医用可降解金属材料,但存在镁合金本身由于快速腐蚀释放镁离子使周围环境碱化而具有的抗菌性和骨植入物需要长期保持力学性能之间的矛盾。本文总结了多种类型的金属及其氧化物、生物活性物质、天然抗菌物质、光热、光动力疗法在抗菌镁合金及表面抗菌涂层领域的研究进展,研究多种镁合金表面耐蚀抗菌涂层的制备方法,重点讨论了不同抗菌载体对镁合金耐蚀抗菌性的影响。结果表明:现阶段抗菌镁合金设计、镁合金抗菌耐蚀涂层制备的研究逐渐成熟,但也存在一些亟需解决的问题。同时,综合阐述了生物医用可降解镁合金表面抗菌涂层未来的发展方向。
文章编号:1004-0609(2021)-11-3071-22 中图分类号:TG174.4 文献标志码:A
引文格式:崔蓝月, 薛 奎, 李硕琦, 等. 可降解骨植入镁合金表面抗菌涂层研究进展[J]. 中国有色金属学报, 2021, 31(11): 3071-3092. DOI: 10.11817/j.ysxb.1004.0609.2021-42297
CUI Lan-yue, XUE Kui, LI Shuo-qi, et al. Research progress of antibacterial coating on surface of biodegradable bone implant magnesium alloys[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(11): 3071-3092. DOI: 10.11817/j.ysxb.1004.0609.2021-42297
近年来,随着科技的发展,越来越多的新型医用金属材料层出不穷,由最初的不锈钢[1-3]与钛合金[4-7]双雄争霸,逐渐演化为镍钛合金(形状记忆金属)[8-11]、贵金属[12]、可降解金属(镁合金、锌合金)[13-25]等遍地开花,特别是可降解镁基、锌基合金可有效避免二次手术,同时还表现出良好的促成骨功能,逐渐成为了现阶段研究的热点[26]。
对于生物医用可降解镁合金,2006年,英国皇家科学院院士威廉姆斯(D. F. Williams)以“New Interesting Magnesium”为题撰写了可降解生物医用镁合金的评论,提出传统的金属植入材料在人体中的腐蚀产物对人体具有潜在的危害性。镁金属的腐蚀产物不仅没有害处,而且可能对人体有益[27-28]。从此,揭开了21世纪镁合金医用研究的序幕。随着研究的深入,镁合金良好的生物相容性、力学相容性、生物可降解性、密度和弹性模量与人体皮质骨相似、一定的抗菌性等优势逐渐被研究人员一一证实,其降解主产物镁离子也作为人体必需的微量元素,参加人体生命活动,并且多余的镁离子能通过人体自主的排泄过程排出体外,不会对人体产生额外的影响[29]。
镁合金作为一种在生物医学领域具有广泛的应用前景的新型材料,可作为心血管支架[30]、骨科内固定材料[31]等。德国Syntellix AG公司的镁合金压缩螺钉(Compression Screw (CS), MAGNEZIX )是获得欧盟临床应用审批资质的可降解医用镁合金产品[32]。2012年,苏州奥芮济医疗科技有限公司研发的胸部固定板获得了国内医用镁合金领域第一个医疗器械产品注册证。2014年11月3日,东莞宜安科技股份有限公司申请的“可降解镁骨内固定螺钉”产品通过国家食品药品监督管理总局创新医疗器械特别审批申请审查。2019年7月19日,该可降解镁骨内固定螺钉获批临床使用,标志着我国生物可降解金属植入物产业化步入了新阶段。2020年5月18日,该可降解镁骨内固定螺钉项目正式获欧盟CE认证,标志着我国镁金属材料医用产业化进程取得重大进展。我国镁资源丰富,镁合金种类繁多,Mg-Al合金、Mg-Ca合金[33]、Mg-Li合金[34]、Mg-Li-Ca合金[35]、Mg-Zn合金[36]、Mg-Zr合金[37]、Mg-Sr合金[38]、Mg-RE合金[30]、Mg-Sc合金[39]、Mg-Ge合金[40]等都是比较典型的医用镁合金。
)是获得欧盟临床应用审批资质的可降解医用镁合金产品[32]。2012年,苏州奥芮济医疗科技有限公司研发的胸部固定板获得了国内医用镁合金领域第一个医疗器械产品注册证。2014年11月3日,东莞宜安科技股份有限公司申请的“可降解镁骨内固定螺钉”产品通过国家食品药品监督管理总局创新医疗器械特别审批申请审查。2019年7月19日,该可降解镁骨内固定螺钉获批临床使用,标志着我国生物可降解金属植入物产业化步入了新阶段。2020年5月18日,该可降解镁骨内固定螺钉项目正式获欧盟CE认证,标志着我国镁金属材料医用产业化进程取得重大进展。我国镁资源丰富,镁合金种类繁多,Mg-Al合金、Mg-Ca合金[33]、Mg-Li合金[34]、Mg-Li-Ca合金[35]、Mg-Zn合金[36]、Mg-Zr合金[37]、Mg-Sr合金[38]、Mg-RE合金[30]、Mg-Sc合金[39]、Mg-Ge合金[40]等都是比较典型的医用镁合金。
骨骼,作为人体中少数具有再生潜力的组织之一[41],比较容易受到伤害后断裂。随着人口老龄化加剧,骨折将会成为我国医疗领域面临的巨大问题。据统计,2010年,我国的骨骼疾病患者多达百万例,由此导致的医疗费用大约为七百亿元。预计到21世纪中叶,每年在此方面所需的花费将会超过两千亿元[42]。因此,新型骨植入材料的研发是医用生物材料领域的重点。镁合金作为骨固定材料的生物相容性已逐渐被证实[43],特别是镁合金在人体中释放出的镁离子能促进成骨细胞的生长、增殖及分化,加速骨愈合[44]。究其原因是镁合金降解产生的镁离子可以刺激感觉神经末端释放出更多的神经递质降钙素基因相关肽(主要为CGRP),促进骨膜内干细胞的成骨分化直至形成新骨。研究表明,高于生理浓度的镁离子可显著提高脊髓背根神经元突触的可塑性,促进大鼠骨质疏松、骨折的愈合。因此,镁及合金可在骨折愈合初期为骨骼修复提供良好的骨细胞生长微环境和力学环境,降低应力遮挡效应、局部骨质疏松和再骨折的可能性。然而,骨折的愈合是一个复杂的过程。简单的骨折术后愈合需要分为四个周期[45]:1) 0~5 d,为骨折之后的血肿形成和炎症多发期;2) 5~16 d,为软组织形成期;3) 16~21 d,为硬组织形成期;4) 21~63 d,为最终愈合阶段,硬骨重塑为成熟的层状骨。
镁及合金应用于骨植入材料领域主要面临两个大问题:过快的腐蚀速度和炎症。镁的标准电极电位为-2.372 V[46],属于化学活性比较高的金属,在人体复杂环境中容易发生降解而失去力学性能。过快的降解速度将直接导致材料的性能不能满足服役期的要求,在创伤未愈合时发生危险。炎症反应分为两种:免疫调节反应和细菌感染[47]。免疫调节反应多发生在植入材料生物相容性较低时,而细菌感染则是镁及合金临床应用面临的一个重大问题。因此,开发可控降解-抗菌一体化的功能镁合金表面十分必要。
1 常用的抗菌物质
具有抗菌性能的镁及合金骨植入材料,不仅能够抑制微生物的繁殖,同时可以避免因微生物繁殖而产生的酸性化境,减弱微生物腐蚀。现阶段,赋予镁及合金抗菌性能的方式主要分为两大类。一是通过在合金中添加具有抗菌性能的金属元素,例如:Cu、Zn、Ag等,在降解过程中释放离子起到杀菌效果。二是通过在镁及合金表面制备抗菌涂层或复合涂层。当然,表面涂层除了可以发挥抗菌作用,还可赋予镁合金表面其他的功能性[48]。图1所示为常用的抗菌物质、特征及抗菌机制。本文中主要总结了金属及其氧化物、多肽与酶、天然抗菌剂、药物、光动力与光热疗法在镁合金表面耐蚀抗菌涂层制备领域的研究现状,所涉及到的抗菌机理包括:抑制合金表面细菌黏附、破坏细胞膜结构导致细胞死亡、抑制DNA复制过程阻止细菌繁殖、抑制RNA转录导致细胞生物活动进程不能正常进行、热杀菌和活性氧杀菌等。下文我们将分类进行详细讨论。
1.1 金属及其氧化物
金属元素在抗菌领域的应用不仅体现在镁及合金(Mg-Ag,Mg-Cu,Mg-Cu)的合金化,同时也可以用于镁及合金的表面改性。研究发现,一些具有抑菌作用的Ag、Cu、Zn及其氧化物可以通过制备涂层的方式负载在镁合金表面,而后通过特殊的方式或者机制释放出来,起到杀菌效果。以下将对这些常用的具有抑菌作用的金属及其氧化物在镁合金抗菌领域的应用进行讨论。
1.1.1 Mg与MgO
镁及合金的降解产物Mg2+本身具有较好的抗菌效果,对此,RODRIGUEZ-SANCHEZ等[29]对镁离子的抗菌机制进行了系统的研究,发现当体系pH不变时,更高的Mg2+浓度会基于作用于细菌的更大的渗透压力,导致细菌生理活动紊乱直至死亡,从而表现出良好的抗菌性。687 mg/L的Mg2+可以杀死50%的革兰氏阳性、阴性菌,因此,口服或者静脉注射适量的Mg2+有助于抑制术后炎症。另外,镁及合金降解导致的碱性微环境也是其具有抗菌性的重要原因[49]。当然尽管MgO在降解过程中由于Mg2+的释放也表现出一定的抗菌性[50-51],但通过在镁合金表面制备MgO涂层实现抗菌功能的研究比较少。而且,由于人体新陈代谢以及体液对pH值的缓冲作用,镁合金本身因为Mg2+和碱环境所产生的抗菌性能在体内并不适用[52]。
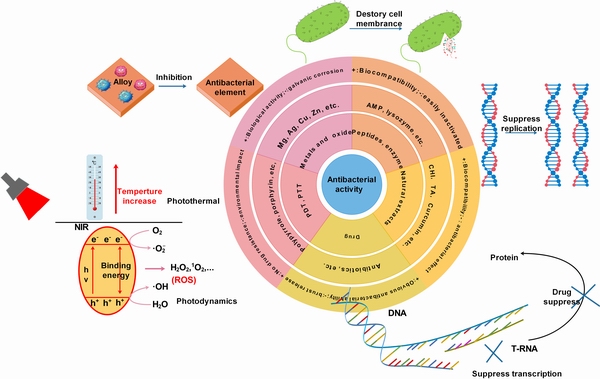
图1 常用抗菌物质特征及机理
Fig. 1 Features and mechanisms of commonly used antimicrobial substances
1.1.2 Ag与Ag2O
银由于可以和细菌酶蛋白形成结合键,使得这些物质不能为微生物所利用,从而破坏细菌细胞膜,导致细菌死亡。研究表明,Mg-Ag合金表现出较低的细胞毒性和良好的细胞相容性,对于金黄色葡萄球菌(S. aureus)和表皮葡萄球菌(S. epidermidis)两种常见致病菌的抑菌活性高于90%[53]。但铸态Mg-4% Ag合金的力学性能较差,降解速度较快,经过T4热处理后,由于树枝状结构和部分析出物的溶解,其降解速率与力学性能略有改善。侧向等径挤压处理也可以显著影响合金的微观组织和力学性能。总的来说,降解速率加快是Ag用于合金化的最大弊端,惰性的Ag会与活性的Mg形成原电池,加快镁及合金的腐蚀速度[54]。
