

朱明飞1,王云燕1, 2,张文超1, 2,罗永健2,孙竹梅1, 3
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083;
3. 中北大学 环境与安全工程学院,太原 030051)
电容去离子技术(Capacitive deionization,CDI)是一种新兴的脱盐技术,通过在电极两端施加外加电场除去水中带电离子或分子,由于其具有低能耗和可持续性的特点而备受关注。储能电池领域的迅猛发展实现了阳离子储存从双电层作用为代表的碳材料到法拉第电极材料的跨越。相较于传统碳材料,法拉第材料的脱盐性能有了显著提升。目前,CDI中针对阴离子,特别是氟离子,高效去除的电极材料研究较少。本文从CDI装置构型的发展演变出发,系统梳理了CDI中关于除氟电极材料的分类,对比不同类型除氟电极材料的特点,并总结氟离子的去除机理以及氟离子电池领域电极材料的研究进展,为CDI除氟电极与装置构型的设计开发提供了理论基础和研究思路。
电容去离子技术;装置构型;除氟电极材料;除氟机理;氟离子电池;
文章编号:1004-0609(2021)-11-3362-18 中图分类号:X703 文献标志码:A
引文格式:朱明飞, 王云燕, 张文超, 等. 电容去离子装置构型演变及除氟电极研究进展[J]. 中国有色金属学报, 2021, 31(11): 3362-3379. DOI: 10.11817/j.ysxb.1004.0609.2021-42258
ZHU Ming-fei, WANG Yun-yan, ZHANG Wen-chao, et al. Evolution of capacitive deionization devices configuration and research progress in fluoride ion removal electrodes[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(11): 3362-3379. DOI: 10.11817/j.ysxb.1004.0609.2021-42258
淡水资源是社会经济发展的核心要素。然而,随着世界人口的快速增长以及城市化进程加速,加之工业化带来的普遍水环境污染,全球对清洁淡水资源的需求不断增加[1-3]。因此,清洁淡水资源获取已成为人类经济社会可持续发展的重要议题。在许多水污染物中,氟化物是一种严重和难以处理的问题。氟离子(F–)半径小,配体作用强,容易在土壤、岩石、空气和植物中形成大量不同的有机和无机化合物。其中一些化合物在水中具有高度可溶性,因此氟化物通常以完全电离的离子形式存在于表面和地下水中[4]。在许多国家,氟化物浓度的增加主要是氟化物废水排放造成的。这些废水通常来自磷肥制造厂、玻璃和陶瓷制造工业、有色冶炼、钢铁生产、铀浓缩设施、燃煤发电厂、铍提取厂、炼油厂、光伏[5]以及太阳能电池行业等[6]。流行病学研究表明,饮用水是每日摄入氟化物的主要来源,饮用水中的氟化物浓度大于1.5 mg/L可导致生殖和免疫缺陷、牙科和骨骼氟病及其他氟中毒[6]。除饮用水外,氟化物还可以通过食品、工业接触、药品、化妆品等进入人体。因此,发展高效、经济、可持续的含氟废水处理技术是长期以来全球科学界面临的一个关键问题。
目前,去除水中氟化物的方法有沉淀法[7-8]、膜法[9-10]、吸附法[11-14]和电絮凝法[15-16]。沉淀法一般用于高氟废水的预处理,操作简单,应用最为广泛,但其处理效果取决于废水pH值和共存离子,经处理后的出水水质不稳定,形成高含水率的污泥,处理不当会造成二次污染[17]。膜技术由于膜材料本身成本高,并且其会造成高的接触电阻,限制了该技术的大规模应用。吸附法受到吸附材料的限制,如容量低、工作酸碱度范围窄、机械强度差、回收再生难及选择性差等缺点[18-19]。电容去离子技术(CDI)是21世纪新兴的水处理技术[1, 20-21],又名电吸附脱盐技术,是基于双电层电容或法拉第电容原理的一种电吸附脱盐技术,其具有能耗低、环境友好及操作方便[1, 22-25]等优势,已成为研究热点,在含氟废水处理方面也引起了人们的兴趣和关注,并且被认为是一种新型除氟技术[26-29]。
CDI单元模块由一对电极(碳电极或法拉第电极)组成,施加一外加电势时,电极处于充电状态(见图1(a))。通常情况下,电池电压在 0.8~2.0 V 之间。水中的离子迁移进入多孔碳电极的双电层(Electrical double layer,EDL),或通过法拉第反应进入电极材料中,直至达到平衡,这一步骤被称为电吸附;随后,当电势反转或外部电源短路时发生解吸,离子离开电极从而电极获得再生,能量也因此获得回收。传统的电容脱盐技术以碳电极为主,如碳气凝胶[30-31]、活性炭[32-35]、活性炭纤维[36-37],及诸如碳化物衍生碳[38-41]、碳纳米管[42-44]、石墨烯[45-46]等新型碳材料。由于碳材料通过表面双电层作用存储电荷,其电荷存储容量低[47],实际脱盐能力一般约为10 mg/g,并且碳材料对于目标离子的选择性低,因此,以碳基材料为主的传统CDI技术难以应用于实际海水淡化及工业废水的处理。
最近研究发现,随着电池领域储能电极材料的迅速发展,电池中常用的电荷转移材料,又称为法拉第电极材料,在低摩尔浓度或高摩尔浓度电解液中均显示出相较于碳基材料大得多的电荷储存容量[48],并具有低能耗[49]及选择性[50-51]的优势,从而引起了研究者们的高度关注[52-54]。法拉第材料主要是通过界面发生的可逆电荷转移过程实现离子的嵌入或脱出,从而达到脱盐和再生的目的,由于其具有较高的电荷存储量,目前在分离纯化、资源回收及重金属离子去除等方面已经实现了应用。得益于储能电极材料的迅速发展,目前法拉第电极材料的研究主要集中于阳离子可逆嵌入脱出电极。相较于阴离子,与其核外电子数相同的阳离子具有较小的离子半径,故阴离子的电荷与原子半径的比值较小,其表现出较弱的静电结合力,同时阴离子在低pH条件下容易与质子结合,阴离子的赋存形态受体系酸碱度影响较大[55],因此,阴离子分离更具挑战性。如图1(b)所示,目前,有关阴离子特异性法拉第电极材料的设计开发非常缺乏,仍以碳基材料为主。
1 CDI装置构型的发展演变
CDI的研究最早可以追溯到20世纪60年代末,当时它被称为“电化学去除矿物质” (Electrochemical demineralization)[56],随后的40年中,这种技术进展缓慢[1]。然而,伴随着近十年各种CDI装置构型的不断涌现,该领域激起了广大研究者的兴趣,这致使CDI领域取得了飞速发展,如图2所示。BLAIR等[57]于1960年首次报道了电容脱盐技术,并且建立了间流式CDI (Flow-by CDI)模式。传统的CDI装置其两端的电极均采用碳材料,工作原理与超级电容器相似。由于该模式便于组装、操作方便,一直被广泛使用,间流式CDI模式也是电吸附除氟研究中使用最普遍的装置构型。但由于碳材料表面同离子效应存在和副反应的发生[58-59],传统以碳基材料作为CDI电极的吸附模式存在电荷效率低及离子选择性差等问题。
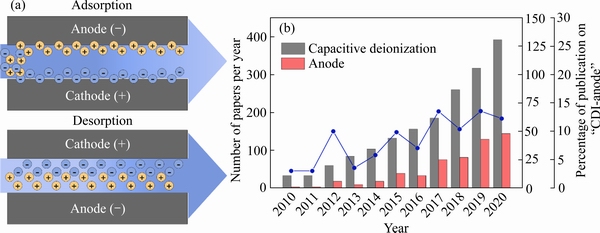
图1 电容去离子的原理示意图以及2010—2020年电容去离子领域发文量及阳极材料的文章比例
Fig. 1 Schematic diagram of prototype of capacitive deionization(a) and numbers of papers with theme of “capacitive deionization” and percentage of papers concerning “anode” materials at 2010—2020(b)
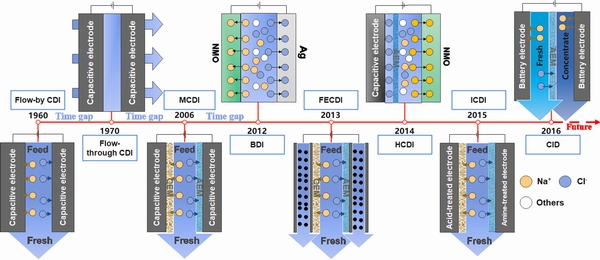
图2 CDI装置构型发展演变示意图
Fig. 2 Schematic diagram of evolution of CDI configurations
通过改进间流式CDI装置,开发了Flow-through CDI装置,其进水方式是以穿透电极的形式,并与外加电场方向平行[60-62],其电极结构如图2所示。有关Flow-through CDI的研究最早可追溯到1970年,该项工作主要是由JOHNSON等[63]开发,与间流式CDI相比,穿透式CDI电极间隔宽度可以实现最小化,通常为200~500 μm,这使得装置更加紧凑,从而提高了脱盐率,但其水流阻力较大,且正极易被氧化。时至今日,穿透式电容脱盐技术进展甚微。
为了降低同离子效应的影响,提高碳材料的电荷效率和离子去除能力,2006年,LEE等[64]通过在电极前面加入离子交换膜,开发了膜电容去离子(Membrane capacitive deionization,MCDI)的新型脱盐技术。MCDI在电吸附选择性去除氟离子方面已有部分研究,但由于碳材料本身的低吸附容量及商业离子交换膜的高接触电阻和高成本,其处理高盐度水的能耗及成本远高于反渗透技术,电极和膜材料结构以及性能优化仍将是该领域研究的重要方向。
对于传统的CDI、MCDI装置,由于电极是固定模式,因此电极的吸附效率容易衰减且难以再 生[65],其脱盐过程以间歇方式运行,脱盐能力很大程度上受到固定电极的限制,这使得传统的CDI、MCDI装置仅适用于处理微咸水或低盐度水。鉴于上述局限,JEON等[66]于2013年率先开发了基于流动电极的CDI装置结构,称为流动电极电容去离子(Electrical double-layer,FECDI),即采用流动电极代替传统的固定电极用于电吸附废水中的离子。与传统CDI、MCDI装置中使用的静态电极相比,FECDI系统可以同时实现电极的脱盐和再生[67],使得FECDI装置的脱盐过程可以持续进行。由于流动电极具有连续性的离子吸附能力[66, 68],因此其在高浓度NaCl溶液中表现出优异的脱盐效率。流动电极中碳粒子的饱和度是流动电极电容脱盐系统的一个限制因素,可通过调整电极流动速率和电极流动的通道尺寸控制碳粒子的饱和度。
