
J. Cent. South Univ. (2020) 27: 2494-2506
DOI: https://doi.org/10.1007/s11771-020-4475-y
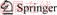
Comparison of microwave and conventional heating routes for kaolin thermal activation
ZHANG Liang-jing(张良静)1, 2, HE Yuan(和媛)1, 2, LU Peng(吕鹏)1, 2,
PENG Jin-hui(彭金辉)1, 2, LI Shi-wei(李世伟)1, 2, CHEN Kai-hua(陈楷华)1, 2,
YIN Shao-hua(尹少华)1, 2, ZHANG Li-bo(张利波)1, 2
1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology,Kunming 650093, China;
2. State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, Kunming University of
Science and Technology, Kunming 650093, China;
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
The effect of activation properties of the precursors of zeolite directly prepared from kaolin influenced by microwave field and conventional heating was investigated. XRD, TG-DSC, FT-IR, SEM, particle size analysis, specific surface area (BET), pore size distribution (BJH) and N2 adsorption-desorption were discussed to determine the optimal activation temperature. It is concluded that the conversion of kaolin to metakaolin in the microwave field is at 500 °C holding for 30 min, which is 100 °C lower than that in conventional calcination and 90 min shorter, and the phase transition process of kaolin under the effect of microwave field is the same as that of conventional heating method. SEM analysis indicates that the particle size is more uniform and agglomeration appears slightly in the microwave field. The N2 adsorption-desorption isotherm, BET and BJH of kaolin indicate that the pore properties are almost invariable regardless of calcination route during the process of calcining kaolin into metakaolin. It indicates that microwave calcination is superior to conventional calcination in the activation pathway of kaolin. It is attributed to microwave heating relying on objects to absorb microwave energy and convert it into thermal energy, which can simultaneously and uniformly heat the entire substance.
Key words:
kaolin; thermal activation; metakaolin; microwave;
Cite this article as:
ZHANG Liang-jing, HE Yuan, LU Peng, PENG Jin-hui, LI Shi-wei, CHEN Kai-hua, YIN Shao-hua, ZHANG Li-bo. Comparison of microwave and conventional heating routes for kaolin thermal activation [J]. Journal of Central South University, 2020, 27(9): 2494-2506.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4475-y1 Introduction
Kaolinite is a clay mineral of chemical formula Al2O3·2SiO2·2H2O contained abundant silicon and aluminum [1] (Figure 1); therefore, it is conveniently used as a silicon source and an aluminum source for synthetic molecular sieves. Clay minerals have been used for the synthesis of zeolites as early as 1961 [2], and now are playing a pivotal role in fabricating zeolites. Simultaneously, the direct synthesis of zeolites from natural aluminosilicate minerals, without experiencing intermediate chemicals, has attracted extensive attention [3-5]. The synthesis of zeolite A from kaolinite comprises two steps: metakaolinization and zeolitization [6]. However, the silicon and aluminum in kaolin are in crystal forms and crystalline kaolinite has high chemical inertness, which cannot be utilized for synthesizing zeolites directly [7]. Therefore, kaolinite has to be calcined at high temperatures (700-900 °C) whereby dehydroxylation takes place, converting to amorphous and more reactive product (metakaolinite) prior to application for the synthesis of zeolites [8-10]. The plate-like structure will decrease the reactivity and surface area of reaction of kaolin, which can be improved by appropriate treatment methods to destroy the crystal structure, such as mechanical activation [11], thermal activation [12], acid-base modification activation [13, 14]. Due to its high passivity, it is impossible to increase the reactivity of kaolin by chemical treatment, even under drastic conditions [15]. Heating to alter kaolin activity has become an important method, which can transform the lamellar crystal structure of kaolin into disordered metakaolin structure by controlling the number of hydroxyl groups. Some original groups in the inner layer of the crystal are exposed to obtain the special physical and chemical properties [16]. The calcined product has the advantages of high strength, good durability and corrosion resistance [17, 18]. However, conventional calcination has the disadvantages of slow heating rate, high energy consumption, and uneven heating inside and outside the material. Therefore, it is essential to seek an alternative heating way to solve the above issues.