因此,研究者大多利用Ag构建涂层,从而最大程度发挥Ag的抗菌作用[55-56]。在镁合金表面,ZHAO等[57]采用层层组装(LbL)技术制备了纳米Ag多层膜,而后通过聚硅烷包裹的方式,使得纳米Ag可以实现缓慢持续释放,在提高镁合金耐蚀性的基础上,有效地实现了抗菌功能。LOPERENA等[58]在镁合金表面通过电沉积法制备了含Ce、Mo的复合涂层,而后浸泡AgNO3溶液,有效改善了AZ91D合金的耐蚀性,同时赋予了其良好的抗菌性。ZHAO等[59]通过一步水热法在镁合金表面制备Ag+掺杂的水滑石涂层,该涂层在提高合金的耐蚀能力的同时,为涂层提供了抗菌功能。但对于载Ag涂层,在Ag+的释放过程中,一旦存在与镁基材接触的机会,也不可避免的存在一定程度的电偶腐蚀。
SONDI等[60]研究发现,除纳米Ag外,Ag2O由于可以破坏细菌DNA复制,也表现出良好的抗菌功能,是一种可以替代纳米Ag的抗菌材料,但其用于表面涂层的构建主要集中在钛合金上[61-62],镁合金表面鲜有研究。
1.1.3 Cu与CuO
铜是人体不可缺少的微量金属元素,主要被胃和小肠吸收,经由胆汁排出体外。铜是体内多种酶和蛋白质的重要成分和催化剂,可以通过多种机制影响人体健康。基于铜的生物功能和益处,越来越多的生物材料研究者开始关注新型含铜生物材料的开发,这些材料在保护心血管系统、促进骨折愈合、发挥抗菌作用等方面具有独特的性能[63]。关于铜的抗菌机制,主要是由于Cu2+破坏细菌的细胞壁和细胞膜,吸附细菌电子产生活性氧(ROS),从而导致细菌和真菌的损伤和死亡[64-65]。具有抗菌效果的Mg-Cu合金能够延长镁合金的抗菌时间,同时不影响合金的可降解性质。但需要注意的是,Cu元素一般作为合金中的杂质元素,其掺杂量上限为1×10-3,过多的Cu掺杂会加速镁及合金的腐蚀速度[66-67]。关晓楠等[68]研究发现,将镁铜合金的表面纳米化到400 nm时,伴随着位错胞及孪晶的形成,镁铜合金在氢氧化钠溶液中会出现明显的活化-钝化-过钝化现象,对腐蚀行为有所改善。
YAN等[69]在铸态及固溶处理的Mg-0.6Cu合金表面构建微弧氧化(MAO)涂层,研究发现,由于Mg2Cu第二相的存在,Mg-0.6Cu合金表现出明显的电偶腐蚀行为,但经固溶处理的Mg-0.6Cu合金表面MAO涂层耐蚀性与抗菌性明显优于铸态合金MAO涂层,其原因在于Mg-0.6Cu合金表面MAO涂层制备过程使得MAO结构外层具有一定量的Cu,其多孔形貌又可以使得细菌与涂层直接接触,继而可以不经过溶液体系的扩散过程,直接起到较好的接触性杀菌的作用。由此可见,载Cu的MAO涂层,可以兼顾较好的耐蚀性与抗菌性。于是,CHEN等[70]在Mg-2Zn-1Gd-0.5Zr合金表面构建了含Cu的MAO涂层,该涂层获得了较MAO涂层更好的耐蚀性,同时基于Cu2+的释放,涂层抑菌率可达96%以上。耐蚀性提高的主因可以归结于涂层中形成的Cu3(PO4)2产物可以稳定涂层结构不被破坏。
与Cu元素相似的,CuO也作为抗菌的掺杂粒子。XU等[71]通过溶胶-凝胶法在镁合金AZ60的羟基磷灰石涂层中掺杂了CuO纳米颗粒,改善了羟基磷灰石涂层多孔的形貌,有效地提高了镁合金的耐蚀能力且使其具有抗菌性能。YANG等[72]将含Cu的生物活性玻璃纳米颗粒(Cu-BGN)载入聚己内酯涂层中,均匀分布的Cu-BGN释放的Cu2+抑制了肉葡萄球菌(S. carnosus)和大肠杆菌(E. coli)的生长,提高了人骨肉瘤细胞(MG-63)的活力和增殖能力。
1.1.4 Zn与ZnO
锌抗菌的途径主要是通过参加细菌的DNA转录过程,进而通过影响有关蛋白质的合成来起到抗菌效果[73],其在镁合金中的掺杂可有效改善镁合金的耐蚀和力学性能。QIN等[74]通过在镁基材中掺杂钕(Nd)、锌(Zn)、锆(Zr)三种金属元素,制备了一种新型的Mg-Nd-Zn-Zr(简称JDBM)合金,有效提高了镁基材的耐蚀抗菌性,不断扩散释放出来的Zn2+也对E. coli、S. epidermidis、S. aureus起到了较好的抑制作用。
利用Zn2+的抗菌性构建抗菌涂层,是实现其抗菌功能的一个重要应用[75-76]。YANG等[77]通过一步水热法在镁合金表面制备了一种Zn/Sr掺杂的磷酸盐涂层,其中的Zn元素为涂层提供了优异的抗菌性能,所得涂层的耐蚀和促成骨功能也显著提高。ZOU等[78]则通过水热法在蒙脱石涂层层板间插入了Zn2+离子,Zn2+作用于细菌的细胞膜,抑制了S. aureus和E. coli的生长并导致细胞质的泄漏和细菌的死亡。Zn2+的释放时间可达144 h,可以应对医用植入物的早期感染。
与Ag、Cu元素不同,ZnO纳米颗粒是一种在镁合金表面应用更为普遍的抗菌氧化物。ZHOU等[79]通过一步法在Mg68Zn28Ca4合金表面制备了纳米羟基磷灰石(HA)/ZnO涂层,该复合涂层内部致密的ZnO对镁合金起到了良好的保护作用,同时对S. aureus和E. coli起到了良好的体外抑菌作用。SUN等[80]利用微波水合成结合热处理制备了单层ZnO涂层,经紫外光辐照,抗菌性能明显提升。在SBF溶液中浸泡14 d后,ZnO涂层表面有含碳酸盐的羟基磷灰石析出,且随着紫外光照射时间的增加,磷灰石的诱导能力显著提高,即生物活性、成骨活性显著提高。这主要是由于紫外光辐照,ZnO涂层表面产生了Zn-OH官能团和羟基自由基。当然,直接将ZnO纳米颗粒掺杂或负载于镁合金表面也是一种不错的方法[81-82]。
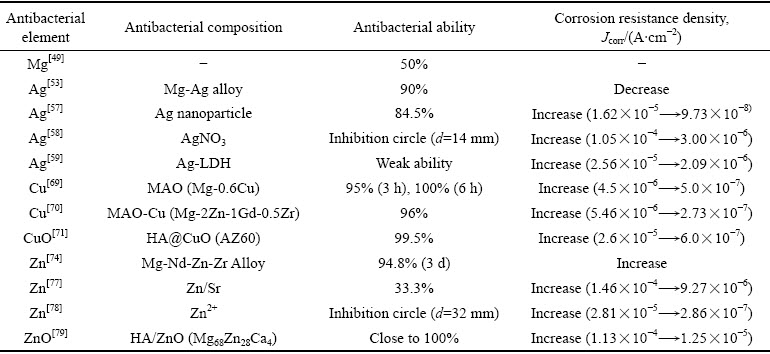
综上所述,抗菌金属及其氧化物可以通过合金化或涂层设计提高镁合金的抗菌性,但普遍存在抗菌金属与镁合金之间的电偶腐蚀问题。金属及其氧化物对镁合金抗菌与耐蚀性能的影响如表1所示,抗菌金属元素含量及合金化处理过程对抗菌镁合金性能具有直接的影响,平衡抗菌镁合金的耐蚀性、力学性能与抗菌性之间的关系至关重要。从涂层设计的角度看,如何在镁合金与抗菌涂层之间设置隔离带,并且实现金属离子向外单向扩散十分关键。尽管人们认为银离子的抗菌性优于铜离子和锌离子,且纳米银在抗菌领域更为常用,但表1所述数据中,铜离子和锌离子抑菌率更优于银离子。由此可见,抑菌性能的优劣不仅与金属抗菌离子的种类有关,更与金属离子在涂层中的存在形式、释放浓度等有关。Cu、Zn氧化物的抑菌性明显优于其离子态,但直接以金属氧化物纳米颗粒作为负载单元,则将进一步衍生出涂层与镁合金表面的结合力问题。聚硅烷等聚合物相较于MAO、HA等无机涂层具有更好的物理阻隔作用,因而所得涂层的耐蚀性优化效果更好。
1.2 多肽与酶
具有抗菌效果的多肽类物质是人类和其他生物体产生免疫反应时的主要参与者。大多数的抗菌多肽是小于50个氨基酸的带电两亲性物质,这类物质的常见抗菌机制在于能够破环细菌的生物膜[83]。迄今为止,已经有超过5000种抗菌多肽可以通过人工合成,抗菌多肽(AMP)的应用也得到了越来越多的关注。在镁合金表面利用抗菌多肽构建抗菌涂层时,往往将多肽进行氨基化修饰,使其更容易与镁基体或者内部涂层结合。TIAN等[84]将抗菌多肽PSI 10(RRWPWWPWRR-NH2)通过浸泡的方式直接吸附在镁合金AZ91表面的微孔板状HA涂层上,随着降解过程中PSI 10的缓慢释放,复合涂层浸泡4 d后依然保持超过50%的抑菌率。近期,WANG等[85]对比了caerin抗菌肽1.9(GLFGVLG- SIAKHVLPHVVPVIAEKL-NH2)和修改后的抗菌肽序列1.1(GLLSVLGSVAKHVLPHVLPHVVPVIAE- HL-NH2)在镁合金聚氨酯涂层表面的抑菌活性,研究发现两种抗菌肽均能提高镁合金的耐蚀、抗菌性,特别是可以有效抑制MRSA细菌,且修改后的抗菌肽1.1比广泛使用的临床抗生素塔佐辛表现出更持久和更显著的抑菌性,这可能与多肽、聚氨酯和金属基体形成的微观结构有关。ZHOU等[83]则通过旋涂法在氧化镁无机涂层表面利用丝纤蛋白负载了CB((NH2)-NGIVKAGPAIAVLGEAALCONH2)抗菌肽,Mg2+与抗菌肽的协同作用抑制了细菌的黏附生长,同时促进了可降解镁合金的成骨分化。抗菌肽在镁合金表面的应用取得了显著成效,且比表面积较大的无机涂层可以提高抗菌肽的负载量并实现缓释功能[84]。
表1 金属及其氧化物对镁合金抗菌与耐蚀性能的影响
Table 1 Effect of metal and its oxide on antibacterial and corrosion resistance of magnesium alloy
另外,酶也是一种具有潜在应用价值的镁合金表面抗菌物质,它可以通过改变生物外基质(ECM)、溶解细胞膜起到抗菌效果[86]。例如:溶菌酶[87]、内溶素[88]等。蛋白酶和DNA解旋酶也具有一定的抗菌性能[89]。研究表明,透明质酸交联溶菌酶对S. aureus具有很好的抑菌能力,同时有助于成骨细胞的增殖和分化[90]。
多肽与酶在镁合金抗菌相关领域的应用仍然还是一个相对空白的领域,且大分子多肽的抗菌机制也不十分明确。如表2所示,所有抗菌多肽均需经过氨基化处理,使其在镁合金表面实现更好的结合,各抗菌多肽的抑菌效果也良莠不齐。另外,由于现有研究中的多肽涂层多为自组装分子膜,涂层厚度较薄,对镁合金耐蚀性的提升较为有限。如何保持抗菌肽、酶在制备、使用过程的生物活性问题是限制其应用的重要原因,通过多肽序列设计或复合涂层设计提高其耐蚀性与抗菌性是未来开展研究的重点。
1.3 天然抗菌剂
自古以来,人们就善于使用大自然中的天然物质来抵抗疾病。随着科学技术的发展,植物中的有效物质逐渐被分离、纯化,特别是一些多酚类提取液和类黄酮类有机物表现出较好的抗炎症、抗过敏、抗血栓、抗癌和促进血脉舒张等作用,图2所示为常用天然活性物质分子式。
1.3.1 壳聚糖及其衍生物