崔屹等[69]于2012年将法拉第反应机制引入CDI领域,首次提出脱盐电池(Desalination battery)的概念。与传统的基于双电层电容效应脱盐原理不同,脱盐电池由Ag负极和Na2Mn5O10正极组成,通过化学键合捕捉离子。电吸附过程如式(1)和(2)所示,电极再生过程如式(3)和(4)所示。体系的总反应过程如式(5)所示。
电化学脱盐过程:
Ag+Cl-→AgCl+e (1)
5MnO2+2Na++2e→Na2Mn5O10 (2)
电极再生过程:
AgCl+e→Ag+Cl- (3)
Na2Mn5O10-2e→5MnO2+2Na+ (4)
总反应方程式:
5MnO2+2Ag+2NaCl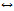 Na2Mn5O10+2AgCl (5)
Na2Mn5O10+2AgCl (5)
采用这种新型电池设计,可实现低能耗海水淡化,即每立方米除盐25%需要消耗的电能为0.29 kW·h,并实现了固液相之间离子的快速交换,这种脱盐技术极大地提高了脱盐量、能量存储和利用效率,同时实现了Na+和Cl-选择性去除。
基于崔屹等[69]的开创性工作,陈福明等[70]提出以AgCl为负极,Na0.44MnO2为正极,氯化钠溶液为电解液,构建了双离子电化学脱盐系统。通过100次循环试验,发现AgCl-Na0.44MnO2体系在100 mA/g电流密度下具有57.4 mg/g稳定可逆的盐吸附能力,远高于传统的CDI装置。在盐的解吸/吸附过程中,平均电荷效率分别高达0.979/0.956。此后,陈福明等[71-74]在双离子电池脱盐领域做了许多开创性的研究工作,均证明电池脱盐系统在能耗、吸附容量及循环性能方面具有显著优势。
LEE等[75]于2014年首次引入(Hybrid capacitive desalination, HCDI)的概念。HCDI电池由钠离子电池的代表性正极材料Na4Mn9O18电极为阴极,多孔活性炭电极为阳极,阴离子交换膜置于碳电极附近。反应如下:
Na4Mn9O18→Na4-9xMn9O18 +9xNa++9xe (6)
采用正电势启动脱盐过程,通过化学反应:
Na4-9xMn9O18 +9xNa++9xe→Na4Mn9O18 (7)
在脱盐试验之前,需要在Na4Mn9O18电极上施加一定的电势(vs. Ag/AgCl)以将Na+脱出,Na+被Na4Mn9O18电极捕获,而Cl-被物理吸附在多孔碳电极表面形成的双电层中。解吸过程是通过施加负电势来释放电极中已经捕获的离子。HCDI体系的除盐吸附容量为31.2 mg/g,与传统CDI的电吸附容量13.5 mg/g相比,提高2.3倍,并且具有离子去除速度快、稳定性好等优点。除Na4Mn9O18外,还有工作探索了其他钠电池电极材料,如Na2FeP2O7[76]、NaTi2(PO4)3[77]、FePO4[78]和普鲁士蓝[79],研究发现,与传统的MCDI电池相比,将这些钠离子电池电极与活性炭电极匹配组合成HCDI系统也能显著提高盐的吸附能力。
除了上述单通道装置构型,为了实现脱盐过程的连续性操作,逐渐出现了基于法拉第阴极材料的双通道装置构型,又称为摇椅式脱盐。由于基于阴离子的法拉第材料研究较少,所以有关阴离子的摇椅式脱盐方式报道较少,其主要为基于Bi-BiOCl[80]以及Ag-AgCl[81]的氯离子摇椅组合模式,有关其他阴离子特别是氟离子的摇椅式脱盐方式鲜有报道。
目前,间流式脱盐和膜电容脱盐装置构型在电吸附除氟领域中使用较为广泛,但大部分电化学脱盐装置仍停留于实验室研究阶段;废水处理量仅为50~100 mL,有关装置的规模化集成、可连续性操作以及实际水处理应用的研究仍十分匮乏,相关方面的技术经济评估也鲜见报道。鉴于目前电吸附装置构型的研究发展现状,针对电吸附除氟领域,需要进一步加强关于摇椅式电吸附除氟以及流动电极电吸附除氟装置构型的研究,从而提升电吸附除氟过程操作的连续性和实际工业场景的适应性,同时,电极材料作为电吸附装置的核心部件,CDI阳极材料与阴极材料同等重要,特别是基于电荷转移的阴离子法拉第电极材料的突破对于电化学脱盐装置构型的发展演变意义更为显著。
2 CDI除氟材料
2.1 碳基材料
目前,电吸附除氟领域主要的电极材料有碳基材料、铋基材料、层状金属氧化物、过渡金属碳化物以及导电聚合物等五种。碳电极主要是通过双电层对离子进行吸附,其电吸附容量一般随着电势的升高而增大,但是由于碳材料本身存在同离子效应及高浓度盐溶液条件下双电层的压缩将导致离子与电极间的静电作用减弱,脱盐容量受到限制[82],同时碳材料本身对目标离子没有选择性。为了改善碳基材料电吸附性能或赋予其新的性能,如离子选择性,对碳材料进行杂原子掺杂及孔径调控是常用的策略。目前,文献报道[83-85]的碳材料对于氟离子的吸附量仅为0.7~1.8 mg/g。由此可知,碳材料对于F-的吸附容量远不能满足实际废水的处理需求。
2.2 铋基材料
由于铋本身的低毒性[86]、良好的生物相容性和高的理论比容量[87],目前其已经在电池[88-89]、电容器[90-91]领域引起了广泛关注。已有研究表明,基于电化学可逆转换反应,铋电极材料能够存储或释放氟离子[92-93]和氯离子[71, 94]。其与氟离子发生转换反应的过程机理如下:
Bi+3F-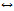 BiF3+3e (8)
BiF3+3e (8)
陈福明等[92]采用BiF3//Na0.44MnO2作为电极对,氟化钠溶液作为电解液,组成双离子脱盐体系用于脱盐。氟化铋材料的表面形貌与循环伏安曲线如图3(a)和(b)所示。该体系具有可逆和稳定的放电容量,库仑效率为98.44%。当电流密度为100 mA/g,经过40次循环后,其比容量仍保持47.28 mA·h/g。
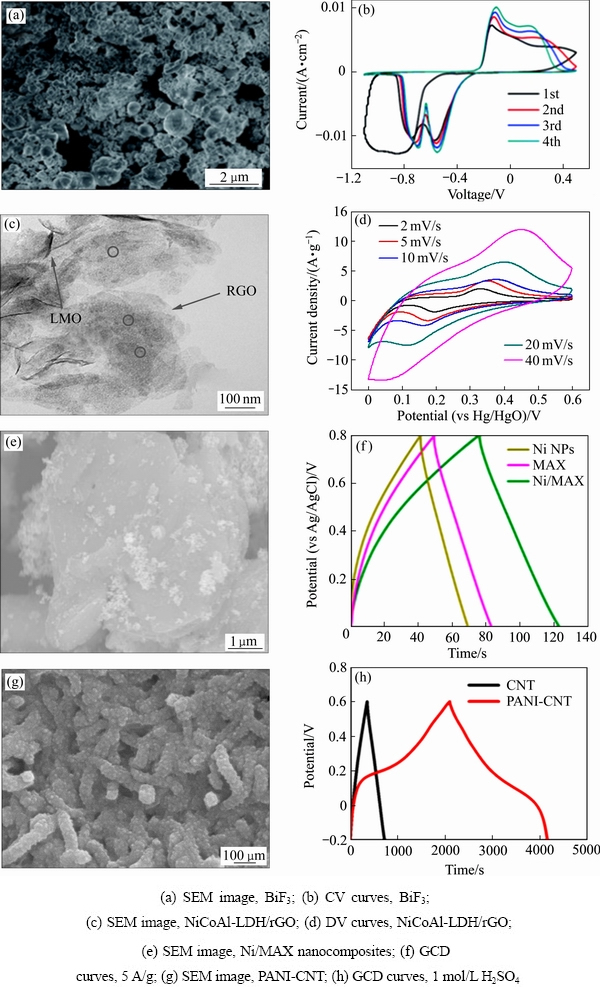
图3 除氟电极材料的SEM、GCD和CV图
Fig. 3 SEM images, GCD and CV curves of fluoride electrode materials
此项工作首次证明了基于水系氟离子和钠离子的双离子电池可以在室温下进行循环充放电,其对未来的储能和离子去除具有重要意义。由氟化铋的循环伏安曲线可知,所采用的氟化铋材料的可逆性较差,需对其进行改性,以期进一步提升其循环稳定性。除此之外,Bi/rGO//AC(Bismuth/reduced graphene oxide//Activated carbon)组成的HCDI脱盐系统[29]在含有NaF、NaCl、NaNO3和Na2SO4,且浓度均为10 mmol/L复合溶液体系中表现出对氟离子、氯离子的高选择性(Cl->F->>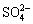 ),其中Bi/rGO复合电极的除氟吸附量为9.11 mg/g。值得注意的是,Bi/BiF3在充放电过程中体积会膨胀,而且产生的BiF3电子导电性较差,研究过程中还发现电极存在溶解的现象,因此,其循环性能的提升也是一个巨大的挑战。此外,铋和BiF3相互转化过程中的中间态尚不明确,铋与其他卤素离子之间的反应机制尚不清楚,均需要进行深入研究,以期为其电化学选择性除氟的应用奠定理论基础。
),其中Bi/rGO复合电极的除氟吸附量为9.11 mg/g。值得注意的是,Bi/BiF3在充放电过程中体积会膨胀,而且产生的BiF3电子导电性较差,研究过程中还发现电极存在溶解的现象,因此,其循环性能的提升也是一个巨大的挑战。此外,铋和BiF3相互转化过程中的中间态尚不明确,铋与其他卤素离子之间的反应机制尚不清楚,均需要进行深入研究,以期为其电化学选择性除氟的应用奠定理论基础。
2.3 层状双金属氢氧化物
层状双金属氢氧化物(Layered double hydroxide,LDH)因具有层板组成、层间阴离子和层板尺寸的可调控性,被认为是具有良好应用前景的无机功能材料[95]。其组成通式[96]可表示为
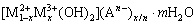
式中:M2+和M3+分别为位于主体层板内的二价和三价金属阳离子;An-是位于层间的阴离子;x为M3+/(M2++M3+)的摩尔比;m为层间水分子数量。
LDH的主要特征由层板的金属元素种类和摩尔比、层间阴离子种类与数量以及层板的堆积形式共同决定。由于LDH层板所带电荷为正,客体离子为负,故是一类非常重要的阴离子交换材料。LDH层板内存在强的共价键作用,层间则以静电力、范德华力或氢键等弱相互作用连接。这种结构使LDH被客体阴离子撑开时其主体层板结构不受影响。在满足层板上的金属离子完好及拥有足够电荷屏蔽LDH层板正电性的条件下,任意阴离子均能够插层进入LDH[97]。阴离子的插层能力取决于其与 LDH 层板及溶剂间相互作用力的差异,同时,还包括其与客体阴离子之间的超分子相互作用。部分阴离子层间插层能力由大到小依次为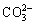 、
、 、
、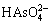 、
、 、
、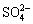 、OH-、F-、Cl-、
、OH-、F-、Cl-、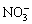 [98]。LDH常见的制备方法有共沉淀法[99-100]、离子交换法、水热法、微波辐射法及溶胶凝胶法[101]。LDH材料具有良好的热稳定性,但当温度达到300 ℃以上时其结构会被破坏,过程大致分为两步:当温度为240 ℃时,LDH表面吸附水和层间吸附水脱出;随后当加热至350~550 ℃时,发生层板脱羟基反应和层间阴离子的分解,形成层状双金属氧化物(Layered double-metal oxide, LDO)。当温度升至 600 ℃后,LDO开始部分烧结并最终得到尖晶石结构。将 LDH煅烧产物 LDO 投入到含有阴离子的水溶液中,其结构可基本恢复到原有 LDH 的有序层状结构。利用该性质可制备得到一系列阴离子插层的 LDH[102]。因此,煅烧还原法由此也成为 LDH 改性的重要方法之一。