Figure 1 Kaolin structure
Microwave heating is a heating route that relies on objects to absorb microwave energy and convert it into heat energy, so that the whole body can heat up at the same time, which is completely different from other conventional heating methods. Microwave thermal [19] has been used as a new type of heating technology on account of the advantages of fast and efficient, uniform interior heating, relatively low reaction temperature, energy saving, environmental protection, high product purity and no direct contact between the heating source and heated materials [20, 21]. ZHONG et al [22] reported a study which can reduce phase transition temperature and prepare finer and more uniform products. ZHANG et al [23] recorded microwave selective heating-enhanced reaction rates for mullite preparation from kaolinite, indicating that conventional heating kaolinite has to be heated at 1400 °C for 1 h to form well-ordered orthorhombic mullite (3Al2O3·2SiO2) that is accompanied by the formation of cristobalite, while microwave heating at 900 °C for 5 min and 1200 °C for 1 min. YOUSSEF et al [24] studied the microwave-assisted versus conventional synthesis of zeolite A from metakaolinite and came to conclusion that the rate of zeolite A formation was found to increase by 2–3 times in microwave treated samples with a notable enhancement in the product crystallinity and yield whether seeded or unseeded. Therefore, an economically viable route has been successfully applied for activating kaolin.
This work describes the thermal activation process of kaolin using conventional and microwave calcination. The effects of the two calcinations on the temperature and time needed for the activation of kaolin were systematically studied, and similarities and difference of products between the two calcination methods were comprehensively compared. Analytical conclusions have been obtained by differential thermogravimetric analysis (TG-DSC), X-ray diffraction (XRD), Fourier transform infrared spectrometry (FT-IR), scanning electron microscopy (SEM), particle size analysis (PSD), specific surface area, pore size distribution and N2 adsorption-desorption.
2 Experimental
2.1 Chemical composition of kaolin
Kaolin was supplied from Yunnan Xishuangbanna Wanxiang Mining Co., Ltd, China, with the composition of elements in their oxide forms of 49.3% SiO2, 37.748% Al2O3, 1.271% K2O, 0.728% Na2O, 0.455% Fe2O3, 0.214% MgO and 0.137% CaO, and was used as the raw material. The main impurities accorded with the standard of high quality kaolin. For example, the content of Fe2O3 was less than 1% [25]. Its compositions are determined by X-ray florescence (XRF, Instrument PANalytical, Rh tube, 2.2 kW). The results are given for elements in their oxide forms in Table 1. The XRD pattern of the kaolin sample used in this study is given in Figure 2. It is noticed that the peaks of kaolinite should be loaded at 12.15°, 20.19°, 24.73°, 26.44° and 28.02°, while that at 26.67° corresponding to quartz and muscovite and albite also is found in sample.
Table 1 Chemical composition of raw kaolin in mass fraction (%)