壳聚糖(CHI)在镁及合金表面改性中的应用较为广泛,依靠接触型杀菌机制,CHI及其衍生物的杀菌主要通过三种方式:1) 依靠自身的正电性与表面负电性的细菌结合,从而破坏细菌的细胞壁和细胞膜;2) 与金属离子结合,从而降低细菌生命活动必须的酶的活性;3) 穿透细菌的细胞膜,从而抑制RNA的转录。CUI等[91-92]利用LbL技术,分别在镁合金表面构建了DNA/CHI、聚谷氨酸/CHI多层,均表现出了较好的耐蚀、抗菌性。更多的研究则是将CHI与无机涂层结合,通过构建复合涂层达到研究目的。YU等[93]以MAO为内层构建了MAO/CHI复合涂层,研究表明,CHI很好地封闭了MAO的多孔结构,降低了镁合金的降解速率,同时降解过程中表现出良好的pH缓冲与抗菌功能。GAO等[94]则利用LbL技术构建了CHI/肝素化氧化石墨烯多层,涂层在提高基材耐蚀性的基础上,降低了溶血率和血小板黏附,同时促进内皮细胞的黏附和增殖。ALAEI等[95]和BAHATIBIEKE等[96]则分别利用CHI与生物活性玻璃以及硅烷结合构建复合涂层,极大地提升了镁合金的耐蚀性和生物相容性。不难发现,研究者们后期更关注CHI对镁合金生物相容性的改善,而不仅仅局限于赋予镁合金抗菌性。另外,由于CHI的溶解性问题,单纯利用CHI改善镁合金的耐蚀性存在较大难度,制备过程往往需要通过调节溶液pH尽可能减小酸性溶液对镁合金的腐蚀。
表2 多肽与酶对镁合金抗菌耐蚀性能的影响
Table 2 Effects of peptides and enzymes on antibacterial and corrosion resistance of magnesium alloys
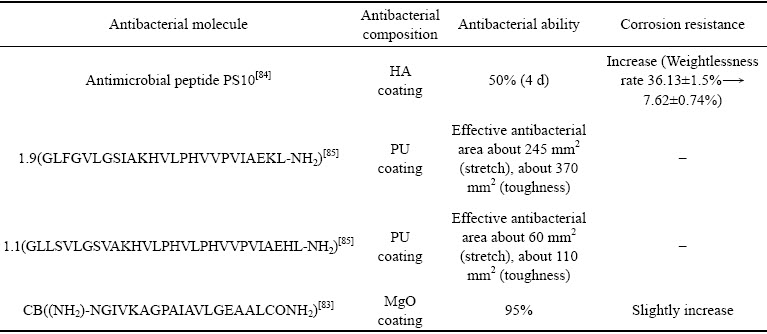
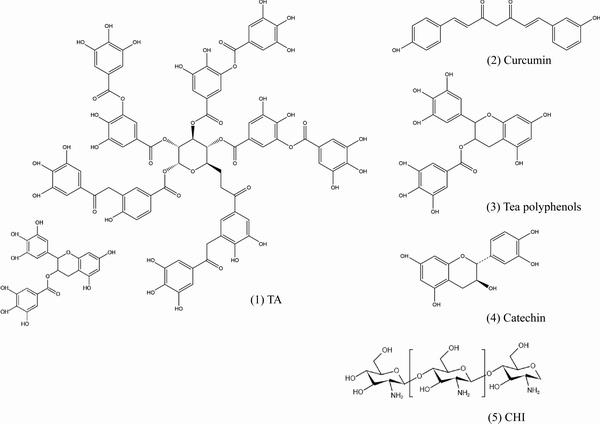
图2 常用天然活性物质分子式
Fig. 2 Molecular formula of commonly used biologically active substances
1.3.2 单宁酸
单宁酸(TA)也称鞣酸,由五倍子中提取,是一种典型的多元酚类有机分子。其抗菌机制主要是基于TA分子中的酚羟基破坏细菌的细胞壁和细胞膜[97]。同时,TA可以络合金属离子,降低细菌中维持正常生命活动的金属酶的活性[98-99]。FACCHI等[100]制备了一种聚阳离子缩合TA/多糖基聚电解质多层膜来防止微生物在基材表面的黏附行为,从而起到较好的抗菌效果。CUI等[101]将TA添加于微弧氧化电解液中制备抗菌MAO陶瓷涂层,研究表明,TA有效提高了植酸系MAO涂层的厚度,降低了涂层的孔径和孔隙率,赋予了涂层较好的抗菌性。WANG等[102]在Mg-Zn-Y-Nd合金表面制备了TA修饰的氟化镁复合涂层,除了TA的酚羟基表现出良好的抗菌性外,TA还表现出优异的自由基清除与血小板排斥能力,并且能够有效促进内皮细胞的生长和分化。另外,TA还可以用做HA等含金属离子无机涂层的诱导剂[103]。
1.3.3 姜黄素
姜黄素是一种从生姜中提取的具有抗菌、消炎、抗癌、促成骨功能的二酮类有机物[104-106],具有优异的生物活性,对于缓解艾兹海默证也有一定的效果[107]。研究发现,姜黄素作为抗菌剂的作用方式主要分为两大途径:1) 参与细菌的调节过程(QS),能够有效的抑制细菌的增殖;2) 能够靶向识别细菌的细胞膜、细胞壁、蛋白质、DNA等,从而影响细菌的生命活动[108]。
最初,姜黄素主要被用于食品防腐领域。而近些年来,姜黄素在抗菌材料领域的应用越来越多,也逐渐延伸到了合金表面的功能化设计。HUANG等[109]设计了一种具有抗菌功能的姜黄素负载纳米MOF结构,这种结构不仅能够有效的杀死细菌,还能控制姜黄素的释放。LI等[110]在镁合金表面设计制备了一种含姜黄素的三层复合涂层,该复合涂层表现出良好的自修复和促成骨分化功能,姜黄素在复合涂层中的缓慢释放主要起到调节免疫微环境的作用,从而促进骨髓干细胞的骨分化和细胞外基质矿化。也有研究发现,姜黄素具有一定的促进伤口愈合的功能[111]。
1.3.4 其他
自古以来,就有用茶叶粉末涂抹伤口来防止伤口发炎的方法。研究者通过对茶叶中的有效成分进行分析提纯后发现,茶多酚和儿茶素等物质具有灭菌功能。例如,早期TAKAHASHI等[112]发现儿茶素对耐甲氧西林金葡萄球菌具有抗菌功能。MARCHESE等[113]通过模仿胃部和肠道的生理活动过程与茶多酚进行比较,发现了茶多酚具有杀死金葡萄球菌的能力。但在镁及合金表面,仅ZHANG等[114]利用绿茶多酚,通过LbL技术诱导富Mg2+多层转化膜,用以增强AZ31镁合金的耐蚀性,促进表面的原位内皮化,但相关抗菌能力并未提及。另外,诸如没食子酸、鞣花酸、芦荟苷、丹参酮等均属于植物提取的天然抗菌剂,值得广大研究者予以关注。
天然抗菌剂因其良好的生物相容性逐渐在镁及合金表面涂层中获得关注,如表3所示,利用天然抗菌剂制备涂层时大多利用其分子结构特征,通过层层组装技术制备多层膜或通过无机涂层负载,其抑菌能力相较于传统抗生素、金属离子等弱,且抑菌范围较为有限,无法应对复杂的感染环境。当出现大量死细菌黏附或者形成细菌生物被膜时,接触型杀菌机制能够发挥的作用也被极大地降低。所得涂层对镁合金的保护能力,取决于其涂层制备方法,原则上结合无机涂层负载相较于层层组装多层的耐蚀性更优,这主要是由于层层组装多层往往较薄,无法屏蔽腐蚀性离子的侵蚀。由此可见,结合一些其他聚合物分子制备涂层对耐蚀性的提高更有利。另外,如何在发挥天然抗菌剂的抗菌能力的同时,更好地利用其促进细胞分化、伤口愈合等其他功能值得广大研究者进一步关注。
1.4 药物
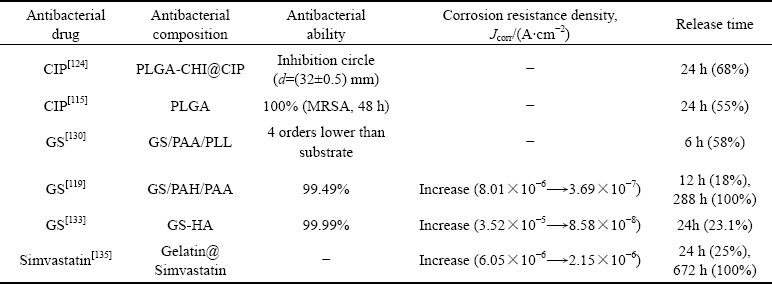
临床环境下的抗感染主要以抗生素等药物为主,即在涂层中负载药物仍是赋予镁及合金抗菌能力最有效的方式,常用的药物包括环丙沙星(盐酸)(CIP)[115-116]、庆大霉素(硫酸)(GS)[117-119]、万古霉素[120-121]、阿莫西林[122]等(见图3)。然而,药物应用到镁及合金表面时面临着两个巨大的挑战:药物递送和药物缓释。
常用的抗菌药物按其水溶性可分为亲水性(盐酸二甲双胍)和疏水性(庆大霉素、万古霉素、环丙沙星等)两种,如果药物本身具有较高的亲水性,则不存在递送问题(存在的靶向识别问题本文不做赘述)。对于较多使用的疏水性药物,其疏水亲脂的特性使其在发挥功能时能够有效地识别细菌生物膜表面的标靶,起到针对性杀灭细菌的作用。同时,疏水性药物穿透磷脂双分子层组成的细胞膜,在细菌内部发挥作用。但是此类药物难以通过细胞液递送到目标位置。因此,制备一种用来递送疏水性药物的载体十分必要。另外,众所周知,伤口的愈合需要一定的周期,如果药物在植入伊始全部释放,不仅会对机体产生毒性,不利于伤口愈合,同时还不利于遏制后期感染。因此,控制药物以一定的速率在一定的时间周期内缓慢释放是极其重要的。
通常,药物的递送和缓释需要载体,如图4所示,纳米纤维[123]、纳米颗粒[124]、脂质[125]、金属有机框架(MOF)结构、明胶[126]、树枝状大分子[127]等都是较好的载体。纳米颗粒的抗生物膜活性取决于它们比现有的游离药物分子在生物膜基质中具有增强的相互作用和穿透能力[128-129]。同样的,纳米颗粒与抗菌药物的结合也能够有效的提高药物的杀菌性能。ARAFA等[124]设计了一种聚乳酸(PLGA)-CHI为基的纳米胶囊来递送不溶于水的环丙沙星有效提高了抗菌性能。同样的,PLGA的微球也被用来向皮肤炎症处递送环丙沙星和人参皂苷Rh2,用来治疗金葡萄球菌的感染[115]。
表3 天然抗菌剂对镁合金抗菌与耐蚀性能的影响
Table 3 Effects of natural antibacterial agents on antibacterial and corrosion resistance of magnesium alloys
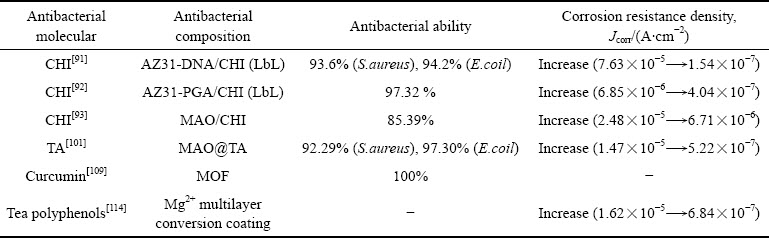
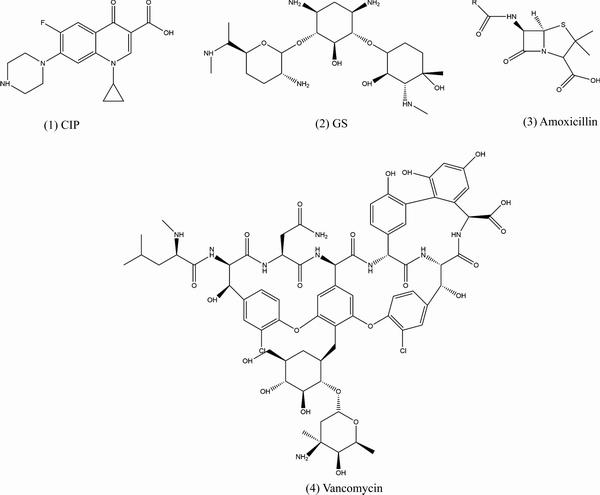
图3 常用药物分子式
Fig. 3 Molecular formula of commonly used drugs
鉴于大量药物递送体系的逐渐完善,把药物负载到镁及合金表面,赋予合金功能化成为可能。ESCOBAR等[130]通过LbL的方法在玻璃表面制备了一种负载了硫酸庆大霉素(GS)的聚丙烯酸/聚赖氨酸(PAA/PLL)涂层,有效地提高了基材抗菌性,涂层在浸泡初期的6 h药物释放量达到58%,而后药物的释放速率保持稳定,释放时间持续一周,符合骨植入术后临床感染预防与治疗的特征。在此基础上,ZHAO等[119]采用LbL技术在镁合金表面制备了负载GS的聚丙烯胺盐酸盐(PAH)/PAA涂层,在提高镁合金耐蚀能力的同时,赋予其良好的抗菌性能。除此之外,聚电解质多层还表现出一定的自修复性能。与此同时,ZHAO等[131]发现利用旋转的剪切力能够提高涂层与合金的结合力。因此,旋涂法成为镁合金表面LbL制备的耐蚀功能性涂层的常见方法,避免了普通浸泡法对镁及合金基体或者内部陶瓷涂层的腐蚀[132]。JI等[133]在镁合金表面通过旋涂法LbL制备了一种GS负载的聚电解质诱导羟基磷灰石涂层(HA),药物释放周期达到了400 h,前期突释造成的药物释放占比减少到了25%。
除了LbL技术,BAKHSHESHI-RAD等[134]利用明胶纳米纤维负载环丙沙星(CIP),有效延长了药物释放时间。QI等[135]同样以明胶(Gelatin)纳米团簇作为辛伐他汀(Simvastatin)的载体,结合CHI通过电沉积技术制备涂层,除了浸泡第一天存在突释现象外,涂层可以在28 d内实现有效的缓释。SUN等[136]利用聚谷氨酸和7-羟基-4-甲基香豆素共聚获得胶体颗粒,实现了维他命M有效负载和缓慢释放。聚乳酸[137-138]、羟基磷灰石[139]等也是可在镁合金表面涂层中获得良好应用的载药纳米颗粒。
药物对镁合金抗菌与耐蚀性能的影响如表4所示,尽管镁合金表面载药涂层的抑菌率均保持在99%以上,有利于抑制植入术后初期易发生的感染问题或者赋予植入物特定的治疗功能,但是药物释放时间、释放量与伤口愈合或者疾病治疗周期的匹配却是亟待解决的问题。一般来说,正常骨折伤口的易感染期为5~7 d,但不同病症所对应的易感染周期是完全不同的,糖尿病等慢性疾病会延长易感染期,另外还有年龄、环境、突发情况以及伤口位置不同等其他因素的影响[41, 140]。此外,植入前期的突释问题以及细菌耐药性等仍制约着载药涂层的应用。
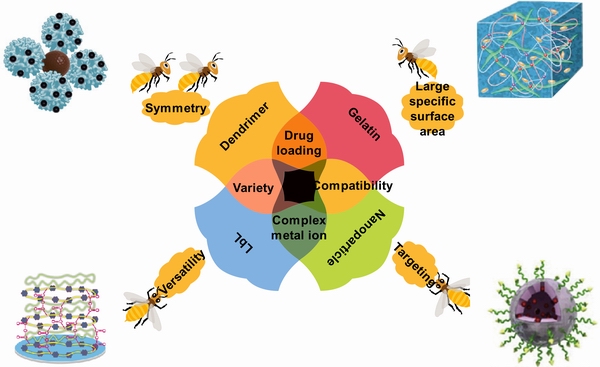
图4 常见载药模型[119, 123, 126-127]