[98]。LDH常见的制备方法有共沉淀法[99-100]、离子交换法、水热法、微波辐射法及溶胶凝胶法[101]。LDH材料具有良好的热稳定性,但当温度达到300 ℃以上时其结构会被破坏,过程大致分为两步:当温度为240 ℃时,LDH表面吸附水和层间吸附水脱出;随后当加热至350~550 ℃时,发生层板脱羟基反应和层间阴离子的分解,形成层状双金属氧化物(Layered double-metal oxide, LDO)。当温度升至 600 ℃后,LDO开始部分烧结并最终得到尖晶石结构。将 LDH煅烧产物 LDO 投入到含有阴离子的水溶液中,其结构可基本恢复到原有 LDH 的有序层状结构。利用该性质可制备得到一系列阴离子插层的 LDH[102]。因此,煅烧还原法由此也成为 LDH 改性的重要方法之一。
层状双金属氢氧化物由于其大的比表面积、良好的化学稳定性和高的比电容而成为一类新兴的CDI电极材料[103-105]。目前,在CDI除氟技术领域,LDH作为除氟电极,其使用也较为普遍。室温下,通过简单的共沉淀反应[106]合成NiFeMn-LDH,然后,通过焙烧制备出NiFeMn-LMO (NiFeMn layered metal oxide),并与硝酸处理过的活性炭结合组装成HCDI模块,用于脱氟处理。在初始氟化钠浓度为500 mg/L、电势为1.4 V时,NiFeMn-LMO除氟容量可达16.7 mg/g,在1.0 V下循环30次后,NiFeMn-LMO除氟容量仍能保持初始容量的93.3%。同时,NiFeMn-LMO在1.4 V下瞬时除氟率为3 mg/(g·min)。锰的引入改善了材料的赝电容行为,从而使三元NiFeMn-LMO具有优异的CDI脱氟性能。该工作为开发高性能CDI脱氟电极材料提供了新思路。另外一项工作用溶胶-凝胶法[107]制备了NiAl-LDH薄膜电极,然后通过煅烧获得NiAl-LMO材料,经过10次电吸附/解吸循环后,电极的氟去除率没有显著降低,由此可见,NiAl-LMO电极可以很好的回收和再生,并且具有良好的稳定性。
LDH本身的结构呈碱性,在酸性条件下很容易破坏其自身结构,从而降低吸附效率[108]。当溶液呈碱性时,溶液中羟基离子的浓度相对较高,与氟竞争吸附,导致氟的吸附容量降低[107]。由于LDH材料本身导电性较差,所以也有研究通过将其与石墨烯材料复合以提高其电吸附除氟性能,即采用尿素沉淀法[109]一步合成制备NiCoAl-LDH/rGO复合材料,然后在500 ℃条件下煅烧获得NiCoAl-LMO/ rGO复合材料,材料的表面形貌图与循环伏安曲线见图3(c)和(d)。通过H-AC//NiCoAl-LMO/rGO组装成CDI模块进行电吸附除氟,实验对比结果表明,rGO的引入增强了除氟效果。同时,钴原子的价态对NiCoAl-LMO/rGO的脱氟性能起着至关重要的作用。当在CDI模块上施加正电势时,Co2+转化为Co3+,致使NiCoAl-LMO/rGO电极的层板带正电,为了保持电荷平衡,阴离子进入夹层,从而达到脱氟效果。因此,NiCoAl-LMO/rGO复合材料是从水溶液中高效经济地吸附氟离子的具有广阔前景的材料之一。
2.4 过渡金属碳化物
MXene是过渡金属碳化物、氮化物、碳氮化物的简称,由于其优异的导电性和高的电荷存储容量而引起了研究人员的高度关注[110-112]。SRIMUK等[113]报告称,基于MXene的CDI电极在1.2 V的外加电势下显示出13 mg/g的脱盐能力,并且其在30个运行周期内显示出优异的再生性能。通过将碳纳米管和聚合物黏合剂结合到MXene的表面,MXene基CDI电极的氯化钠去除效率进一步提高[114],这些材料也可通过CDI技术有效地用于去除重金属[115]。此外,为了提高电吸附容量、充电效率、表面润湿性和机械稳定性,MXene基复合电极可以通过掺入各种纳米材料来实现。BHARATH等[116]通过一步微波辐射法制备了Ni/MXene杂化纳米复合材料,图3(e)显示了Ni/MAX (Ni/Ti3AlC2)纳米复合材料的形态。Ni/MAX基电极在1 mol/L Na2SO4中以10 mV/s的扫描速率显示出准矩形形状,与由纯的镍纳米颗粒(260 F/g)和MAX (349 F/g)制成的电极相比,所制造的Ni/MAX复合电极表现出更高的比电容值(385 F/g)。其电化学性能的提高是镍和金属间化合物层间间距共同作用的结果。此外,如图3(f)所示,在5 A/g的固定电流密度下,Ni/MAX表现出更长的充电/放电时间。使用Ni/MAX//pRGO(porous graphene oxide)作为电极组装成CDI装置,在1.4 V下运行160 min,F-、Pb2+和As3+的电吸附容量分别达到68、76和51 mg/g。溶液的pH影响其电吸附容量,在接近中性时电吸附容量最大。用Ni/MAX//pRGO电极组成的系统去除地下水中的F-和工业废水中的Pb2+/As3+,其对F(T)、Pb(T)和As(T)的去除率分别为79%、89.1%和81%。总上所述可知,MXene是一类具有F-储存能力的二维材料,同时其对阳离子也存在吸附作用,但其存储容量有待进一步提高。
2.5 导电聚合物
导电聚合物也具有一定的脱盐能力,例如聚 吡咯[117-118]与聚苯胺[119-120]。聚苯胺因其具有低成本、易于可控合成、高导电性和环境稳定性,在选择性去除水中阴离子(如F-)方面显示出良好的性 能[121-122]。然而,纯聚苯胺通常表现出非常低的目标离子吸附能力,其除氟吸附量为0.04 mmol/g[123]。除此之外,具有导电基底的复合材料,如碳纳米管,是增加导电性和加速各种氧化还原活性过程电动力学的很有前景的材料[124-126]。鉴于聚苯胺和碳纳米管的良好性能,侯嘉红等[28]通过在碳纳米管上电沉积聚苯胺制备聚苯胺-碳纳米管复合电极,其表面形貌如图3(g)所示。该工作合成的材料具有高比例的中孔和亲水性表面,以增强反应过程中的传质。在电位为1.6 V,pH为7的最佳条件下,由于聚苯胺的氧化还原反应,聚苯胺-碳纳米管电极氟的去除能力(0.52 mmol/g)优于碳纳米管电极(0.34 mmol/g),如图3(h)所示,聚苯胺与碳纳米管复合材料的充放电容量明显高于碳纳米管。此外,在含有氟化钠和氯化钠的混合溶液中,聚苯胺-碳纳米管电极的选择性系数为1.22,这表明由于氟化物的强电负性,电极倾向于优先结合氟化物而不是氯化物。在4个电吸附除氟循环周期中,聚苯胺-碳纳米管电极表现出高稳定性和易再生的特点。总之,聚苯胺在碳纳米管复合电极上的电沉积通过电化学调制有效地提高了氟的选择性去除。此外,聚苯胺-碳纳米管与其他先进材料的结合,如MOF前驱体衍生碳,可为开发新型高效的除氟电极材料提供一个很好的途径。
3 电吸附除氟机制
针对CDI除氟电极领域现有研究,本节将对其机理进行梳理归纳。如图4所示,目前,除氟材料电吸附氟离子机理共分为4种类型,分别是双电层吸附、离子插层、转换反应和氧化还原反应。碳电极主要是通过材料表面形成的双电层进行吸附,即当在电极两端施加电压时,海水或苦咸水中的阴离子和阳离子会分别向正极和负极迁移,从而在电极界面处形成双电层,当把正负极短接或者接反向电压时,阴阳离子分别从正负极脱附重新进入溶液,从而电极获得再生。该过程属于电场辅助下的物理吸脱附形式,因此,具有长使用寿命和低维护成本的特点,但不适用于高浓度废水的处理并且针对目标离子电吸附选择性较差。转换反应指的是氟离子和材料之间发生电化学转换反应生成新相的过程,该过程需要克服转化反应中所涉及的化学键的断裂和重组以及材料自身结构转变所需的较大的活化能。在充电过程中,氟离子与过渡金属发生氧化还原反应生成结构完全不同的金属氟化物,在放电过程中,金属氟化物会转变为金属,氟离子进入溶液中,在充放电过程当中,宿主材料的结构本身会发生较大变化,如何避免材料失活并确保转化过程反应的可逆性是今后研究者的主要研究方向。如Bi/BiF3[92],该类反应与Ag/AgCl[127-128]及Bi/BiOCl[94]机理相同。与转化反应不同,离子插层是指在一定条件下,某些离子可以克服层状固体物质各层之间的作用力而插入其层间空隙,但不破坏原有的层状结构,因此,插层反应通常是一个可逆过程。由于LDH二维材料的特殊结构,其相邻片层之间可以存储离子,由于片层之间的正电性,其能够在层间储存阴离子,尤其对于氟离子还表现出优异的选择性[129]。氧化还原反应主要涉及导电聚合物,通过氮元素的价态变化实现氟离子的吸附与解吸[28]。除碳材料外,其他涉及电子转移的材料统称为法拉第电极材料。
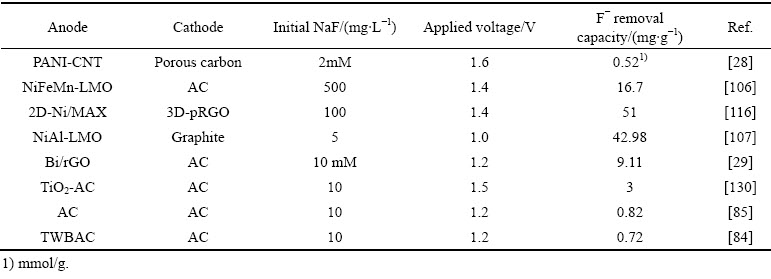
表1针对目前电吸附除氟领域不同类型电极进行了系统地比较。通过对比可知,碳电极容量最低,基于离子插层和转换反应的电极具有较高的除氟容量,说明其更具实际应用前景。除吸附容量单一指标外,该表后续更应关注除氟电极循环性能和去除速率的指标对比,同时后续电吸附操作还应注重研究恒流模式对电吸附过程的影响,目前基本操作模式为恒压操作。
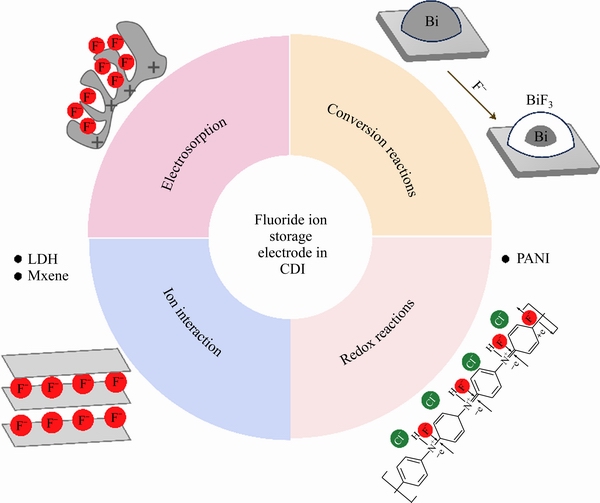
图4 电吸附除氟机理示意图
Fig. 4 Schematic diagram of mechanisms for fluorine ion storage in CDI
表1 电化学脱盐体系中不同除氟电极的脱盐性能对比
Table 1 Performance comparison of different fluorine ion removal electrodes in different electrochemistry deionization system
4 氟离子电池电极材料