Figure 2 XRD pattern of kaolin samples
2.2 Calcination of raw kaolinite
Block kaolinite was subjected to grind on a portable multifunctional powder machine. After grinding, 50 g kaolin was charged into crucible of d60 mm×75 mm to heat with microwave nor conventional field.
The thermal treatment of the kaolin was carried out in a laboratory muffle furnace at a heating rate of 10 °C/min in air, and heated in the range of 600-1200 °C and then remained at these temperatures for 2 h before cooling in the furnace. Then sample was determined by TG-DSC analysis and the characterization was analyzed to make the change of the phase certain.
A certain amount of kaolin was contained in the corundum crucible and covered with a microwave absorbing medium (shown in Figure 3). The microwave frequency was 2.45 GHz with the maximum input power of 4.5 kW. Temperature was measured by a thermocouple and the calcination conditions were selected at 500- 1000 °C holding for 30 min.
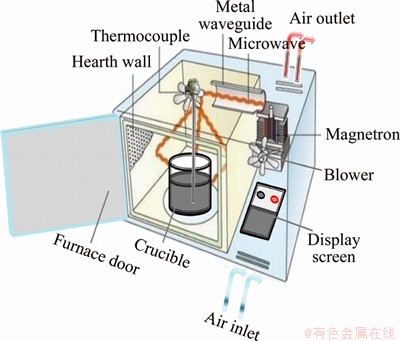
Figure 3 Microwave heating device diagram
2.3 Characterization
The composition of the starting ore was identified by XRF on an AXios max spectrometry (PANalytical). The sample phases and compositions at different temperature were identified by the X-ray diffraction analysis on a diffractometer (XRD, RU-200B/D/MAX-RB RU-200B, Japan; CuKα, λ=1.5418  , 40 mA and 40 kV). The thermal behavior of kaolin was investigated by thermal gravity analysis and differential, scanning calorimetry (TG-DSC, Netzsch STA449F3) at a ramping rate of 20 °C/min in 25-1400°C under argon atmosphere. IR spectra (Nicolet iS50, Thermo Nicolet, American) with KBr as diluent were scanned in the wavelength range of 400-4000 cm-1 to analyze the functional groups. The microstructures of the kaolin and fired bodies were studied with scanning electron microscope (SEM, FEIQUANTA 600) with a field emission gun and without the need to conduct enhanced conductivity treatment on the sample. Microparticle size and distribution of the powders were obtained by laser particle size distribution instrument (Sympatec Helos-Rodos, Sichuan, China) with hexametaphosphate as a dispersing solvent. The specific surface area and pore size distribution were determined by N2 adsorption/desorption isotherms at 350 °C using automated surface-area & pore size analyzer (Quadrasorb-evo, American), prior to N2 physisorption samples were degassed for 4-5 h using helium.
, 40 mA and 40 kV). The thermal behavior of kaolin was investigated by thermal gravity analysis and differential, scanning calorimetry (TG-DSC, Netzsch STA449F3) at a ramping rate of 20 °C/min in 25-1400°C under argon atmosphere. IR spectra (Nicolet iS50, Thermo Nicolet, American) with KBr as diluent were scanned in the wavelength range of 400-4000 cm-1 to analyze the functional groups. The microstructures of the kaolin and fired bodies were studied with scanning electron microscope (SEM, FEIQUANTA 600) with a field emission gun and without the need to conduct enhanced conductivity treatment on the sample. Microparticle size and distribution of the powders were obtained by laser particle size distribution instrument (Sympatec Helos-Rodos, Sichuan, China) with hexametaphosphate as a dispersing solvent. The specific surface area and pore size distribution were determined by N2 adsorption/desorption isotherms at 350 °C using automated surface-area & pore size analyzer (Quadrasorb-evo, American), prior to N2 physisorption samples were degassed for 4-5 h using helium.
3 Results and discussion
3.1 Thermal analysis
The thermal behavior of the starting kaolin is very simple from a qualitative point of view (Figure 4) and Table 2 clearly clarifies the relevant chemical reactions during the activation of kaolin. The DSC curves show the de-hydroxylation reaction and transformation of kaolinite, which can be divided into the following stages:
Stage I: The stage is from room temperature to 250 °C. There are two endothermic peaks at 40.7 and 230.3 °C ascribed to the removal of physical absorbed water and the removal of layer hydroxyl water, resulting in the mass loss of 0.49 wt% and 1.38 wt%, corresponding 0.068 unity and 0.19 unity of H2O, respectively.
Stage II: In the temperature range of 250- 800 °C, there is an intensive endothermic peak occurring at 471.9 °C attributed to de-hydroxylation and convert to meta-kaolin, and the mass loss is about 9.77 wt% with a loss of 1.37 unity of H2O.
Stage III: The stage is from 800 to 1400 °C.