Fig. 4 Common drug loading models[119, 123, 126-127]
表4 不同药物对镁合金抗菌与耐蚀性能的影响
Table 4 Effects of different drugs on antibacterial and corrosion resistance of magnesium alloys
1.5 光热和光动力抗菌
近年来,由于副作用小、效果明显、作用条件可控制等优点,光热和光动力抗菌成为一种新型高效可循环利用的抗菌方法。光热疗法(PTT)通过光热剂将光能转化为热能进而导致细胞生物膜通透性改变、破坏细胞结构起到抗菌效果[141]。光动力疗法(PDA)的作用原理为材料在接受特定波长的光激发后能产生杀死细菌的活性氧(ROS)[142]。
目前,具有光敏效果的物质大致可分为无机物和有机物两大类。一些金属氧化物(TiO2[143]等)、金属硫化物(CuS[144]、MoS2[145]等)、金属离子(Au2+[146]、Zn2+[147]等)在受光刺激后能将光能转化为热能或者产生活性氧杀死细菌,但是这些无机物往往生物相容性低、造价高、难控制。有机物相对于无机物来说,结构容易控制、制备涂层简单,在光热、光动力抗菌领域已经得到广泛应用,例如聚吡咯[148]、菁染料[149]、聚多巴胺[150]、卟啉[151-152]等。其特点在于结构中的大π键容易吸收光能转化为热量释放,从而起到光热效果。同样的,有机分子π键从光子中吸收能量后从基态跃迁到单线态,不稳定的状态下快速释放能量达到低能量的稳定状态,释放的能量会产生ROS抗菌。
HUANG等[148]在镁合金AZ31表面通过旋涂LbL法制备了一种聚几内酯/聚吡咯多层,涂层不仅具有耐腐蚀、自愈合功能,同时利用了物质的光热性能。LOCATELLI等[153]则合成了一种PLGA/聚乙二醇(PEG)包裹的锂-萘还原Mg纳米颗粒,以还原Mg为核,以有机物为壳的结构在810 nm近红外光的照射下表面温度能够至少提高4~5 ℃,对镁合金表面制备光热涂层具有启示意义。但总体来说,具有光热、光动力抗菌能力的涂层在镁合金表面的应用还比较少见,需要研究者们进一步开发和研究。
2 可降解镁合金表面抗菌涂层制备方法
基于上述不同抗菌物质的不同特性,为了更好地解决镁及合金降解速率过快的问题,同时赋予表面抗菌功能,研究者们重点关注以下几种涂层制备方法(见图5)。
2.1 自组装法
利用有机涂层的物理屏障作用提高镁及合金的耐蚀性,同时通过交联、共聚等反应机制负载抗菌物质,赋予表面抗菌活性是一种常见的抗菌涂层设计思路。自组装(Self-assembly)法则是在镁合金表面制备有机涂层的常用方法。当然,现今的涂层设计与制备已经不仅仅限于单一涂层,对于有机涂层而言,其往往会与内部无机涂层相结合,进一步提高涂层的结合力。CHEN等[154]利用自组装法辅以高温,通过交联反应在纯镁表面构建了具有良好耐蚀性、pH稳定性、抗菌活性、细胞相容性、光致发光和成骨分化能力的聚柠檬酸硅有机涂层,该涂层可以通过—COO—Mg离子键实现与镁基体的良好结合。WANG等[155]和YU等[93]则均以MAO涂层作为复合涂层内层,分别利用聚乙烯亚胺负载纳米Ag和CHI,改善了镁合金的耐蚀性,同时赋予其较好的抗菌性,特别是MAO涂层特殊的多孔状形貌为外部有机涂层的成功制备提供了机械咬合作用,更有利于提高外部有机涂层与基材的结合。
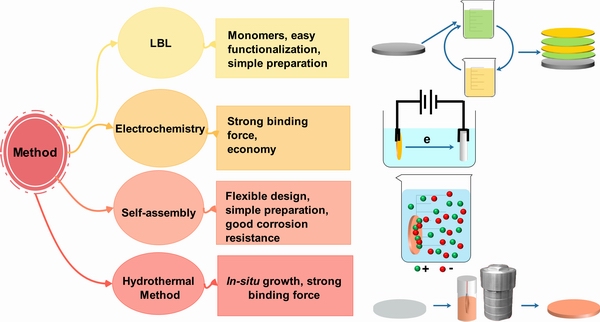
图5 可降解镁合金表面抗菌涂层制备过程示意图
Fig. 5 Schematic diagram of preparation process for antibacterial coating on Mg alloy surface
2.2 层层组装法
层层组装(LbL)技术是通过利用不同离子之间的静电引力、共价键、氢键等分子间作用力,将聚电解质、聚合物、蛋白质、多肽、脂质、核酸、纳米颗粒和超结构等逐层交替制备涂层的一种方法,所得涂层往往为分子膜且质地均匀,这为制备复杂的功能化涂层提供了一种简单有效、环境友好的方式[156]。自2012年,镁合金表面制备LbL涂层的技术逐渐成熟,组装前通常需要将镁合金表面进行碱处理,而后利用聚乙烯亚胺等作为前驱体,为后续涂层的制备提供条件[157-159]。另外,组装单体和结合方式的多样性使得镁合金表面负载金属纳米粒子、金属氧化物、天然抗菌剂、药物等变得更为灵活。ZHANG等[160]利用LbL技术将纳米银载入共聚物多层中,从而改善了镁合金的耐蚀抗菌性。同时,组装单体官能团的多样性对涂层降解过程中控制腐蚀速率也十分关键。CUI等[91]通过LbL的方式,以DNA和CHI为组装单体,在氢氧化镁涂覆的镁合金表面制备复合涂层,涂层表现出良好的抗腐蚀性能和抗菌性能,复合涂层降解过程中,DNA的磷酸基团和CHI的氨基可以通过诱导形成钙磷沉积产物进一步保护镁基体。但是,LbL制备的涂层往往太薄,无法对镁合金提供长效的保护。在LbL技术实施过程中,浸泡法往往会损伤内部无机涂层或基体,旋涂或喷涂法则无法实现对形状复杂试样的制备。
2.3 电化学法
电化学方法制备复合涂层是镁合金表面改性的一种较为简单便捷的方法。通常使用的方法有阳极氧化[161]、MAO[162]、电沉积等。阳极氧化是把镁合金作为阳极,在低电压下制备氧化物涂层的方式。而MAO是在阳极氧化基础上的升级,通过提高电压在样品表面产生微电弧,促进基体与电解质溶液反应生成陶瓷涂层。常用的MAO电解液有植酸系、硅酸系和磷酸系。通过调整电解液的配方,可以直接制备抗菌MAO涂层。CUI等[101]通过在植酸系电解液中添加TA的方法制备了改性MAO涂层,减少了涂层表面的孔径和孔隙率,并且赋予了涂层一定的抗菌性能。另外,MAO涂层往往表现为典型的多孔结构,这种结构一方面在降解过程中会成为离子交换的通道,导致电偶腐蚀,同时,也为构建复合涂层提供了有利条件(此处参见自组装法)。
电沉积法是通过电荷移动,把溶液中的带电离子或分子沉积到样品表面,从而制备出具有特定功能的涂层,涂层性能依赖于电解液的成分、pH、温度、电流密度等条件。SUN等[98]将丙烯酸异冰片酯和甲基丙烯酸二甲氨基乙酯共聚物P(ISA-co-DMA)与TA制备的胶体颗粒电沉积在镁合金表面,提高了镁合金耐蚀性,同时赋予了镁合金良好的抗炎和细胞黏附能力。
2.4 水热法
水热处理法主要是指在密闭的容器中,涂层的有效成分经过不断的沉淀、粉碎、再结晶,形成一系列晶粒细小、分布均匀且颗粒团聚较轻的纳米颗粒的过程[163]。目前,利用水热处理法制备抗菌涂层主要是包括直接原位生长抗菌无机涂层或者在自组装/LbL的基础上诱导钙磷、水滑石等无机涂层,进而控制药物的缓慢释放。ZHAO等[59]和ZOU等[78]均通过一步法分别在镁合金表面原位生长纳米银-水滑石涂层和载锌蒙脱石涂层,在提高耐蚀性的同时,赋予镁合金良好的抗菌能力。JI等[133, 164]则通过层层组装GS、环丙沙星等药物诱导钙磷涂层,在赋予镁合金抗菌性的同时,实现了药物的缓慢释放。
2.5 几种制备方法的性能比较
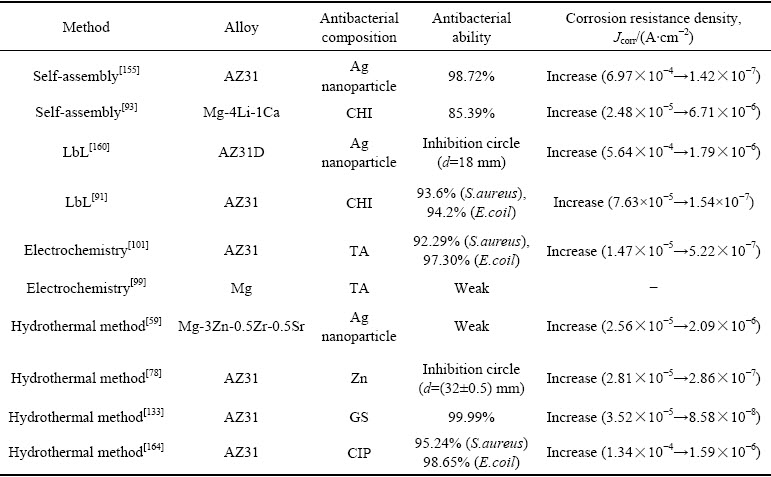
不同制备方法和抑菌因子对不同种类镁合金耐蚀抗菌能力的影响如表5所示,自组装、LbL、电化学、水热法等均可导致镁合金自腐蚀电流密度降低2~3个数量级,即镁合金耐蚀性的提升与制备方法关系不大,而主要取决于合金种类、涂层结构和成分。同一种抑菌因子,通过不同制备方法实现负载时,其抑菌性能不同,这主要是由于抑菌因子在涂层中的分布与释放形式不同。因此,涂层设计过程中,需要研究者们根据需求,选择适宜的抑菌因子和涂层制备方法,平衡涂层可控降解与抗菌性之间的关系。
表5 不同制备方法和抑菌因子对不同种类镁合金耐蚀抗菌能力的影响
Table 5 Effects of different preparation methods and antibacterial factors on corrosion and antibacterial ability of different kinds of magnesium alloys
3 结语
近年来,尽管可降解抗菌镁合金及其表面抗菌涂层越来越多样化,但仍无法解决复杂的感染问题,特别是兼顾镁基体的降解可控、顽固多样的菌种和多变的感染周期。突出的问题以及未来的发展方向体现如下:
1) 引入抗菌因子导致的腐蚀问题
对于引入金属离子导致的电偶腐蚀问题可以通过构建镁基体与抗菌涂层之间的物理屏障,控制金属离子单向往溶液扩散来解决。而对于层层组装等由于制备过程导致的腐蚀问题,则应通过调控pH值、浓度、温度等参数,创造更为温和的制备条件。
2) 抑菌有效性问题
多肽、酶、CHI等天然抑菌剂可利用其分子结构,结合光动力疗法、光热疗法等新型抗菌机制改善其抗菌能力。对于载药涂层,则应遵循感染规律,通过选取适宜的药物载体,通过控制载药量、降解速率等条件实现药物的可控释放。另外,多数涂层的抑菌机制依靠与细菌的直接接触,通过涂层结构设计,实现逐层降解来移除黏附死细菌,同时抑制细菌生物被膜形成十分关键。
3) 抗菌涂层的多功能化
作为骨固定材料,在赋予镁合金抗菌能力的同时,改善其促骨生长能力或者赋予其治疗特异性疾病(如骨髓炎等)的能力值得广大研究者们关注。
4) 抗菌涂层的智能化与适配化
不同年龄段、不同身体状态的个体的感染周期、伤口愈合周期各不相同,通过涂层结构设计、设置可供调整的涂层制备参数或者利用微环境变化(如pH值、离子浓度、温度等)使得抗菌涂层智能化,从而提高镁合金表面抗菌涂层在植入术后的适配性将是未来抗菌涂层设计需要解决的问题。
REFERENCES
[1] AYDEMIR T, LIVERANI L, PASTORE J I, et al. Functional behavior of chitosan/gelatin/silica-gentamicin coatings by electrophoretic deposition on surgical grade stainless steel[J]. Materials Science and Engineering C, 2020, 115: 111062.