锂离子电池和钠离子电池中正极材料的发展为CDI中领域中法拉第电极材料的引入提供了有益的指导与借鉴,为电吸附脱盐领域的发展带来了新的机遇。同样地,通过借鉴氟离子电池领域中的电极材料能够为CDI中除氟电极的开发提供了更多有利的选择。
氟离子电池(Fluoride ion batteries,FIBs)在1979年被首次提出,当时被称为氟离子原电池(Fluoride galvanic cells)[131]。氟离子由于其较小的半径和原子质量而成为出色的电荷载体,其具有高能量密度及低成本的特点,理论容量可达5000 W·h/L,远高于锂空气电池与传统的锂离子电池[132]。FIBs主要依靠氟离子在阴极与阳极之间的迁移实现可逆的充放电,其组成如图5所示,充放电过程中,在阴极与阳极发生的电极反应如式(9)和(10)。目前,氟离子电池在电极(插层与转换型电极)、电解质(包括固体电解质和液体电解质)和电池结构设计开发方面取得了很多进展,主要集中梳理氟离子电池中的电极材料的研究进展,以期给电化学除氟电极材料的设计开发提供启发。
阴极:
xe+MFx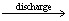 M+xF- (9)
M+xF- (9)
阳极:
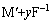
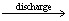 xe+MFx (10)
xe+MFx (10)
目前,氟离子电池的电极材料根据其反应机制主要可分为两类,一类是基于转换反应的金属氟化物电极材料,GSCHWIND等[133]基于材料的安全性、毒性以及理论性能,遴选了BiF3、CuF2、FeF3、AlF3、CoF3、TiF4、ZnF2等7种主要材料。尽管金属氟化物具有较高的理论容量,但由于在电化学循环过程中,其自身存在的体积膨胀、低的离子电导率以及溶解等问题限制了其实际的电化学性能。为了解决上述问题,人们在电极设计开发方面也做了一些改进的工作,针对单纯的金属氟化物具有非常差的电子电导率或离子电导率问题,目前的研究主要是通过与其他材料复合以增强其电化学性能,通过将电极材料与碳复合增强其电子电导率,在某些情况下将其与固态电解质复合增强其离子电导率。还有研究[134-136]表明,可以通过高能球磨制造缺陷并合成纳米材料以增强材料的离子电导率。
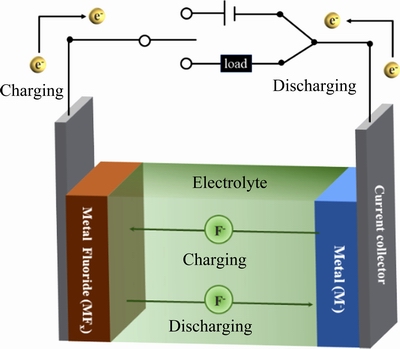
图5 氟离子电池原理示意图
Fig. 5 Schematic diagram of principle of fluoride ion battery
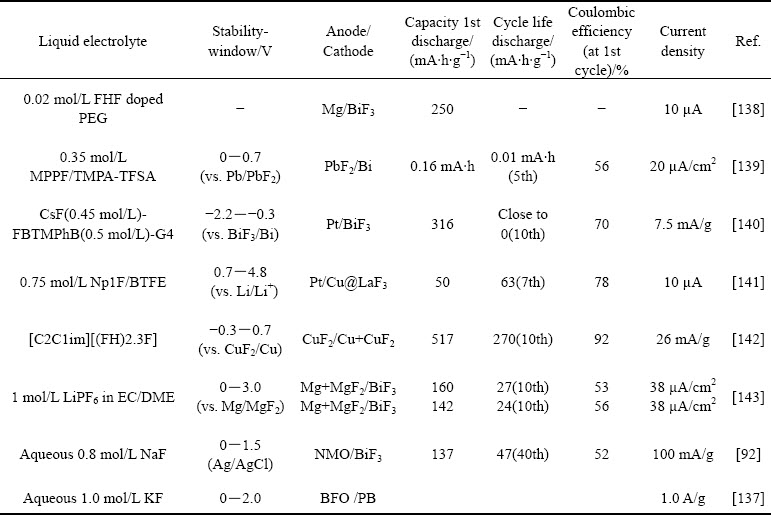
水溶液体系中,氟离子与电极界面间不稳定的电化学界面反应一直是水系FIBs发展的障碍。目前,基于电子转移的水系氟离子储存电极主要为铋基材料。然而,铋电极发生转换反应生成的氟化铋电极存在导电性差、电极溶解以及循环过程中体积变化等系列问题。同时,在水溶液体系中,铋基材料与氟离子的电化学反应过程及机理还有待深入研究。已经有研究[92-93]表明,在水溶液体系中,铋基材料与氟离子之间的电化学转换反应过程具有可逆性,并且通过对材料进行纳米化及与碳纳米管复合可以提高其电化学可逆性。LI等[137]通过构建BiF3@Bi7F11O5@RGO(BFO) 复合材料作为电极,该结构有效缓解了循环过程中材料的体积膨胀,同时还原氧化石墨烯的引入增强了材料电子导电性。通过电极材料缓冲相设计,可在室温下水性电解质中实现稳定的电化学循环。由于Bi7F11O5的体积变化较小且电子电导率较高,BiF3和Bi7F11O5相的交错分布可以提高BFO的结构稳定性和可逆性。此外,石墨烯涂层还改善了劣质的电子电导率问题和活性物质的粉碎,这得益于合理的设计,组装后的电池在室温下表现出高容量(在1 A/g时为218 mA·h/g)和长寿命(超过1000次循环)。这种水性电池的高可逆性和优异的倍率性能远远优于传统的固态电解质氟离子电池以及有机液态氟离子电池。BFO在水系KF电解质中的稳定电化学性能为水性FIB的发展铺平了道路。由于KF电解液的离子电导率很高(25 ℃时为0.092 S/cm),因此电池在室温下工作良好。相反,由于固体电解质的离子电导率低(10–5~ 10–4 S/cm),常规氟离子电池必须在高温(>150 ℃)下运行。铋基材料作为CDI中的氟离子存储电极其可行性已得到验证和报道,这些结果将有助于推进氟离子电池在室温下工作并使用水溶液作为电解质的氟离子电池的发展。在环境温度下可逆和稳定的充电/放电性能有利于扩展FIB的实际应用,例如大型储能系统和可穿戴设备。表2所列为文献中报道的液态电解质不同电极所组成氟离子电池体系的电化学性能。目前的研究主要集中于有机体系,氟离子电池系统的循环性能仍有待进一步提高,新型电极材料开发与电解质优化设计仍是有机氟离子电池未来研究的重点。更加重要的是,其发展也为新型脱盐体系的构建及脱盐性能提升带来了更多的可能性。
表2 不同电极所组成氟离子电池体系的电化学性能
Table 2 Liquid electrolytes for FIBs and their electrochemical property
5 结论与展望
一直以来,有关电吸附领域的电极研究主要集中于脱除Na+相关的材料,而涉及F-去除的电极材料研究较少。一方面,由于可供选择的F-储存材料较少,另一方面,研究者们对电吸附过程中F-的去除机理认识不够全面和深入。本文系统总结了目前电吸附装置构型的演变以及有关除氟领域电极材料的研究进展,希望为后续新型氟离子存储装置与电极的开发提供新的思路。
1) 碳基电极具有较好的循环再生性能,但其主要是基于双电层吸附机理,以物理吸附形式为主,因此碳基电极的吸附容量和选择性表现较差,可适用于含单一且低浓度氟离子废水中氟的去除,但不适用于高浓度且组分复杂的含氟废水中氟离子去除的实际应用。
2) 基于转化反应机理的铋基材料脱盐容量较高,因此,Bi/BiF3的界面电化学反应过程及机制应进一步受到关注,且材料设计的重点应该放在如何提高铋基电极的转化效率,同时其与阳离子法拉第电极材料组合构建双离子脱盐装置是今后需要研究的重点方向。
3) MXene由于其优异的导电性和高的电荷存储容量而引起了研究人员的高度关注。MXene是一类具有F-储存能力的二维材料,同时其对阳离子也存在吸附作用。目前已有用Ni/MAX//pRGO电极组成的系统去除地下水中的F-和工业废水中的Pb2+/As3+,因此MXene也是一个具有较好实际应用场景的电极材料。
4) 基于离子插层和氧化还原反应机理的材料在除氟电极中也具有很好的应用前景。二维材料中LDH具有很强的化学可调控性,通过改变或添加过渡金属可获得不同的电吸附性能。然而,目前LDH并没有较高的稳定的脱氟容量,其本身结构受溶液pH的影响较大,同时其对于氟离子选择性受其他共存阴离子干扰较大。导电聚合物直接应用于CDI除氟的性能虽然较差,但是其优异的导电性、柔性以及黏结性应该在新型电极设计时应被充分考虑并利用。
5) 高吸附容量、高循环稳定性以及具有选择性的氟离子去除电极的设计开发有望解决工业生产中高含氟废水的处理问题,具有重要的应用价值。未来的研究应该主要集中在三个方向,即改善现有电极材料除氟性能及其循环稳定性;开发新的CDI高效除氟材料,研究工作与氟离子电池领域的最新研究相一致;同时研发选择性去除氟离子的电极也具有重要的科学与工程意义。
6) 充分拓展除氟电极的应用场景。法拉第除氟电极的发展将使得CDI除氟性能得到进一步提升,并在除氟领域有着更加广泛的应用,其对于电吸附除氟的装置构型演变也具有重要的促进意义,今后应当注重流动电极和摇椅式电吸附除氟装置构型与法拉第除氟电极的结合开发与研究,同时装置的规模化集成也是未来研究的重点方向,以此进一步拓展电吸附技术在实际场景中的应用。
REFERENCES
[1] PORADA S, ZHAO R, VAN DER WAL A, et al. Review on the science and technology of water desalination by capacitive deionization[J]. Progress in Materials Science, 2013, 58(8): 1388-1442.
[2] ELIMELECH M, PHILLIP W A. The future of seawater desalination: energy, technology, and the environment[J]. Science, 2011, 333(6043): 712-717.
[3] TANG Wang-wang, HE Di, ZHANG Chang-yong, et al. Comparison of Faradaic reactions in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) water treatment processes[J]. Water Research, 2017, 120(5): 229-237.
[4] PODGORSKI J E, LABHASETWAR P, SAHA D, et al. Prediction modeling and mapping of groundwater fluoride contamination throughout India[J]. Environmental Science & Technology, 2018, 52(17): 9889-9898.
[5] 王俊杰, 赵娇娇, 孟旭超, 等. 光伏光电行业含氟废水及污泥利用处置研究现状及展望[J]. 环境工程技术学报, 2018, 8(3): 333-342.WANG Jun-jie, Zhou Jiao-jiao, MENG Xu-chao, et al. Research status and prospect of fluorinated wastewater and sludge utilization in photovoltaic industry[J]. Journal of Environmental Engineering Technology, 2018, 8(3): 333-342.