Figure 4 DSC-TG curve of kaolin
The exothermic peak at 982.8 °C may be attributed to the transformation of AlSi spinel to pseudomullite.
It can be seen that kaolin is accompanied by a large number of physical and chemical reactions in the process of calcination when it undergoes convertion from kaolin to metakaolin, spinel type phase and format mullite.
3.2 X-ray diffraction analysis
The XRD of the kaolinite calcination products at different temperatures using conventional and microwave heating are shown in Figures 5(a) and (b). It is indicated that well-defined reflections at the two values of 12.15° and 26.44° (corresponding to the d values of 7.2243  , these peaks correspond to the reflections from (001)) are ascribed to kaolin. At 500 °C, practically all peaks corresponding to kaolinite have disappeared in the microwave field holding for 30 min, generating a featureless band of X-ray amorphous metakaolin and quartz that could be identified. However, it requires 600 °C holding for 120 min by the conventional route, suggesting that the calcined kaolin has removed a large amount of hydroxyl groups in the structure when the crystal structure is destroyed. The quartz and muscovite peaks remain for the kaolin after calcination, suggesting that the muscovite structure remains intact and is not dehydrated. As the temperature of calcination increases, the diffraction peak of mullite begins to appear in the pattern of calcined samples at 900 °C for 30 min in the microwave field, and become distinct at 1000 °C. The mullite diffraction peaks are visible eventually in the two calcination routes, but the difference between the two is that the conventional calcination must be kept at 1100 °C for 120 min, while the microwave calcination only needs to be kept at 1000 °C for 30 min. The patterns exhibit no significant change at 700-900 °C and 600-800 °C, and characteristics of a quasi- amorphous material in conventional and microwave heating systems. The above analysis shows that well-ordered kaolinite is transformed to less reactive metakaolinite by conventional calcination at 600 °C for 120 min as well as by microwave at 500 °C for 30 min. Thus, the microwave calcination time is significantly shortened, which is sufficient to show the superiority of microwave heating, energy saving and high efficiency.
, these peaks correspond to the reflections from (001)) are ascribed to kaolin. At 500 °C, practically all peaks corresponding to kaolinite have disappeared in the microwave field holding for 30 min, generating a featureless band of X-ray amorphous metakaolin and quartz that could be identified. However, it requires 600 °C holding for 120 min by the conventional route, suggesting that the calcined kaolin has removed a large amount of hydroxyl groups in the structure when the crystal structure is destroyed. The quartz and muscovite peaks remain for the kaolin after calcination, suggesting that the muscovite structure remains intact and is not dehydrated. As the temperature of calcination increases, the diffraction peak of mullite begins to appear in the pattern of calcined samples at 900 °C for 30 min in the microwave field, and become distinct at 1000 °C. The mullite diffraction peaks are visible eventually in the two calcination routes, but the difference between the two is that the conventional calcination must be kept at 1100 °C for 120 min, while the microwave calcination only needs to be kept at 1000 °C for 30 min. The patterns exhibit no significant change at 700-900 °C and 600-800 °C, and characteristics of a quasi- amorphous material in conventional and microwave heating systems. The above analysis shows that well-ordered kaolinite is transformed to less reactive metakaolinite by conventional calcination at 600 °C for 120 min as well as by microwave at 500 °C for 30 min. Thus, the microwave calcination time is significantly shortened, which is sufficient to show the superiority of microwave heating, energy saving and high efficiency.
Table 2 Related chemical reactions of kaolin transformation


Figure 5 XRD pattern of kaolin at different temperatures:
3.3 Fourier infrared spectroscopy (FT-IR)
Distinctly different from the starting ore, the FT-IR bands of calcination products show extreme distinctions in Figures 6 and 7. The FT-IR spectra of the crude clay show peaks at 3695, 3619, 1634, 1030, 911, 789, 753, 693, 536, 468 and 429 cm-1,characteristic of kaolinite [23]. The 3619 cm-1 peak has been appointed to the internal hydroxyl group; the 3695 cm-1 peak corresponds to the internal surface O-H group; the 1098 and 1030 cm-1 peaks are attributed to Si-O stretching. The Al-OH is assigned to 911 cm-1; the peaks at 789 and 753 cm-1 are assigned to vs (Si-O-Si), Si-O-Al vibration bands lies in 536 cm-1; and finally the peaks at 468 and 429 cm-1 are adapted to the deformation vibration of Si-O [26, 27].
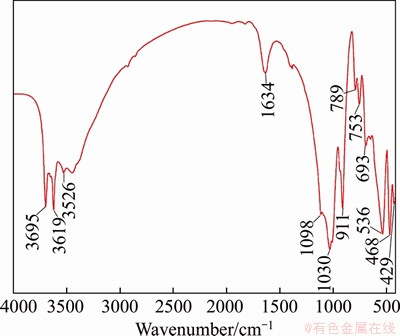
Figure 6 Infrared spectrum of original kaolin