[2] PERUMAL G, GREWAL H S, ARORA H S. Enhanced durability, bio-activity and corrosion resistance of stainless steel through severe surface deformation[J]. Colloids and Surfaces B-Biointerfaces, 2020, 194: 111197.
[3] GARCíA E, LOUVIER-HERNáNDEZ J F, MENDOZA- LEAL G, et al. Tribological study of HAp/CTS coatings produced by electrodeposition process on 316L stainless steel[J]. Materials Letters, 2020, 277: 128336.
[4] CHEN C, ENRICO A, PETTERSSON T, et al. Bactericidal surfaces prepared by femtosecond laser patterning and layer-by-layer polyelectrolyte coating[J]. Journal of Colloid and Interface Science, 2020, 575: 286-297.
[5] XU Yi-dong, GAO Jun-heng, HUANG Yu-he, et al. A low-cost metastable beta Ti alloy with high elastic admissible strain and enhanced ductility for orthopaedic application[J]. Journal of Alloys and Compounds, 2020, 835: 155391.
[6] ZHANG Wei-dong, YANG Peng, LIANG Xiao-peng, et al. Strength-ductility trade-off deviation in a pre-deformed metastable β titanium alloy[J]. Journal of Alloys and Compounds, 2020, 835:155332.
[7] 于晓明, 谭丽丽, 刘宗元, 等. Ti6Al4V表面生物功能纯Mg薄膜制备及性能研究[J]. 金属学报, 2018, 54(6): 943-949.YU Xiao-ming, TAN Li-li, LIU Zong-yuan, et al. Preparation and properties of biological functional magnesium coating on Ti6Al4V substrate[J]. Acta Metallurgica Sinica, 2018, 54(6): 943-949.
[8] ZHOU Ya-jun, LI Yun-heng, ZHANG Ling-xia, et al. Fe-leaching induced surface reconstruction of Ni-Fe alloy on N-doped carbon to boost oxygen evolution reaction[J]. Chemical Engineering Journal, 2020, 394: 124977.
[9] MANSOR A F, AZMI A I, ZAIN M Z M, et al. Parametric evaluation of electrical discharge coatings on nickel-titanium shape memory alloy in deionized water[J]. Heliyon, 2020, 6(8): e04812.
[10] IBRAHIM M Z, SARHAN A A D, KUO T Y, et al. Developing a new laser cladded FeCrMoCB metallic glass layer on nickel-free stainless-steel as a potential superior wear-resistant coating for joint replacement implants[J]. Surface & Coatings Technology, 2020, 392: 125755.
[11] CATANIO B C, PATERNOSTER C, TURGEON S, et al. Plasma-immersion ion implantation surface oxidation on a cobalt-chromium alloy for biomedical applications[J]. Biointerphases, 2020, 15(4): 041004.
[12] WANG Yi, CAO Qi-gao, JIA Zhi-hua, et al. Application and development of precious metal materials for medical application[J]. Rare Metal Materials and Engineering, 2014, 43(S1): 165-170.
[13] SUN Jia-yue, CAI Shu, WEI Jie-ling, et al. Long-term corrosion resistance and fast mineralization behavior of micro-nano hydroxyapatite coated magnesium alloy in vitro[J]. Ceramics International, 2020, 46(1): 824-832.
[14] SHI Xiao-ting, ZHU Yuan-yuan, ZHANG Shu-feng, et al. Characteristics of selenium-containing coatings on WE43 magnesium alloy by micro-arc oxidation[J]. Materials Letters, 2019, 261: 126944.
[15] RAJKUMAR K, RAMRAJI K, NAMRATHA G, et al. A study of bio and tribo modifier on degradation of magnesium-calcium carbonate biomaterial[J]. Materials Today: Proceedings, 2019, 27: 691-695.
[16] MOUSA H M, TIWARI A P, KIM J, et al. A novel in situ deposition of hydroxyapatite nanoplates using anodization/ hydrothermal process onto magnesium alloy surface towards third generation biomaterials[J]. Materials Letters, 2016, 164: 144-147.
[17] HoHN S, VIRTANEN S, BOCCACCINI A R. Protein adsorption on magnesium and its alloys: A review[J]. Applied Surface Science, 2019, 464: 212-219.
[18] GAO Fan, HU Yong-dong, GONG Zhi-hao, et al. Fabrication of chitosan/heparinized graphene oxide multilayer coating to improve corrosion resistance and biocompatibility of magnesium alloys[J]. Materials Science and Engineering C, 2019, 104: 109947.
[19] DEHGHANIAN C, ABOUDZADEH N, SHOKRGOZAR M A. Characterization of silicon- substituted nano hydroxyapatite coating on magnesium alloy for biomaterial application[J]. Materials Chemistry and Physics, 2018, 203: 27-33.
[20] COSTANTINO M D, SCHUSTER A, HELMHOLZ H, et al. Inflammatory response to magnesium-based biodegradable implant materials[J]. Acta Biomaterialia, 2019, 101: 598-608.
[21] CARANGELO A, ACQUESTA A, MONETTA T. In-vitro corrosion of AZ31 magnesium alloys by using a polydopamine coating[J]. Bioactive Materials, 2019, 4(1): 71-78.
[22] 李 峰, 殷正正, 张 芬, 等. 生物医用金属表面水滑石涂层的研究进展[J]. 表面技术, 2021, 50(2): 1-12.LI Feng, YIN Zheng-zheng, ZHANG Fen, et al. Research advances of layered double hydroxides coatings on biomedical metals[J]. Surface Technology, 2021, 50(2): 1-12.
[23] 曾荣昌. “生物医用金属材料表面改性”专题序言[J]. 表面技术, 2021, 50(2): 11.ZENG Rong-chang. Preface to the special topic “surface modification of biomedical metallic materials”[J]. Surface Technology, 2021, 50(2): 11.
[24] 郑玉峰, 吴远浩. 处在变革中的医用金属材料[J]. 金属学报, 2017, 53(3): 257-297. ZHENG Yu-feng , WU Yuan-hao. Revolutionizing Metallic Biomaterials[J]. Acta Metallurgica Sinica, 2017, 53(3): 257-297.
[25] 谢 中, 羊明智, 薛静波, 等. 可降解锌合金植入材料的体内抗菌性[J]. 中国组织工程研究, 2019, 23(14): 2196-2201.XIE Zhong, YANG Ming-zhi, XUE Jing-bo, et al. Antibacterial properties of biodegradable zinc alloys in vivo[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(14): 2196-2201.
[26] 林 潇, 葛 隽, 吴水林, 等. 兼具成骨和抗感染性能的医用金属材料研究进展[J]. 金属学报, 2017, 53(10): 1284-1302.LIN Xiao, GE Jun, WU Shui-lin, et al. Advances in metallic biomaterials with both osteogenic and anti-Infection properties[J]. Acta Metallurgica Sinica, 2017, 53(10): 1284-1302.
[27] WILLIAMS D. New interests in magnesium[J]. Medical Device Technology, 2006, 17(3): 9-10.
[28] 袁广银, 牛佳林. 可降解医用镁合金在骨修复应用中的研究进展[J]. 金属学报, 2017, 53(10): 1168-1180.YUAN Guang-yin, NIU Jia-lin. Research progress of biodegradable magnesium alloys for orthopedic applications[J]. Acta Metallurgica Sinica, 2017, 53(10): 1168-1180.
[29] RODRIGUEZ-SANCHEZ J, PACHA-OLIVENZA M A, GONZALEZ-MARTIN M L. Bactericidal effect of magnesium ions over planktonic and sessile staphylococcus epidermidis and escherichia coli[J]. Materials Chemistry and Physics, 2019, 221: 342-348.
[30] YI S, BOHLEN J, HEINEMANN F, et al. Mechanical anisotropy and deep drawing behaviour of AZ31 and ZE10 magnesium alloy sheets[J]. Acta Materralia, 2010, 58(2): 592-605.
[31] XIA Dan-dong, LIU Yang, WANG Si-yi, et al. In vitro and in vivo investigation on biodegradable Mg-Li-Ca alloys for bone implant application[J]. Science China Materials, 2018: 62(2): 256-272.
[32] SEITZ J M, LUCAS A, KIRSCHNER M. Magnesium-based compression screws: A novelty in the clinical use of implants[J]. JOM, 2016, 68(4): 1177-1182.
[33] ZENG Rong-chang, QI Wei-chen, CUI Hong-zhi, et al. In vitro corrosion of as-extruded Mg-Ca alloys-the influence of Ca concentration[J]. Corrosion Science, 2015, 96: 23-31.
[34] MINARIK P, KRAL R, PESICKA J, et al. Evolution of mechanical properties of LAE442 magnesium alloy processed by extrusion and ECAP[J]. Journal of Materials Research and Technology, 2015, 4(1): 75-78.
[35] CUI Lan-yue, SUN Lu, ZENG Rong-chang, et al. In vitro degradation and biocompatibility of Mg-Li-Ca alloys—The influence of Li content[J]. Science China Materials, 2017, 61(4): 607-618.
[36] ZHANG Shao-xiang, ZHANG Xiao-nong, ZHAO Chang-li, et al. Research on an Mg-Zn alloy as a degradable biomaterial[J]. Acta Biomaterialia, 2010, 6(2): 626-640.