[6] BHATNAGAR A, KUMAR E, SILLANPAA M. Fluoride removal from water by adsorption—A review[J]. Chemical Engineering Journal, 2011, 171(3): 811-840.
[7] CALIN C, STOICA L, CONSTANTIN C. Fluoride ion removal from liquid media by precipitation-flotation[J]. Revista de Chimie, 2014, 65(4): 475-480.
[8] SAHA S. Treatment of aqueous effluent for fluoride removal[J]. Water Research, 1993, 27(8): 1347-1350.
[9] KEBA DIAWARA C, PAUGAM L, PONTIE M, et al. Influence of chloride, nitrate, and sulphate on the removal of fluoride ions by using nanofiltration membranes[J]. Separation Science and Technology, 2005, 40(16): 3339-3347.
[10] GRZEGORZEK M, MAJEWSKA-NOWAK K, AHMED A E. Removal of fluoride from multicomponent water solutions with the use of monovalent selective ion-exchange membranes[J]. Science of the Total Environment, 2020, 722: 137681-137693.
[11] ZHANG T, LI Q, LIU Y, et al. Equilibrium and kinetics studies of fluoride ions adsorption on CeO2/Al2O3 composites pretreated with non-thermal plasma[J]. Chemical Engineering Journal, 2011, 168(2): 665-671.
[12] ZHANG S, LYU Y, SU X, et al. Removal of fluoride ion from groundwater by adsorption on lanthanum and aluminum loaded clay adsorbent[J]. Environmental Earth Sciences, 2016, 75(5): 401-1-9.
[13] YANG Kai, LI Yi-fan, TIAN Zhong-liang, et al. Removal of fluoride ions from ZnSO4 electrolyte by amorphous porous Al2O3 microfiber clusters: Adsorption performance and mechanism[J]. Hydrometallurgy, 2020, 197: 105455-105464.
[14] WANG Fan, WANG Kai-tuo, MUHAMMAD Y, et al. Preparation of CeO2@SiO2 microspheres by a non-sintering strategy for highly selective and continuous adsorption of fluoride ions from wastewater[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(17): 14716-14726.
[15] GOVINDAN K, RAJA M, UMA MAHESHWARI S, et al. Comparison and understanding of fluoride removal mechanism in Ca2+, Mg2+ and Al3+ ion assisted electrocoagulation process using Fe and Al electrodes[J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 1784-1793.
[16] DAS D, NANDI B K. Simultaneous removal of fluoride and Fe(Ⅱ) ions from drinking water by electrocoagulation[J]. Journal of Environmental Chemical Engineering, 2020, 8(1): 103643-103652.
[17] RAO S M, MAMATHA P. Water quality in sustainable water management[J]. Curr Sci India, 2004, 87(7): 942-947.
[18] CHAI Li-yuan, WANG Yun-yan, ZHAO Na, et al. Sulfate- doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water[J]. Water Research, 2013, 47(12): 4040-4049.
[19] CHO D W, HAN Y S, LEE J, et al. Water defluorination using granular composite synthesized via hydrothermal treatment of polyaluminum chloride (PAC) sludge[J]. Chemosphere, 2020, 247: 125899-125907.
[20] SUSS M E, PORADA S, SUN X, et al. Water desalination via capacitive deionization: what is it and what can we expect from it?[J]. Energy & Environmental Science, 2015, 8(8): 2296-2319.
[21] OREN Y. Capacitive deionization (CDI) for desalination and water treatment—past, present and future (a review)[J]. Desalination, 2008, 228(1/2/3): 10-29.
[22] WU Ting-ting, WANG Gang, DONG Qiang, et al. Starch derived porous carbon nanosheets for high-performance photovoltaic capacitive deionization[J]. Environmental Science & Technology, 2017, 51(16): 9244-9251.
[23] LI Guo-xian, HOU Peng-xiang, ZHAO Shi-yong, et al. A flexible cotton-derived carbon sponge for high-performance capacitive deionization[J]. Carbon, 2016, 101: 1-8.
[24] AHMED M A, TEWARI S. Capacitive deionization: Processes, materials and state of the technology[J]. Journal of Electroanalytical Chemistry, 2018, 813: 178-192.
[25] KALFA A, SHAPIRA B, SHOPIN A, et al. Capacitive deionization for wastewater treatment: Opportunities and challenges[J]. Chemosphere, 2020, 241: 125003-125015.
[26] CUI Hao, QIAN Yu, AN Hao, et al. Electrochemical removal of fluoride from water by PAOA-modified carbon felt electrodes in a continuous flow reactor[J]. Water Research, 2012, 46(12): 3943-3950.
[27] PARK G, HONG S P, LEE C, et al. Selective fluoride removal in capacitive deionization by reduced graphene oxide/hydroxyapatite composite electrode[J]. Journal of Colloid and Interface Science, 2021, 581: 396-402.