Figure 7 Infrared spectra of kaolin samples after calcination at different calcination temperatures:
As the temperature increases, the absorption peaks of 3695, 3619 and 911 cm-1 disappeared, indicating that the internal structure water of kaolin is lost and the kaolin structure has been destroyed and transformed into metakaolin. A low, broad peak at ~3440 cm-1 is attributed to the absorption of associated hydroxyls formed by the coupling agent molecules on the surface of the mineral [28]. The peak near 1637 cm-1 ascribed to the bending vibration mode of physisorbed water on the surface of free silica produced is quite intense [29]. The presence of the vibration band at 1000-1100 cm-1 for metakaolinite is assigned to the stretching Si-O bonds in amorphous silica [30]. The broad band of metakaolinite, located at 791 cm-1 assigned to the Al-O bonds in Al2O3 is observed. The broad Al-O octahedral stretching band at 555-558 cm-1 can be clearly identified. The results are probably associated with the formation of four-coordinated Al species [31]. The framework of mullite phase formed completely when the thermal treatment temperature was increased to near 1000 °C, which is in good agreement with the XRD characterization results. These bands all prove the conversion of kaolin to metakaolin, which needs to be calcined to 600 °C for 120 min in the conventional route, while that only needs to be kept at 500 °C for 30 min in the microwave field. In comparison, the peak at 555-558 cm-1 occurred in conventional as well as microwave field at different temperatures, indicating that there is a heat gap between microwave heating and conventional calcaination, where microwave heating efficiency is higher, and the required temperature is lower.
3.4 SEM analysis
The SEM images of the crude ore at different magnifications are shown in Figure 8. The surface morphology of crude ore is a mixture of flakes and rods, wherein the flaky crystal forms are pseudo hexagons, exhibiting an irregular shape and relatively poor crystallinity [32]. As shown in Figures 9 and 10, with the increase of temperature, the surface morphology of kaolin changed significantly. For example, the rod structure decreased; the flaky structure increased, the slab-like fragments increased; and varying degrees of stacking and agglomeration appeared. The kaolin desorbs the internal and external hydroxyl groups; at the same time the structure of the aluminoxy octahedron is destroyed during the calcination process, but the silicon tetrahedron still maintains a layered structure, resulting in the crystal lattice of the kaolin change. However, agglomeration appeared slightly in the microwave field compared to the conventional route. It is attributed to the difference of the mechanism of conventional heating and microwave heating, where microwave heating makes the material be more evenly heated, resulting in agglomeration less.

Figure 8 SEM images of crude ore:

Figure 9 SEM images of calcined kaolin at different temperatures by conventional route:
Figure 10 SEM images of calcined kaolin at different temperatures in microwave field:
3.5 Particle size distribution analysis
The particle size distributions of kaolin at 500 °C in the microwave field and conventional calcination at 600 °C are shown in Figures 11(a) and (b). It can be seen that particle size distributions are (D90-D10)/(2×D50)=1.095 and 1.351 in the microwave field and conventional calcination, respectively; the value is closer to 1, showing that the particle size distribution is narrower. The particle size distribution of the calcination kaolin in the microwave field is mostly distributed in the range of 10-50 μm, and the particle size distribution range is narrow; while it is relatively divergent for that obtained by conventional calcination at 600 °C. It is attributed to the fact that specific surface area of the microwave treated product is relatively stable as the temperature increases, while the conventional calcination specific surface area fluctuates greatly, resulting in uneven particle growth.
3.6 Pore size analysis
The N2 adsorption-desorption isotherms of the kaolin raw material used in the experiment are shown in Figure 12. At low pressure (P/P0<0.8), the adsorption amount (W) slowly increases; however, a sharp increase occurs posterior to P/P0>0.8, with capillary condensation due to pore size limitation. In addition, the adsorption curve and desorption curve in Figure 12 do not completely coincide, and the existence of a hysteresis loop can be observed. The adsorption and desorption isotherm of the kaolin belongs to the type IV adsorption and desorption isotherm, and the hysteresis ring belongs to the type A hysteresis loop. It is known from calculation that the specific surface area of kaolin is 20.065 m2/g (Table 3). It can be seen from Figure 12 that the pore size distribution of raw kaolin is relatively wide and there are abundant of mesopores and a handful of macropores.