[37] LI Yun-cang, WEN Cui-e, MUSHAHARY DOLLY, et al. Mg-Zr-Sr alloys as biodegradable implant materials[J]. Acta Biomaterialia, 2012, 8(8): 3177-3188.
[38] BORNAPOUR M, CELIKIN M, PEKGULERYUZ M. Thermal exposure effects on the in vitro degradation and mechanical properties of Mg-Sr and Mg-Ca-Sr biodegradable implant alloys and the role of the microstructure[J]. Materials Science and Engineering C, 2015, 46: 16-24.
[39] ZHENG Yu-feng. Magnesium alloys as degradable biomaterials[M]. Boca Raton: CRC Press, 2015: 547-578.
[40] BIAN Dong, ZHOU Wei-rui, DENG Jiu-xu, et al. Development of magnesium-based biodegradable metals with dietary trace element germanium as orthopaedic implant applications[J]. Acta Biomaterialia, 2017, 64: 421-436.
[41] GIANNOUDIS P V, EINHORN T A, MARSH D. Fracture healing: The diamond concept[J]. Injury, 2007, 38: S3-S6.
[42] CUI Lan-yue, CHENG Shen-cong, LIANG Lu-xian, et al. In vitro corrosion resistance of layer-by-layer assembled polyacrylic acid multilayers induced Ca-P coating on magnesium alloy AZ31[J]. Bioactive Materials, 2020, 5(1): 153-163.
[43] ERDMANN N, ANGRISANI N, REIFENRATH J, et al. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: A comparative in vivo study in rabbits[J]. Acta Biomaterialia, 2011, 7(3): 1421-1428.
[44] ZHANG Yi-feng, XU Jian-kun, RUAN Ye-chun, et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats[J]. Nature Medicine, 2016, 22(10): 1160-1169.
[45] MUIRE P J, MANGUM L H, WENKE J C. Time course of immune response and immunomodulation during normal and delayed healing of musculoskeletal wounds[J]. Frontiers in Immunology, 2020, 11: 1056.
[46] ZHANG Lin-cai, XU Ming-zhu, HU You-dong, et al. Biofunctionization of biodegradable magnesium alloy to improve the in vitro corrosion resistance and biocompatibility[J]. Applied Surface Science, 2018, 451: 20-31.
[47] METSEMAKERS W J, MORGENSTERN M, MCNALLY M A, et al. Fracture-related infection: A consensus on definition from an international expert group[J]. Injury-international Journal of the Care of the Injured, 2018, 49(3): 505-510.
[48] 冯名城, 李 卫, 符青云, 等. 镁合金表面可降解聚合物改性涂层研发及应用进展[J]. 稀有金属材料与工程, 2021, 50(9): 3366-3374.FENG Ming-cheng, LI Wei, FU Qing-yun, et al. Progress on development and application of degradable polymer modified coating on magnesium alloy surface[J]. Rare Metal Materials and Engineering, 2021, 50(9): 3366-3374.
[49] QIN Hui, ZHAO Yao-chao, CHENG Meng-qi, et al. Anti-biofilm properties of magnesium metal via alkaline pH[J]. RSC Advances, 2015, 5(28): 21434-21444.
[50] DIZAJ S M, LOTFIPOUR F, BARZEGAR-JALALI M, et al. Antimicrobial activity of the metals and metal oxide nanoparticles[J]. Materials Science and Engineering C, 2014, 44: 278-284.
[51] COELHO C C, ARAUJO R, QUADROS P A, et al. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections[J]. Materials Science and Engineering C, 2019, 97: 529-538.
[52] BROOKS E K, AHN R, TOBIAS M E, et al. Magnesium alloy AZ91 exhibits antimicrobial properties in vitro but not in vivo[J]. Journal of Biomedical Materials Research, 2018, 106(1): 221-227.
[53] TIE D, FEYERABEND F, MULLER W D, et al. Antibacterial biodegradable Mg-Ag alloys[J]. European Cells & Materials, 2013, 25: 284-298.
[54] BRYLA K, HORKY J, KRYSTIAN M, et al. Microstructure, mechanical properties, and degradation of Mg-Ag alloy after equal-channel angular pressing[J]. Materials Science and Engineering C, 2020, 109: 110543.
[55] 刘继光, 王艳艳, 李慕勤, 等. 纯镁载银微弧氧化生物涂层的体外抗菌作用[J]. 中国体视学与图像分析, 2015, 20(2): 128-135.LIU Ji-guang, WANG Yan-yan, LI Mu-qin, et al. In vitro antimicrobial properties of Ag-containing biological coating prepared by micro-arc oxidation on pure magnesium[J]. Chinese Journal of Stereology and Image Analysis, 2015, 20(2): 128-135.
[56] 向红亮, 郭培培, 刘东福. 含Ag抗菌双相不锈钢组织及抗菌性能研究[J]. 金属学报, 2014, 50(10): 1210-1216.XIANG Hong-liang, GUO Pei-pei, LIU Dong-fu. Microstructure and antibacterial properties of Ag-bearing duplex stainless steel[J], Acta Metallurgica Sinica, 2014, 50(10): 1210-1216
[57] ZHAO Yan-bin, SHI Li-qian, JI Xiao-jing, et al. Corrosion resistance and antibacterial properties of polysiloxane modified layer-by-layer assembled self-healing coating on magnesium alloy[J]. Journal of Colloid and Interface Science, 2018, 526: 43-50.
[58] LOPERENA A P, LOPEZ A D F, BRUGNONI L I, et al. Electroformation of coatings modified with silver on magnesium alloys for biomedical applications[J]. Portugaliae Electrochimica Acta, 2020, 38(6): 351-364.
[59] ZHAO Yun, CHEN Yang-ping, WANG Wei, et al. One-step in situ synthesis of nano silver-hydrotalcite coating for enhanced antibacterial and degradation property of magnesium alloys[J]. Materials Letters, 2020, 265: 127349.
[60] SONDI I, SALOPEK-SONDI B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria[J]. Journal of Colloid and Interface Science, 2004, 275(1): 177-182.
[61] VU A A, ROBERTSON S F, KE D, et al. Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications[J]. Acta Biomater, 2019, 92: 325-335.
[62] SARRAF M, DABBAGH A, ABDUL RAZAK B, et al. Highly-ordered TiO2 nanotubes decorated with Ag2O nanoparticles for improved biofunctionality of Ti6Al4V[J]. Surface and Coatings Technology, 2018, 349: 1008-1017.
[63] WANG Peng, YUAN Yong-hui, XU Ke, et al. Biological applications of copper-containing materials[J]. Bioactive Materials, 2021, 6(4): 916-927.
[64] ESPIRITO SANTO C, LAM E W, ELOWSKY C G, et al. Bacterial killing by dry metallic copper surfaces[J]. Applied and Environmental Microbiology, 2011, 77(3): 794-802.
[65] LIU Rui, TANG Yu-long, LIU Hui, et al. Effects of combined chemical design (Cu addition) and topographical modification (SLA) of Ti-Cu/SLA for promoting osteogenic, angiogenic and antibacterial activities[J]. Journal of Materials Science & Technology, 2020, 47: 202-215.
[66] YAN Xu-dong, WAN Peng, TAN Lili, et al. Corrosion and biological performance of biodegradable magnesium alloys mediated by low copper addition and processing[J]. Materials Science and Engineering C, 2018, 93: 565-581.
[67] 周 苗, 刘楚明, 高永浩, 等. 含铜AZ31镁合金的腐蚀行为[J]. 中国有色金属学报, 2019, 29(1): 18-26.ZHOU Miao, LIU Chu-Ming, GAO Yong-hao, et al. Corrosion behavior of Cu-containing AZ31 magnesium alloy[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(1): 18-26.
[68] 关晓楠, 江静华, 陈建清, 等. 表面纳米化对铜镁合金电化学腐蚀行为的影响[J]. 中国有色金属学报, 2017, 27(3): 477-485.GUAN Xiao-nan, JIANG Jing-hua, CHEN Jian-qing, et al. Effect of surface nanocrystallization on electrochemical corrosion behaviours of Cu-Mg alloy[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(3): 477-485.
[69] YAN Xu-dong, ZHAO Ming-chun, YANG Yi, et al. Improvement of biodegradable and antibacterial properties by solution treatment and micro-arc oxidation (MAO) of a magnesium alloy with a trace of copper[J]. Corrosion Science, 2019, 156: 125-138.
[70] CHEN Jun-xiu, ZHANG Yi, IBRAHIM M, et al. In vitro degradation and antibacterial property of a copper-containing micro-arc oxidation coating on Mg-2Zn-1Gd-0.5Zr alloy[J]. Colloids Surface B Biointerfaces, 2019, 179: 77-86.
[71] XU Ying-chao, WANG Tian-xiao, GUO Yun-ting, et al. Improvements of corrosion resistance and antibacterial properties of hydroxyapatite/cupric oxide doped titania composite coatings on degradable magnesium alloys[J]. Langmuir, 2020, 36(46): 13937-13948.
[72] YANG Yu-yun, ZHENG Kai, LIANG Rui-fang, et al. Cu-releasing bioactive glass/polycaprolactone coating on Mg with antibacterial and anticorrosive properties for bone tissue engineering[J]. Biomedical Materials, 2017, 13(1): 015001.
[73] SHAO Yang, ZENG Rong-chang, LI Shuo-qi, et al. Advance in antibacterial magnesium alloys and surface coatings on magnesium alloys: A review[J]. Acta Metallurgica Sinica (English Letters), 2020, 33(5): 615-629.
[74] QIN Hui, ZHAO Yao-chao, AN Zhi-quan, et al. Enhanced antibacterial properties, biocompatibility, and corrosion resistance of degradable Mg-Nd-Zn-Zr alloy[J]. Biomaterials, 2015, 53: 211-220.
[75] 张 鹏, 周立波, 张慧明, 等. 镁合金微弧氧化掺杂Zn-HA复合涂层细胞相容性与抗菌性[J]. 口腔医学研究, 2019, 35(10): 1001-1004.ZHANG Peng, ZHOU Li-bo, ZHANG Hui-ming, et al. Cell compatibility and antibacterial properties of magnesium alloy after ultrasonic microarc oxidation-doped Zn-HA silane-phytic acid treatment[J]. Journal of Oral Science Research, 2019, 35(10): 1001-1004.
[76] 邹玉红, 王庆昭, 曾荣昌, 等. 载锌蒙脱石抗菌中间体的制备及镁合金表面的应用研究[J]. 功能材料, 2016, 47(7): 7121-7129. ZOU Yu-hong, WANG Qing-zhao, ZENG Rong-chang, et al. Synthesis and properties of Zn-MMT antimicrobial composites and application on the surface of magnesium alloy[J]. Journal of Functional Materials, 2016, 47(7): 7121-7129.
[77] YANG Guang-zheng. Enhancing corrosion resistance, osteoinduction, and antibacterial properties by Zn/Sr additional surface modification of magnesium alloy[J]. ACS Biomaterials Science & Engineering, 2018, 4: 4289-4298.
[78] ZOU Yu-hong, WANG Jian, CUI Lan-yue, et al. Corrosion resistance and antibacterial activity of zinc-loaded montmorillonite coatings on biodegradable magnesium alloy AZ31[J]. Acta Biomaterialia, 2019, 98: 196-214.
[79] ZHOU Jie, LI Ke, WANG Biao, et al. Nano-hydroxyapatite/ ZnO coating prepared on a biodegradable Mg-Zn-Ca bulk metallic glass by one-step hydrothermal method in acid situation[J]. Ceramics International, 2020, 46(5): 6958-6964.
[80] SUN Jin-e, CAI Shu, LI Qian-qian, et al. UV-irradiation induced biological activity and antibacterial activity of ZnO coated magnesium alloy[J]. Material Science and Engineering C, 2020, 114: 110997.
[81] GUO Yun-ting, JIA Si-qi, QIAO Lu, et al. A multifunctional polypyrrole/zinc oxide composite coating on biodegradable magnesium alloys for orthopedic implants[J]. Colloids and Surface B, 2020, 194: 111186.
[82] MOUSA H M, ABDAL-HAY A, BARTNIKOWSKI M, et al. A multifunctional zinc oxide/poly(lactic acid) nanocomposite layer coated on magnesium alloys for controlled degradation and antibacterial function[J]. ACS Biomaterials Science & Engineering, 2018, 4(6): 2169-2180.