[28] WU J C, CHEN S S, YU T C, et al. Effective electrochemically controlled removal of fluoride ions using electrodeposited polyaniline-carbon nanotube composite electrodes[J]. Separation and Purification Technology, 2021, 254: 117561-117570.
[29] MIN Xiao-bo, ZHU Ming-fei, HE Yin-jie, et al. Selective removal of Cl- and F- from complex solution via electrochemistry deionization with bismuth/reduced graphene oxide composite electrode[J]. Chemosphere, 2020, 251: 126319-126329.
[30] XU Pei, DREWES J E, HEIL D, et al. Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology[J]. Water Research, 2008, 42(10/11): 2605-2617.
[31] JUNG H H, HWANG S W, HYUN S H, et al. Capacitive deionization characteristics of nanostructured carbon aerogel electrodes synthesized via ambient drying[J]. Desalination, 2007, 216(1/2/3): 377-385.
[32] CHOI J H. Fabrication of a carbon electrode using activated carbon powder and application to the capacitive deionization process[J]. Separation and Purification Technology, 2010, 70(3): 362-366.
[33] CHANG Li-ming, YU Yan-yan, DUAN Xiao-yue, et al. Capacitive deionization performance of activated carbon electrodes prepared by a novel liquid binder[J]. Separation Science and Technology, 2012, 48(2): 359-365.
[34] HOU C H, HUANG C Y. A comparative study of electrosorption selectivity of ions by activated carbon electrodes in capacitive deionization[J]. Desalination, 2013, 314: 124-129.
[35] LIU N L, SUN S H, HOU C H. Studying the electrosorption performance of activated carbon electrodes in batch-mode and single-pass capacitive deionization[J]. Separation and Purification Technology, 2019, 215: 403-409.
[36] WANG Gang, DONG Qiang, LING Zheng, et al. Hierarchical activated carbon nanofiber webs with tuned structure fabricated by electrospinning for capacitive deionization[J]. Journal of Materials Chemistry, 2012, 22(41): 21819-21823.
[37] WANG Gang, PAN Chao, WANG Liu-ping, et al. Activated carbon nanofiber webs made by electrospinning for capacitive deionization[J]. Electrochimica Acta, 2012, 69: 65-70.
[38] SHEN Jia-ming, LI Yang, WANG Chao-hai, et al. Hollow ZIFs-derived nanoporous carbon for efficient capacitive deionization[J]. Electrochimica Acta, 2018, 273: 34-42.
[39] ZHANG H, ZHANG W, SHEN J, et al. Ag-doped hollow ZIFs-derived nanoporous carbon for efficient hybrid capacitive deionization[J]. Desalination, 2020, 473: 114173-114182.
[40] ZHANG Jing, YAN Ting-ting, FANG Jian-hui, et al. Enhanced capacitive deionization of saline water using N-doped rod-like porous carbon derived from dual-ligand metal-organic frameworks[J]. Environmental Science: Nano, 2020, 7(3): 926-937.
[41] OSCHATZ M, ZEIGER M, JAECKEL N, et al. Emulsion soft templating of carbide-derived carbon nanospheres with controllable porosity for capacitive electrochemical energy storage[J]. Journal of Materials Chemistry A, 2015, 3(35): 17983-17990.
[42] WANG Lei, WANG Ming, HUANG Zheng-hong, et al. Capacitive deionization of NaCl solutions using carbon nanotube sponge electrodes[J]. Journal of Materials Chemistry, 2011, 21(45): 18295-18299.
[43] NIE Chun-yang, PAN Li-kun, LI Hai-bo, et al. Electrophoretic deposition of carbon nanotubes film electrodes for capacitive deionization[J]. Journal of Electroanalytical Chemistry, 2012, 666: 85-88.
[44] YANG Ling-fang, SHI Zhou, YANG Wen-hao. Enhanced capacitive deionization of lead ions using air-plasma treated carbon nanotube electrode[J]. Surface and Coatings Technology, 2014, 251: 122-127.
[45] LI Hai-bo, LU Ting, PAN Li-kun, et al. Electrosorption behavior of graphene in NaCl solutions[J]. Journal of Materials Chemistry, 2009, 19(37): 6773-6779.
[46] DUAN Hai-yan, YAN Ting-ting, CHEN Guo-rong, et al. A facile strategy for the fast construction of porous graphene frameworks and their enhanced electrosorption performance[J]. Chemical Communications, 2017, 53(54): 7465-7468.
[47] SRIMUK P, SU X, YOON J, et al. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements[J]. Nature Reviews Materials, 2020, 5(7): 517-538.
[48] CHOI S, CHANG B, KIM S, et al. Battery electrode materials with omnivalent cation storage for fast and charge-efficient ion removal of asymmetric capacitive deionization[J]. Advanced Functional Materials, 2018, 28(35): 1802665.
[49] KIM T, GORSKI C A, LOGAN B E. Low energy desalination using battery electrode deionization[J]. Environmental Science & Technology Letters, 2017, 4(10): 444-449.
[50] SHI Wei, NIE Peng-fei, SHANG Xiao-hong, et al. Berlin green-based battery deionization-highly selective potassium recovery in seawater[J]. Electrochimica Acta, 2019, 310: 104-112.
[51] ZHAO Xiao-yu, FENG Ming-hui, JIAO Yao-xin, et al. Lithium extraction from brine in an ionic selective desalination battery[J]. Desalination, 2020, 481: 114360-114371.
[52] SHI Wen-hui, GAO Xin-long, MAO Jing, et al. Exploration of energy storage materials for water desalination via next-generation capacitive deionization[J]. Frontiers in Chemistry, 2020, 8: 415-424.
[53] XU Dong-chuan, WANG Wen-hui, ZHU Ming-yue, et al. Recent advances in desalination battery: an initial review[J]. ACS Applied Materials & Interfaces, 2020, 12(52): 57671-57685.
[54] LIU Zhen-zhen, SHANG Xu, LI Hai-bo, et al. A brief review on high-performance capacitive deionization enabled by intercalation electrodes[J]. Global Challenges, 2021, 5(1): 2000054-2000072.
[55] BEER P D, GALE P A. Anion recognition and sensing: the state of the art and future perspectives[J]. Angewandte Chemie International Edition, 2001, 40(3): 486-516.
[56] MURPHY G W, CAUDLE D D. Mathematical theory of electrochemical demineralization in flowing systems[J]. Electrochimica Acta, 1967, 12(12): 1655-1664.
[57] BLAIR J W, MURPHY G W. Electrochemical demineralization of water with porous electrodes of large surface area[M]//Advances in Chemistry. Washington, D. C.: American Chemical Society, 1960: 206-223.
[58] SUSS M E, PRESSER V. Water desalination with energy storage electrode materials[J]. Joule, 2018, 2(1): 10-15.
[59] ZHANG Chang-yong, HE Di, MA Jin-xing, et al. Faradaic reactions in capacitive deionization (CDI)-problems and possibilities: A review[J]. Water Research, 2018, 128: 314-330.
[60] GUYES E N, SHOCRON A N, SIMANOVSKI A, et al. A one-dimensional model for water desalination by flow- through electrode capacitive deionization[J]. Desalination, 2017, 415: 8-13.
[61] GUYES E N, SIMANOVSKI A, SUSS M E. Several orders of magnitude increase in the hydraulic permeability of flow-through capacitive deionization electrodes via laser perforations[J]. RSC Advances, 2017, 7(34): 21308-21313.
[62] QU Ya-tian, CAMPBELL P G, HEMMATIFAR A, et al. Charging and transport dynamics of a flow-through electrode capacitive deionization system[J]. The Journal of Physical Chemistry B, 2018, 122(1): 240-249.
[63] JOHNSON A M, NEWMAN J. Desalting by means of porous carbon electrodes[J]. Journal of the Electrochemical Society, 1971, 118(3): 510-517.
[64] LEE J B, PARK K K, EUM H M, et al. Desalination of a thermal power plant wastewater by membrane capacitive deionization[J]. Desalination, 2006, 196(1/2/3): 125-134.
[65] 高新源, 李爱民, 刘佩春, 等. 流动电极电吸附去除水中Na+、SO42-、Fe3+及Ca2+[J]. 环境工程学报, 2021, 15(4): 1252-1259.GAO Xin-yuan, LI Ai-min, LIU Pei-chun, et al. Removal of Na+, SO42-, Fe3+ and Ca2+ from water by means of flow-electrode capacitive deionization[J]. Chinese Journal of Environmental Engineering, 2021, 15(4): 1252-1259.
[66] JEON S I, PARK H R, YEO J G, et al. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes[J]. Energy & Environmental Science, 2013, 6(5): 1471-1475.
[67] JEON S I, YEO J G, YANG S C, et al. Ion storage and energy recovery of a flow-electrode capacitive deionization process[J]. Journal of Materials Chemistry A, 2014, 2(18): 6378-6383.
[68] DOORNBUSCH G J, DYKSTRA J E, BIESHEUVEL P M, et al. Fluidized bed electrodes with high carbon loading for water desalination by capacitive deionization[J]. Journal of Materials Chemistry A, 2016, 4(10): 3642-3647.
[69] PASTA M, WESSELLS C D, CUI Yi, et al. A desalination battery[J]. Nano Letters, 2012, 12(2): 839-843.
[70] CHEN Fu-ming, HUANG Yin-xi, GUO Lu, et al. A dual-ion electrochemistry deionization system based on AgCl-Na0.44MnO2 electrodes[J]. Nanoscale, 2017, 9(28): 10101-10108.
[71] CHEN Fu-ming, HUANG Yin-xi, GUO Lu, et al. Dual-ions electrochemical deionization: A desalination generator[J]. Energy & Environmental Science, 2017, 10(10): 2081-2089.
[72] CHEN Fu-ming, HUANG Yin-xi, KONG De-zhi, et al. NaTi2(PO4)3-Ag electrodes based desalination battery and energy recovery[J]. FlatChem, 2018, 8: 9-16.
[73] ZUBAIR M, IHSANULLAH I, ABDUL AZIZ H, et al. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook[J]. Bioresource Technology, 2021, 319: 124128-124140.