Figure 11 Particle size distribution:
Figures 13 and 14 show the N2 adsorption- desorption isotherm, BET specific surface area curve, and BJH pore size distribution curve of kaolin under the conventional condition (600 °C) and microwave field (500 °C), respectively. It can be seen that the adsorption-desorption isotherms, the BET specific surface area curve, and the BJH pore size distribution curve of kaolin are not significantly different from that prior to calcination. After calcination, the adsorption-desorption isotherm is still considered to be the type IV of adsorption-desorption isotherm, and the hysteresis ring belongs to the type A hysteresis ring, indicating that the pore structure is a cylindrical capillary with open ends. In addition, the pore size distribution of both routes after calcination is relatively extensive. In the conventional calcination,the distribution is concentrated at about 3.835 nm, while that is more concentrated near 3.83 nm in the microwave field. After 15 nm, the curve gradually flattens, indicating that there are still substantial of mesopores and a small fraction of macropores after calcination. By the calculation of the specific surface area graph drawn according to the BET equation, a conclusion is drawn that the specific surface area of kaolin is 22.383 m2/g after conventional calcination, while that is 23.486 m2/g in the microwave field. Tables 4 and 5 show the pore performance analysis of calcined kaolin. The specific surface area, pore volume, average pore size, and external surface area of the calcined kaolin and the raw materials are not significantly different, which indicates that calcination will not significantly affect the pore performance of kaolin.

Figure 12 N2 adsorption and desorption isotherm (a), BET specific surface area curve (b) and BJH pore size distribution chart (c) of raw kaolin

Figure 13 N2 adsorption and desorption isotherm (a), BET specific surface area curve (b) and BJH pore size distribution chart (c) of conventional calcined kaolin (600 °C)
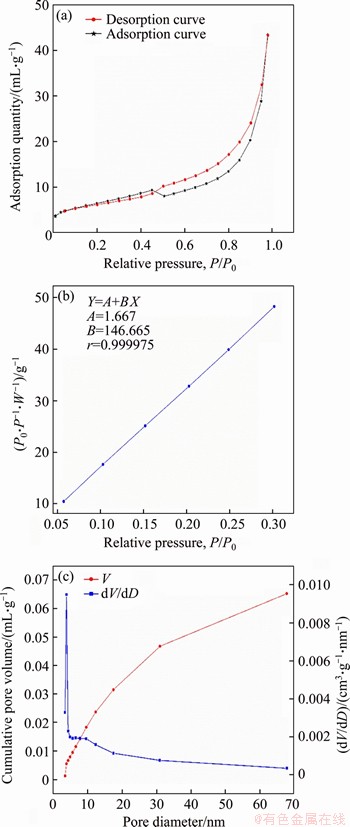
Figure 14 N2 adsorption and desorption isotherm (a), BET specific surface area curve (b) and BJH pore size distribution chart (c) of microwave calcined kaolin (500 °C)
Table 3 Pore property analysis of kaolin raw material