[83] ZHOU Wen-hao, YAN Jiang-long, LI Yang-yang, et al. Based on the synergistic effect of Mg(2+) and antibacterial peptides to improve the corrosion resistance, antibacterial ability and osteogenic activity of magnesium-based degradable metals[J]. Biomaterials Science, 2021, 9(3): 807-825.
[84] TIAN J, SHEN S, ZHOU C, et al. Investigation of the antimicrobial activity and biocompatibility of magnesium alloy coated with HA and antimicrobial peptide[J]. Journal of Materials Science, 2015, 26(2): 66.
[85] WANG T, NI G, FURUSHIMA T, et al. Mg alloy surface immobilised with caerin peptides acquires enhanced antibacterial ability and putatively improved corrosion resistance[J]. Materials Science and Engineering C, 2021, 121: 111819.
[86] LIU Ping, HAO Yan-sha, DING Yao, et al. Fabrication of enzyme-responsive composite coating for the design of antibacterial surface[J]. Journal of Materials Science- Materials in Medicine, 2018, 29(10): 160.
[87] DE F K J, KUMMER N, REN Q, et al. Assembly of cellulose nanocrystal-lysozyme composite films with varied lysozyme morphology[J]. Biomacromolecules, 2020, 21(12): 5139-5147.
[88] DONG Hong-ling, ZHU Choa-yang, CHEN Jingyi, et al. Antibacterial activity of stenotrophomonas maltophilia endolysin P28 against both gram-positive and gram-negative bacteria[J]. Frontiers in Microbiology, 2015, 6: 1299.
[89] ZHAO Xiao-wei, WU Hong-yu, LU Hai-rong, et al. LAMP: A database linking antimicrobial peptides[J]. Plos One, 2013, 8(6): e66557.
[90] AGARWAL S, RIFFAULT M, HOEY D, et al. Biomimetic hyaluronic acid-lysozyme composite coating on AZ31 Mg alloy with combined antibacterial and osteoinductive activities[J]. ACS Biomaterials Science & Engineering, 2017, 3(12): 3244-3253.
[91] CUI Lan-yue, GAO Ling, ZHANG Jing-chao, et al. In vitro corrosion resistance, antibacterial activity and cytocompatibility of a layer-by-layer assembled DNA coating on magnesium alloy[J]. Journal of Magnesium and Alloys, 2020, 9(1): 266-280.
[92] CUI Lan-yue, XU Ji, LU Na, et al. In vitro corrosion resistance and antibacterial properties of layer-by-layer assembled chitosan/poly-L-glutamic acid coating on AZ31 magnesium alloys[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1081-1086.
[93] YU Chi, CUI Lan-yue, ZHOU Yong-feng, et al. Self- degradation of micro-arc oxidation/chitosan composite coating on Mg-4Li-1Ca alloy[J]. Surface and Coatings Technology, 2018, 344: 1-11.
[94] GAO Fan, HU You-dong, GONG Zhi-hao, et al. Fabrication of chitosan/heparinized graphene oxide multilayer coating to improve corrosion resistance and biocompatibility of magnesium alloys[J]. Materials Science and Engineering C, 2019, 104: 109947.
[95] ALAEI M, ATAPOUR M, LABBAF S. Electrophoretic deposition of chitosan-bioactive glass nanocomposite coatings on AZ91 Mg alloy for biomedical applications[J]. Progress in Organic Coatings, 2020, 147: 105803.
[96] BAHATIBIEKE A, QIN H, CUI T, et al. In vivo and in simulated body fluid degradation behavior and biocompatibility evaluation of anodic oxidation-silane- chitosan-coated Mg-4.0Zn-0.8Sr alloy for bone application[J]. Materials Science and Engineering C, 2021, 120: 111771.
[97] WIDSTEN P, HEATHCOTE C, KANDELBAUER A, et al. Enzymatic surface functionalisation of lignocellulosic materials with tannins for enhancing antibacterial properties[J]. Process Biochemistry, 2010, 45(7): 1072-1081.
[98] SUN Jia-di, ZHU Ye, MENG Long, et al. Electrophoretic deposition of colloidal particles on Mg with cytocompatibility, antibacterial performance, and corrosion resistance[J]. Acta Biomaterialia, 2016, 45: 387-398.
[99] EJIMA H, RICHARDSON J J, LIANG K, et al. One-step assembly of coordination complexes for versatile film and particle engineering[J]. Science, 2013, 341(6142): 154-157.
[100] FACCHI S P, DE OLIVEIRA A C, BEZERRA E O T, et al. Polycationic condensed tannin/polysaccharide-based polyelectrolyte multilayers prevent microbial adhesion and proliferation[J]. European Polymer Journal, 2020, 130: 109677.
[101] CUI Lan-yue, LIU Han-peng, XUE Kui, et al. In vitro corrosion and antibacterial performance of micro-arc oxidation coating on AZ31 magnesium alloy: effects of tannic acid[J]. Journal of the Electrochemical Society, 2018, 165(11): C821-C829.
[102] WANG Pei, LIU Jing, LUO Xu-Jiang, et al. A tannic acid-modified fluoride pre-treated Mg-Zn-Y-Nd alloy with antioxidant and platelet-repellent functionalities for vascular stent application[J]. Journal of Materials Chemistry B, 2019, 7(46): 7314-7325.
[103] ZHU Bo-wu, WANG Shi-meng, WANG Lei, et al. Preparation of hydroxyapatite/tannic acid coating to enhance the corrosion resistance and cytocompatibility of AZ31 magnesium alloys[J]. Coatings, 2017, 7(7): 105.
[104] GHOSH S, DUTTA S, SARKAR A, et al. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency[J]. Colloids and Surfaces B, 2021, 197: 111404.
[105] ZAHEDIPOUR F, HOSSEINI S A, SATHYAPALAN T, et al. Potential effects of curcumin in the treatment of COVID-19 infection[J]. Phytotherapy Research, 2020, 34(11): 2911-2920.
[106] BANEZ M J, GELUZ M I, CHANDRA A, et al. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health[J]. Nutrition Research, 2020, 78: 11-26.
[107] SHABBIR U, RUBAB M, TYAGI A, et al. Curcumin and its derivatives as theranostic agents in Alzheimer’s disease: The implication of nanotechnology[J]. International Journal of Molecular Sciences, 2021, 22(1): 196.
[108] ZHENG Dan-tong, HUANG Chong-xing, HUANG Hao-he, et al. Antibacterial mechanism of curcumin: A review[J]. Chemistry & Biodiversity, 2020, 17(8): e2000171.
[109] HUANG Guo-huan, YAN Yu-ping, XU Dan-xia, et al. Curcumin-loaded nanoMOFs@CMFP: A biological preserving paste with antibacterial properties and long-acting, controllable release[J]. Food Chemistry, 2021, 337: 127987.
[110] LI Bo, HUANG Run, YE Jing, et al. A self-healing coating containing curcumin for osteoimmunomodulation to ameliorate osseointegration[J]. Chemical Engineering Journal, 2021, 403: 126323.
[111] ZAHIRI M, KHANMOHAMMADI M, GOODARZI A, et al. Encapsulation of curcumin loaded chitosan nanoparticle within poly (epsilon-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution[J]. International Journal of Biological Macromolecules, 2020, 153: 1241-1250.
[112] TAKAHASHI O, CAI Z, TODA M, et al. Appearance of antibacterial activity of oxacillin against methicillin resistant Staphylococcus aureus (MRSA) in the presence of catechin[J]. The Journal of the Japanese Association for Infectious Diseases, 1995, 69(10): 1126-1134.
[113] MARCHESE A, COPPO E, SOBOLEV A P, et al. Influence of in vitro simulated gastroduodenal digestion on the antibacterial activity, metabolic profiling and polyphenols content of green tea (Camellia sinensis)[J]. Food Research International, 2014, 63: 182-191.
[114] ZHANG Bo, YAO Rui-juan, LI Lin-hua, et al. Green tea polyphenol induced Mg(2+)-rich multilayer conversion coating: toward enhanced corrosion resistance and promoted in situ endothelialization of AZ31 for potential cardiovascular applications[J]. ACS Applied Materials & Interfaces, 2019, 11(44): 41165-41177.
[115] SUN Ming-hao, ZHU Chun-e, LONG Jie-yu, et al. PLGA microsphere-based composite hydrogel for dual delivery of ciprofloxacin and ginsenoside Rh2 to treat Staphylococcus aureus-induced skin infections[J]. Drug Delivery, 2020, 27(1): 632-641.
[116] KORZENIOWSKA A, STRZEMPEK W, MAKOWSKI W, et al. Incorporation and release of a model drug, ciprofloxacin, from non-modified SBA-15 molecular sieves with different pore sizes[J]. Microporous and Mesoporous Materials, 2020, 294: 109903.
[117] HE Li-jun, HAO Jian-chao, DAI Lin, et al. Layer-by-layer assembly of gentamicin-based antibacterial multilayers on Ti alloy[J]. Materials Letters, 2020, 261: 127001.
[118] BALLARRE J, AYDEMIR T, LIVERANI L, et al. Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: Improving early stage performance of titanium implants[J]. Surface and Coatings Technology, 2020, 381: 125138.
[119] ZHAO Yan-bin, CHEN Xue-yang, LI Shou-qi, et al. Corrosion resistance and drug release profile of gentamicin-loaded polyelectrolyte multilayers on magnesium alloys: Effects of heat treatment[J]. Journal of Colloid and Interface Science, 2019, 547: 309-317.
[120] DU Min-ting, HUANG Lin-lin, PENG Meng-ke, et al. Preparation of vancomycin-loaded alginate hydrogel coating on magnesium alloy with enhanced anticorrosion and antibacterial properties[J]. Thin Solid Films, 2020, 693: 137679.
[121] ZHANG Teng, ZHOU Wen-hao, JIA Zhao-jun, et al. Polydopamine-assisted functionalization of heparin and vancomycin onto microarc-oxidized 3D printed porous Ti6Al4V for improved hemocompatibility, osteogenic and anti-infection potencies[J]. Science China Materials, 2018, 61(4): 579-592.
[122] HEAKAL F E-T, BAKRY A M. Role of amoxicillin in enhancing AZ31 alloy degradation resistance and its monitoring using nano-Pd electrochemical sensor[J]. Materials Chemistry and Physics, 2019, 234: 224-236.
[123] LIANG Yu-ting, ZHU Hong-xiang, WANG Lei, et al. Biocompatible smart cellulose nanofibres for sustained drug release via pH and temperature dual-responsive mechanism[J]. Carbohydr Polym, 2020, 249: 116876.
[124] ARAFA M G, MOUSA H A, AFIFI N N. Preparation of PLGA-chitosan based nanocarriers for enhancing antibacterial effect of ciprofloxacin in root canal infection[J]. Drug Delivery, 2019, 27(1): 26-39.
[125] ALLEN T M, CULLIS P R. Liposomal drug delivery systems: from concept to clinical applications[J]. Advance Drug Delivery Reviews, 2013, 65(1): 36-48.
[126] KOWALCZYK D, PYTKA M, SZYMANOWSKA U, et al. Release kinetics and antibacterial activity of potassium salts of iso-α-acids loaded into the films based on gelatin, carboxymethyl cellulose and their blends[J]. Food Hydrocolloids, 2020, 109: 106104.
[127] WANG Yin-zhu, HAO Nan, FENG Qiu-mei, et al. A ratiometric electrochemiluminescence detection for cancer cells using g-C3N4 nanosheets and Ag-PAMAM-luminol nanocomposites[J]. Biosens Bioelectron, 2016, 77: 76-82.
[128] MU Hai-bo, TANG Jiang-jiang, LIU Qian-jin, et al. Potent antibacterial nanoparticles against biofilm and intracellular bacteria[J]. Scientific Reports, 2016, 6: 18877.
[129] CHAUDHARI P R, MASURKAR S A, SHIDORE V B, et al. Effect of biosynthesized silver nanoparticles on staphylococcus aureus biofilm quenching and prevention of biofilm formation[J]. Nano-Micro Letters, 2012, 4(1): 34-39.
[130] ESCOBAR A, MUZZIO N E, ANDREOZZI P, et al. Antibacterial layer-by-layer films of poly(acrylic acid)– gentamicin complexes with a combined burst and sustainable release of gentamicin[J]. Advanced Materials Interfaces, 2019, 6(22): 1901373.