[74] HUANG Yin-xi, CHEN Fu-ming, GUO Lu, et al. Low energy consumption dual-ion electrochemical deionization system using NaTi2(PO4)3-AgNPs electrodes[J]. Desalination, 2019, 451(2): 241-247.
[75] LEE J, KIM S, KIM C, et al. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques[J]. Energy Environ Sci, 2014, 7(11): 3683-3689.
[76] KIM S, LEE J, KIM C, et al. Na2FeP2O7 as a novel material for hybrid capacitive deionization[J]. Electrochimica Acta, 2016, 203: 265-271.
[77] HUANG Yin-xi, CHEN Fu-ming, GUO Lu, et al. Ultrahigh performance of a novel electrochemical deionization system based on a NaTi2(PO4)3/rGO nanocomposite[J]. Journal of Materials Chemistry A, 2017, 5(34): 18157-18165.
[78] GUO Lu, HUANG Yin-xi, DING Meng, et al. A high performance electrochemical deionization method to desalinate brackish water with an FePO4/RGO nanocomposite[J]. Journal of Materials Chemistry A, 2018, 6(19): 8901-8908.
[79] GUO Lu, MO Run-wei, SHI Wen-hui, et al. A Prussian blue anode for high performance electrochemical deionization promoted by the faradaic mechanism[J]. Nanoscale, 2017, 9(35): 13305-13312.
[80] LIU Yong, GAO Xin, WANG Zi-ping, et al. Controlled synthesis of bismuth oxychloride-carbon nanofiber hybrid materials as highly efficient electrodes for rocking-chair capacitive deionization[J]. Chemical Engineering Journal, 2021, 403: 126326-126336.
[81] SRIMUK P, HUSMANN S, PRESSER V. Low voltage operation of a silver/silver chloride battery with high desalination capacity in seawater[J]. RSC Advances, 2019, 9(26): 14849-14858.
[82] LI Yuan-yuan, CHEN Nan, LI Zeng-ling, et al. Frontiers of carbon materials as capacitive deionization electrodes [J]. Dalton Transactions, 2020, 49(16): 5006-5014.
[83] TANG Wang-wang, KOVALSKY P, CAO Bai-chuan, et al. Investigation of fluoride removal from low-salinity groundwater by single-pass constant-voltage capacitive deionization[J]. Water Research, 2016, 99: 112-121.
[84] GAIKWAD M S, BALOMAJUMDER C. Tea waste biomass activated carbon electrode for simultaneous removal of Cr(Ⅵ) and fluoride by capacitive deionization[J]. Chemosphere, 2017, 184: 1141-1149.
[85] GAIKWAD M S, BALOMAJUMDER C. Simultaneous electrosorptive removal of chromium(Ⅵ) and fluoride ions by capacitive deionization (CDI): Multicomponent isotherm modeling and kinetic study[J]. Separation and Purification Technology, 2017, 186: 272-281.
[86] SAFARDOUST-HOJAGHAN H, SALAVATI-NIASARI M, MOTAGHEDIFARD M H, et al. Synthesis of micro sphere- like bismuth nanoparticles by microwave assisted polyol method; designing a novel electrochemical nanosensor for ultra-trace measurement of Pb2+ ions[J]. New Journal of Chemistry, 2015, 39(6): 4676-4684.
[87] WU Ming-guang, XU Bao-lin, ZHANG Yue-feng, et al. Perspectives in emerging bismuth electrochemistry[J]. Chemical Engineering Journal, 2020, 381: 122558-122574.
[88] WANG Chen-chen, WANG Liu-bin, LI Fujun, et al. Bulk bismuth as a high-capacity and ultralong cycle-life anode for sodium-ion batteries by coupling with glyme-based electrolytes[J]. Advanced Materials, 2017, 29(35): 1702212-1702218.
[89] CHENG Xiao-long, LI Dong-jun, WU Ying, et al. Bismuth nanospheres embedded in three-dimensional (3D) porous graphene frameworks as high performance anodes for sodium- and potassium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(9): 4913-4921.
[90] KONISHI H, KUCUK A C, MINATO T, et al. Improved electrochemical performances in a bismuth fluoride electrode prepared using a high energy ball mill with carbon for fluoride shuttle batteries[J]. Journal of Electroanalytical Chemistry, 2019, 839: 173-176.
[91] GONG Qiu-fang, DING Pan, XU Ming-quan, et al. Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction[J]. Nature Communications, 2019, 10: 2807-2816.
[92] ZHANG Zi-shuai, HU Xiao-qiao, ZHOU Yu, et al. Aqueous rechargeable dual-ion battery based on fluoride ion and sodium ion electrochemistry[J]. Journal of Materials Chemistry A, 2018, 6(18): 8244-8250.
[93] HU Xiao-qiao, CHEN Fu-ming, ZHANG Zi-shuai, et al. The electrochemical behaviors of NaF dual battery based on the hybrid electrodes of nano-bismuth@CNTs[J]. Materials Letters, 2018, 233: 332-335.
[94] NAM D H, CHOI K S. Bismuth as a new chloride-storage electrode enabling the construction of a practical high capacity desalination battery[J]. Journal of the American Chemical Society, 2017, 139(32): 11055-11063.
[95] MITTAL J. Recent progress in the synthesis of Layered Double Hydroxides and their application for the adsorptive removal of dyes: A review[J]. Journal of Environmental Management, 2021, 295: 113017-113056.
[96] PAVLOVIC M, ROUSTER P, ONCSIK T, et al. Tuning colloidal stability of layered double hydroxides: from monovalent ions to polyelectrolytes[J]. Chem Plus Chem, 2017, 82(1): 121-131.
[97] SHAO Ming-fei, ZHANG Rui-kang, LI Zhen-hua, et al. Layered double hydroxides toward electrochemical energy storage and conversion: Design, synthesis and applications[J]. Chemical Communications, 2015, 51(88): 15880-15893.
[98] YAN Kai, LIU Yu-qian, LU Yi-ran, et al. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules[J]. Catalysis Science & Technology, 2017, 7(8): 1622-1645.
[99] 汪爱河, 周康根, 刘 行, 等. Mg-Al-Me(Me=La,Ce,Zr)复合氧化物制备及其除氟性能[J]. 环境科学, 2016, 37(12): 4874-4881.WANG Ai-he, ZHOU Kang-gen, LIU Xing, et al. Preparation of Mg-Al-Me(Me=La, Ce, Zr) composite oxides for efficient fluoride uptake[J]. Environmental Science, 2016, 37(12): 4874-4881.
[100] 汪爱河, 周康根, 刘 行, 等. 双滴共沉淀法合成MgO-LDH的脱氟性能[J]. 中国有色金属学报, 2017, 27(4): 869-875.WANG Ai-he, ZHOU Kang-gen, LIU Xing, et al. Deflouridation efficiency of MgO-LDH prepared by double drop co-precipitation[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(4): 869-875.
[101] QU Jun, ZHANG Qi-wu, LI Xue-wei, et al. Mechanochemical approaches to synthesize layered double hydroxides: A review[J]. Applied Clay Science, 2016, 119: 185-192.
[102] WANG Qiang, O’HARE D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets[J]. Chemical Reviews, 2012, 112(7): 4124-4155.
[103] REN Qi-di, WANG Gang, WU Ting-ting, et al. Calcined MgAl-layered double hydroxide/graphene hybrids for capacitive deionization[J]. Industrial & Engineering Chemistry Research, 2018, 57(18): 6417-6425.
[104] HONG Xiao-ting, ZHU En-hao, YE Zhuo-liang, et al. Enhanced phosphate removal under an electric field via multiple mechanisms on MgAl-LDHs/AC composite electrode[J]. Journal of Electroanalytical Chemistry, 2019, 836: 16-23.
[105] ZHU En-hao, HONG Xiao-ting, YE Zhuo-liang, et al. Influence of various experimental parameters on the capacitive removal of phosphate from aqueous solutions using LDHs/AC composite electrodes[J]. Separation and Purification Technology, 2019, 215: 454-462.
[106] WANG Gang, LI Duan-zheng, WANG Shi-yong, et al. Ternary NiFeMn layered metal oxide (LDO) compounds for capacitive deionization defluoridation: The unique role of Mn[J]. Separation and Purification Technology, 2021, 254: 117667-117675.
[107] BAI Zhao-yu, HU Cheng-zhi, LIU Hui-juan, et al. Selective adsorption of fluoride from drinking water using NiAl-layered metal oxide film electrode[J]. Journal of Colloid and Interface Science, 2019, 539: 146-151.
[108] PANG Hong-wei, WU Yi-han, WANG Xiang-xue, et al. Recent advances in composites of graphene and layered double hydroxides for water remediation: A review[J]. Chemistry-an Asian Journal, 2019, 14(15): 2542-2552.
[109] LI Duan-zheng, WANG Shi-yong, WANG Gang, et al. Facile fabrication of NiCoAl-layered metal oxide/graphene nanosheets for efficient capacitive deionization defluorination[J]. ACS Applied Materials & Interfaces, 2019, 11(34): 31200-31209.
[110] RASOOL K, PANDEY R P, RASHEED P A, et al. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes)[J]. Materials Today, 2019, 30: 80-102.
[111] IHSANULLAH I. Potential of MXenes in water desalination: current status and perspectives[J]. Nano-Micro Letters, 2020, 12(1): 1-20.
[112] SUVINA V, KOKULNATHAN T, WANG T J, et al. Lanthanum cobaltite supported on graphene nanosheets for non-enzymatic electrochemical determination of catechol[J]. Microchimica Acta, 2020, 187(3): 1-7.
[113] SRIMUK P, HALIM J, LEE J, et al. Two-dimensional molybdenum carbide (MXene) with divacancy ordering for brackish and seawater desalination via cation and anion intercalation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 3739-3747.
[114] KUMAR E A, KOKULNATHAN T, WANG T J, et al. Two-dimensional titanium carbide (MXene) nanosheets as an efficient electrocatalyst for 4-nitroquinoline N-oxide detection[J]. Journal of Molecular Liquids, 2020, 312: 113354-113361.