[131] ZHAO Yan-bin, LIU Han-peng, LI CHang-yang, et al. Corrosion resistance and adhesion strength of a spin-assisted layer-by-layer assembled coating on AZ31 magnesium alloy[J]. Applied Surface Science, 2018, 434: 787-795.
[132] HE Li-jun, SHAO Yang, LI Shuo-qi, et al. Advances in layer-by-layer self-assembled coatings upon biodegradable magnesium alloys[J]. Science China Materials, 2021, 64(9): 2093-2106.
[133] JI Xiao-jing, GAO Ling, LIU Jia-cheng, et al. Corrosion resistance and antibacterial properties of hydroxyapatite coating induced by gentamicin-loaded polymeric multilayers on magnesium alloys[J]. Colloids and Surfaces B: Biointerfaces, 2019, 179: 429-436.
[134] BAKHSHESHI-RAD H R, HADISI Z, HAMZAH E, et al. Drug delivery and cytocompatibility of ciprofloxacin loaded gelatin nanofibers-coated Mg alloy[J]. Materials Letters, 2017, 207: 179-182.
[135] QI H F, HEISE S, ZHOU J C, et al. Electrophoretic deposition of bioadaptive drug delivery coatings on magnesium alloy for bone repair[J]. ACS Appl Mater Interfaces, 2019, 11(8): 8625-8634.
[136] SUN Jia-di, ZHU Ye, MENG Long, et al. Controlled release and corrosion protection by self-assembled colloidal particles electrodeposited onto magnesium alloys[J]. Journal of Materials Chemistry B, 2015, 3(8): 1667-1676.
[137] XU Xin-hua, LU Ping, GUO Mei-qing, et al. Cross-linked gelatin/nanoparticles composite coating on micro-arc oxidation film for corrosion and drug release[J]. Applied Surface Science, 2010, 256(8): 2367-2371.
[138] LEE W S, PARK M, KIM M H, et al. Nanoparticle coating on the silane-modified surface of magnesium for local drug delivery and controlled corrosion[J]. Journal of Biomaterials Applications, 2016, 30(6): 651-661.
[139] JIANG Yan-an, WANG Bi, JIA Zhan-rong, et al. Polydopamine mediated assembly of hydroxyapatite nanoparticles and bone morphogenetic protein-2 on magnesium alloys for enhanced corrosion resistance and bone regeneration[J]. Journal of Biomedical Materials Research Part A, 2017, 105(10): 2750-2761.
[140] SONG Jiang-feng, SHE Jia, CHEN Dao-lun, et al. Latest research advances on magnesium and magnesium alloys worldwide[J]. Journal of Magnesium and Alloys, 2020, 8(1): 1-41.
[141] LI Jun, LIU Xiang-mei, ZHOU Zi-ao, et al. Lysozyme- assisted photothermal eradication of methicillin-resistant Staphylococcus aureus infection and accelerated tissue repair with natural melanosome nanostructures[J]. ACS Nano, 2019, 13(10): 11153-11167.
[142] SU Kun, TAN Lei, LIU Xiang-mei, et al. Rapid photo-sonotherapy for clinical treatment of bacterial infected bone implants by creating oxygen deficiency using sulfur doping[J]. ACS Nano, 2020, 14(2): 2077-2089.
[143] HARADA Y, OGAWA K, IRIE Y, et al. Ultrasound activation of TiO2 in melanoma tumors[J]. Journal of Controlled Release, 2011, 149(2): 190-195.
[144] LI Mu, LIU Xiang-mie, TAN Lei, et al. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel[J]. Biomaterials Science, 2018, 6(8): 2110-2121.
[145] FENG Zi-zhou, LIU Xiang-mei, TAN Lei, et al. Electrophoretic deposited stable chitosan@MoS2 coating with rapid in situ bacteria-killing ability under dual-light irradiation[J]. Small, 2018, 14(21): 1704347.
[146] MU W Y, AKROFI R, CHEN Q Y. Near-infrared- driven Au-decorated polymer-metal protein microfibers with bacterial filtration ability for use in photothermal sterilization[J]. Chemical Engineering Journal, 2020, 388: 124236.
[147] LI Yuan, LIU Xiang-mei, TAN Lei, et al. Rapid sterilization and accelerated wound healing using Zn2+ and graphene oxide modified g-C3N4 under dual light irradiation[J]. Advanced Functional Materials, 2018, 28(30): 1800299.
[148] HUANG Lei, LI Jun, YUAN Wei, et al. Near-infrared light controlled fast self-healing protective coating on magnesium alloy[J]. Corrosion Science, 2020, 163: 108257.
[149] WANG Hong-yu, CHANG Jin-jie, SHI Ming-wan, et al. A dual-targeted organic photothermal agent for enhanced photothermal therapy[J]. Angewandte Chem-International Edition, 2019, 58(4): 1057-1061.
[150] LIU Yan-lan, AI Ke-long, LU Le-hui. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields[J]. Chemical Reviews, 2014, 114(9): 5057-5115.
[151] TIAN Jia, XIA Lei, WU Jian, et al. Linear alternating supramolecular photosensitizer for enhanced photodynamic therapy[J]. ACS Applied Materials & Interfaces, 2020, 12(29): 32352-32359.
[152] ZENG Jin-feng, YANG Wen-di, SHI Dong-jian, et al. Porphyrin derivative conjugated with gold nanoparticles for dual-modality photodynamic and photothermal therapies in vitro[J]. ACS Biomaterials Science & Engineering, 2018, 4(3): 963-972.
[153] LOCATELLI E, MATTEINI P, SASDELLI F, et al. Surface chemistry and entrapment of magnesium nanoparticles into polymeric micelles: A highly biocompatible tool for photothermal therapy[J]. Chemical Communications, 2014, 50(58): 7783-7786.
[154] CHEN Mi, WANG Min, LI Bo, et al. Visual and antibacterial magnesium implants with low biocorrosion and bioactive surface for in vivo tracking and treating MRSA infection[J]. Chemical Engineering Journal, 2021, 417: 129198.
[155] WANG Xin, YAN Hong-guang, HANG Rui-qiang, et al. Enhanced anticorrosive and antibacterial performances of silver nanoparticles/polyethyleneimine/MAO composite coating on magnesium alloys[J]. Journal of Materials Research and Technology, 2021, 11: 2354-2364.
[156] CUI Lan-yue, ZENG Rong-chang, ZHU Xiao-xiao, et al. Corrosion resistance of biodegradable polymeric layer-by-layer coatings on magnesium alloy AZ31[J]. Frontiers of Materials Science, 2016, 10(2): 134-146.
[157] CUI Lan-yue, ZENG Rong-chang, LI SHuo-Qi, et al. Corrosion resistance of layer-by-layer assembled polyvinylpyrrolidone/polyacrylic acid and amorphous silica films on AZ31 magnesium alloys[J]. RSC Advances, 2016, 6(68): 63107-63116.
[158] OSTROWSKI N, LEE B, ENICK N, et al. Corrosion protection and improved cytocompatibility of biodegradable polymeric layer-by-layer coatings on AZ31 magnesium alloys[J]. Acta Biomaterialia, 2013, 9(10): 8704-8713.
[159] KUNJUKUNJU S, ROY A, RAMANATHAN M, et al. A layer-by-layer approach to natural polymer-derived bioactive coatings on magnesium alloys[J]. Acta Biomaterialia, 2013, 9(10): 8690-8703.
[160] ZHANG Xiao-dan, YI Jin-hong, ZHAO Guo-wei, et al. Layer-by-layer assembly of silver nanoparticles embedded polyelectrolyte multilayer on magnesium alloy with enhanced antibacterial property[J]. Surface and Coatings Technology, 2016, 286: 103-112.
[161] DAROONPARVAR M, MAT YAJID M A, KUMAR GUPTA R, et al. Antibacterial activities and corrosion behavior of novel PEO/nanostructured ZrO2 coating on Mg alloy[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(8): 1571-1581.
[162] 李慕勤, 张爱琴, 彭书浩, 等. 镁合金表面超声微弧氧化载氟生物涂层耐磨性和耐蚀性[J]. 表面技术, 2017, 46(3): 40-46.LI Mu-qin, ZHANG Ai-qin, PENG Shu-hao, et al. Wear and corrosion resistance of ultrasound micro-arc oxidized fluorine-carrying biological coating on surface of magnesium alloy[J]. Surface Technology, 2017, 46(3): 40-46.
[163] ZENG Rong-chang, LIU Li-jun, LUO Kai-jie, et al. In vitro corrosion and antibacterial properties of layer-by-layer assembled GS/PSS coating on AZ31 magnesium alloys[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4028-4039.
[164] JI Xiao-jing, GAO Ling, LIU Jia-cheng, et al. Corrosion resistance and antibacterial activity of hydroxyapatite coating induced by ciprofloxacin-loaded polymeric multilayers on magnesium alloy[J]. Progress in Organic Coatings, 2019, 135: 465-474.
[165] ZHAO Yun, CHEN Yang-ping, WANG Wei, et al. One-step in situ synthesis of nano silver-hydrotalcite coating for enhanced antibacterial and degradation property of magnesium alloys[J]. Materials Letters, 2020, 265: 127349.
CUI Lan-yue1, XUE Kui1, LI Shuo-qi1, ZOU Yu-hong2, ZHANG Fen1, LIU Cheng-bao1, ZENG Rong-chang1
(1. College of Materials Science and Engineering, Shandong University of Science and Technology, Qingdao 266590, China;
2. College of Chemical and Biological Engineering, Shandong University of Science and Technology, Qingdao 266590, China)
Abstract: In recent years, the application of biodegradable implant materials has a fast development. Magnesium (Mg) and its alloys have become a new generation of biodegradable metal materials with broad prospects due to their good biocompatibility and biodegradability. However, the contradiction between the antibacterial properties, resulted from the releasing of Mg2+ ions and the alkalization of the surrounding environment in the process of rapid corrosion, and the long-term mechanical properties as the bone implants needs to be resolved urgently. This paper summarized the research progress of various metals and its oxides, biologically active substances, natural antibacterial substances and photothermal and photodynamic therapy on antibacterial Mg alloys and antibacterial coatings on the surface of Mg alloys. The preparation methods of various corrosion-resistant and antibacterial coatings on the surface of Mg alloys were studied, and the influence of different antibacterial carriers on the corrosion resistance and antibacterial activity of Mg alloys was discussed. The results show that the evolution of antibacterial Mg alloys and antibacterial coatings on the surface of Mg alloys are gradually matured, but there still are some problems need to be solved. Finally, the way forward of the antibacterial coating on the surface of biomedical degradable Mg alloys is elaborated.
Key words: magnesium alloys; antibacterial property; coating; biomaterials
Foundation item: Project(5210010178) supported by the National Natural Science Foundation of China; Project(ZR2020QE009) supported by the Natural Science Foundation of Shandong Province, China; Project(0104060541112) supported by the “Elite Plan” Foundation of Shandong University of Science and Technology, China; Project(01040125219) supported by the Research Start-up Foundation of Shandong University of Science and Technology, China
Received date: 2021-09-08; Accepted date: 2021-10-22
Corresponding author: ZENG Rong-chang; Tel: +86-18754280969; E-mail: rczeng@foxmail.com
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(5210010178);山东省自然科学基金资助项目(ZR2020QE009);山东科技大学“菁英计划”资助项目(0104060541112);山东科技大学材料学院科研启动基金资助项目(01040125219)
收稿日期:2021-09-08;修订日期:2021-10-22
通信作者:曾荣昌,教授,博士;电话:18754280969;E-mail:rczeng@foxmail.com
摘 要:近年来,生物可降解植入材料的应用发展迅速,其中镁及合金因其具有良好的生物相容性与生物可降解性,成为新一代具有广阔发展前景的生物医用可降解金属材料,但存在镁合金本身由于快速腐蚀释放镁离子使周围环境碱化而具有的抗菌性和骨植入物需要长期保持力学性能之间的矛盾。本文总结了多种类型的金属及其氧化物、生物活性物质、天然抗菌物质、光热、光动力疗法在抗菌镁合金及表面抗菌涂层领域的研究进展,研究多种镁合金表面耐蚀抗菌涂层的制备方法,重点讨论了不同抗菌载体对镁合金耐蚀抗菌性的影响。结果表明:现阶段抗菌镁合金设计、镁合金抗菌耐蚀涂层制备的研究逐渐成熟,但也存在一些亟需解决的问题。同时,综合阐述了生物医用可降解镁合金表面抗菌涂层未来的发展方向。