[115] SHEN Xiao-jie, XIONG Yue-cheng, HAI Re-ti, et al. All-MXene-based integrated membrane electrode constructed using Ti3C2Tx as an intercalating agent for high-performance desalination[J]. Environmental Science & Technology, 2020, 54(7): 4554-4563.
[116] BHARATH G, HAI A, RAMBABU K, et al. Designed assembly of Ni/MAX (Ti3AlC2) and porous graphene-based asymmetric electrodes for capacitive deionization of multivalent ions[J]. Chemosphere, 2021, 266: 129048-129060.
[117] ZHANG Yu-jie, WANG Yue, XU Shi-chang, et al. Polypyrrole nanowire modified graphite (PPy/G) electrode used in capacitive deionization[J]. Synthetic Metals, 2010, 160(13/14): 1392-1396.
[118] 刘艳辉, 徐 克, 张晓晨. 聚吡咯/活性炭复合电极对不同离子电吸附性研究[J]. 工业水处理, 2016, 36(10): 28-31.LIU Yan-hui, XU Ke, ZHANG Xiao-chen. Research on polypyrrole/activated carbon composite electrode used for the electro-adsorbability for different ions[J]. Industrial Water Treatment, 2016, 36(10): 28-31.
[119] WANG Yue, WANG Ru-guo, XU Shi-chang, et al. Polypyrrole/polyaniline composites with enhanced performance for capacitive deionization[J]. Desalination and Water Treatment, 2015, 54(12): 3248-3256.
[120] EVANS S F, IVANCEVIC M R, WILSON D J, et al. Carbon polyaniline capacitive deionization electrodes with stable cycle life[J]. Desalination, 2019, 464: 25-32.
[121] CIRIC-MARJANOVIC G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications[J]. Synthetic Metals, 2013, 177: 1-47.
[122] WANG Huan-huan, LIN Jian-yi, SHEN Ze-xiang. Polyaniline (PANI) based electrode materials for energy storage and conversion[J]. Journal of Science: Advanced Materials and Devices, 2016, 1(3): 225-255.
[123] KARTHIKEYAN M, SATHEESHKUMAR K K, ELANGO K P. Defluoridation of water via doping of polyanilines[J]. Journal of Hazardous Materials, 2009, 163(2/3): 1026-1032.
[124] CAPEK I. Dispersions, novel nanomaterial sensors and nanoconjugates based on carbon nanotubes[J]. Advances in Colloid and Interface Science, 2009, 150(2): 63-89.
[125] WANG Yi-fan, KUANG Cui-fang, CAI Huan-qing, et al. Sub-diffraction imaging with confocal fluorescence microscopy by stochastic photobleaching[J]. Optics Communications, 2014, 312: 62-67.
[126] JIN Chen, NAGAIAH T C, XIA Wei, et al. Metal-free and electrocatalytically active nitrogen-doped carbon nanotubes synthesized by coating with polyaniline[J]. Nanoscale, 2010, 2(6): 981-987.
[127] YOON H, LEE J, KIM S, et al. Hybrid capacitive deionization with Ag coated carbon composite electrode[J]. Desalination, 2017, 422: 42-48.
[128] HE Ying-jie, HUANG Lei, ZHAO Yi-xian, et al. A newly synthesized highly stable Ag/N-carbon electrode for enhanced desalination by capacitive deionization[J]. Environmental Science: Nano, 2020, 7(10): 3007-3019.
[129] ZHANG X, ZUO K, ZHANG X, et al. Selective ion separation by capacitive deionization (CDI) based technologies: A state-of-the-art review[J]. Environmental Science: Water Research & Technology, 2020, 6(2): 243-257.
[130] WU Peng, XIA Ling, DAI Min, et al. Electrosorption of fluoride on TiO2-loaded activated carbon in water[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 502: 66-73.
[131] NOWROOZI M A, MOHAMMAD I, MOLAIYAN P, et al. Fluoride ion batteries—Past, present, and future[J]. Journal of Materials Chemistry A, 2021, 9(10): 5980-6012.
[132] RONGEAT C, ANJI REDDY M, DIEMANT T, et al. Development of new anode composite materials for fluoride ion batteries[J]. Journal of Materials Chemistry A, 2014, 2(48): 20861-20872.
[133] GSCHWIND F, RODRIGUEZ-GARCIA G, SANDBECK D J S, et al. Fluoride ion batteries: Theoretical performance, safety, toxicity, and a combinatorial screening of new electrodes[J]. Journal of Fluorine Chemistry, 2016, 182: 76-90.
[134] KUCUK A C, YAMANAKA T, ABE T. Fluoride shuttle batteries: On the performance of the BiF3 electrode in organic liquid electrolytes containing a mixture of lithium bis(oxalato)borate and triphenylboroxin[J]. Solid State Ionics, 2020, 357: 115499-115508.
[135] KONISHI H, MINATO T, ABE T, et al. Electrochemical properties of lead fluoride electrode in fluoride shuttle battery[J]. Journal of Electroanalytical Chemistry, 2018, 826: 60-64.
[136] RONGEAT C, REDDY M A, WITTER R, et al. Nanostructured fluorite-type fluorides as electrolytes for fluoride ion batteries[J]. The Journal of Physical Chemistry C, 2013, 117(10): 4943-4950.
[137] LI Xue-jin, TANG Yong-chao, ZHU Jia-xiong, et al. Initiating a room-temperature rechargeable aqueous fluoride- ion battery with long lifespan through a rational buffering phase design[J]. Advanced Energy Materials, 2021, 11(14): 2003714-2003724.
[138] GSCHWIND F, ZAO-KARGER Z, FICHTNER M. A fluoride-doped PEG matrix as an electrolyte for anion transportation in a room-temperature fluoride ion battery[J]. Journal of Materials Chemistry A, 2014, 2(5): 1214-1218.
[139] OKAZAKI K I, UCHIMOTO Y, ABE T, et al. Charge- discharge behavior of bismuth in a liquid electrolyte for rechargeable batteries based on a fluoride shuttle[J]. ACS Energy Letters, 2017, 2(6): 1460-1464.
[140] KONISHI H, MINATO T, ABE T, et al. Electrochemical performance of a bismuth fluoride electrode in a reserve-type fluoride shuttle battery[J]. Journal of the Electrochemical Society, 2017, 164(14): A3702-A3708.
[141] DAVIS V K, BATES C M, OMICHI K, et al. Room- temperature cycling of metal fluoride electrodes: Liquid electrolytes for high-energy fluoride ion cells[J]. Science, 2018, 362(6419): 1144-1148.
[142] YAMAMOTO T, MATSUMOTO K, HAGIWARA R, et al. Room-temperature fluoride shuttle batteries based on a fluorohydrogenate ionic liquid electrolyte[J]. ACS Applied Energy Materials, 2019, 2(9): 6153-6157.
[143] MOHAMMAD I, WITTER R. Testing Mg as an anode against BiF3 and SnF2 cathodes for room temperature rechargeable fluoride ion batteries[J]. Materials Letters, 2019, 244: 159-162.
ZHU Ming-fei1, WANG Yun-yan1, 2, ZHANG Wen-chao1, 2, LUO Yong-jian2, SUN Zhu-mei1, 3
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control and Treatment of Heavy Metals Pollution, Central South University, Changsha 410083, China;
3. School of Environmental and Safety Engineering, North University of China, Taiyuan 030051, China)
Abstract: The capacitive deionization (CDI) is an emerging desalination technology that removes charged ions and molecules from water by applying an applied electric field at both ends of the electrode, which is concerned due to its low energy consumption and sustainability. Because of the rapid development in the field of energy storage batteries, the cationic electrode materials have leaped carbon materials, represented by the bilayer mechanism of action, to Faraday electrode materials, resulting in a significant improvement in the desalination performance of CDI. Currently, less research attention has been paid to electrode materials for the efficient removal of anions, especially fluoride ions in CDI. This paper systematically sorted out the classification of fluoride removal electrode materials in CDI, compared the characteristics of different types of fluoride removal electrode materials, summarized the mechanism of fluoride ion removal and the research progress of electrode materials in the field of fluorine ion battery, and provided the theoretical basis and research ideas for the design and development of CDI fluoride removal electrodes and device configurations.
Key words: capacitive deionization (CDI); device configuration; fluoride removal electrode material; fluoride removal mechanism; fluoride ion battery
Foundation item: Project(52004256) supported by the National Natural Science Foundation for Young Scientists of China; Project (201901D211212) supported by the Shanxi Province Science Foundation for Youths, China; Project(2018SK2026) supported by the Key Research and Development Program of Hunan Province, China; Project(2019L0574) supported by Scientific and Technological Innovative Program of Higher Education Institutions in Shanxi Province, China; Project(QX202004) supported by Young academic leader of North University of China
Received date: 2021-08-31; Accepted date: 2021-10-14
Corresponding author: SUN Zhu-mei; Tel: +86-351-3920504; E-mail: sunzhumei41@163.com
(编辑 李艳红)
基金项目:国家自然科学基金青年基金资助项目(52004256);山西省面上青年基金资助项目(201901D211212);湖南省重点研发计划资助项目(2018SK2026);山西省高等学校科技创新资助项目(2019L0574);中北大学青年学科带头人资助项目(QX202004)
收稿日期:2021-08-31;修订日期:2021-10-14
通信作者:孙竹梅,讲师,博士;电话:0351-3920504;E-mail:sunzhumei41@163.com
摘 要:电容去离子技术(Capacitive deionization,CDI)是一种新兴的脱盐技术,通过在电极两端施加外加电场除去水中带电离子或分子,由于其具有低能耗和可持续性的特点而备受关注。储能电池领域的迅猛发展实现了阳离子储存从双电层作用为代表的碳材料到法拉第电极材料的跨越。相较于传统碳材料,法拉第材料的脱盐性能有了显著提升。目前,CDI中针对阴离子,特别是氟离子,高效去除的电极材料研究较少。本文从CDI装置构型的发展演变出发,系统梳理了CDI中关于除氟电极材料的分类,对比不同类型除氟电极材料的特点,并总结氟离子的去除机理以及氟离子电池领域电极材料的研究进展,为CDI除氟电极与装置构型的设计开发提供了理论基础和研究思路。