Table 4 Pore property analysis of calcined kaolin

Table 5 Pore property analysis of kaolin raw material

4 Conclusions
A comparison of the properties of the calcination kaolin samples subjected to conventional thermal treatment and microwave heated is studied, and the conclusions are drawn as follows:
1) The phase transition process of kaolin under the effect of microwave field is the same as that of conventional heating method: from crystal phase (kaolin) to amorphous phase (metakaolin) and finally to crystal phase (mullite).
2) Compared with conventional calcination, the time required for kaolin to transform into amorphous metakaolin under microwave field is reduced by 90 min; the optimum temperature of kaolin transformed to metakaolin in the microwave field is 500 °C, which is correspondingly reduced by 100 °C.
3) Through SEM and laser particle size analysis, the products obtained by microwave heating are uniformly distributed of the overall particle sizes and agglomeration appears less in the microwave field compared to the conventional thermal treatment.
4) The N2 adsorption-desorption isotherm, BET specific surface area curve, and BJH pore size distribution curve of kaolin indicate that the pore properties are almost invariable regardless of calcination route during the process of calcining kaolin into metakaolin.
Contributors
ZHANG Liang-jing, LU Peng and HE Yuan performed the experiments; LI Shi-wei and YIN Shao-hua conceived and designed the study; YIN Shao-hua and CHEN Kai-hua reviewed the manuscript; ZHANG Liang-jing edited and reviewed the manuscript; Peng Jin-hui and Zhang Li-bo reviewed the whole manuscript; all the authors have read and reviewed this manuscript.
Conflict of interest
ZHANG Liang-jing, HE Yuan, LU Peng, PENG Jin-hui, LI Shi-wei, CHEN Kai-hua, YIN Shao-hua, ZHANG Li-bo declare that they have no conflict of interest.
References
[1] ALIREZA E, MOHAMMAD H, SOGAND A. Effect of calcination temperature and composition on the spray-dried microencapsulated nanostructured SAPO-34 with kaolin for methanol conversion to ethylene and propylene in fluidized bed reactor [J]. Microporous and Mesoporous Materials, 2020, 297: 110046. DOI: 10.1016/j.micromeso.2020.110046.
[2] STERNE E J, REYNOLDS R C, ZANTOP H. Natural ammonium illites from black shales hosting a stratiform base metal deposit, Delong mountains, Northern Alaska [J]. Clays & Clay Minerals, 1982, 30: 161-166. DOI: 10.1346/CCMN. 1982.0300301.
[3] LIU H, SHEN T, LI T, YUAN P, SHI G, BAO X. Green synthesis of zeolites from a natural aluminosilicate mineral rectorite: Effects of thermal treatment temperature [J]. Apply Clay Science, 2014, 90: 53-60. DOI: 10.1016/j.clay.2014.01. 006.
[4] MELDA L B, SALIH K K, BURCU A. Development of antibacterial powder coatings using single and binary ion-exchanged zeolite A prepared from local kaolin [J]. Applied Clay Science, 2019, 182: 105251.
[5] YUE Y Y, GUO X X, LIU T, LIU H Y, WANG T H, YUAN P, ZHU H B, BAI Z S, BAO X J. Template free synthesis of hierarchical porous zeolite Beta with natural kaolin clay as alumina source [J]. Microporous and Mesoporous Materials, 2020, 293: 109772. DOI: 10.1016/j.micromeso.2019.109772.
[6] YOUSSEF H, IBRAHIM D, KOMARNENI S. Microwave- assisted versus conventional synthesis of zeolite A from metakaolinite [J]. Microporous and Mesoporous Materials, 2008, 115: 527-534. DOI: 10.1016/j.micromeso.2008.02. 030.
[7] CHANDRASEKHAR S, PRAMADA P N. Kaolin-based zeolite Y, a precursor for cordierite ceramics [J]. Apply Clay Science, 2004, 27: 187-198. DOI: 10.1016/j.clay.2004.07. 001.
[8] LI N, LI T S, LIU H Y, YUE Y Y, BAO X J. A novel approach to synthesize in-situ crystallized zeolite/kaolin composites with high zeolite content [J]. Applied Clay Science, 2017, 144: 150-156. DOI: 10.1016/j.clay.2017.05. 010.
[9] WANG P, SUN A Q, ZHANG Y J, CAO J. Effective removal of methane using nano-sized zeolite 4A synthesized from kaolin [J]. Inorganic Chemistry Communications, 2020, 111: 107639. DOI: 10.1016/j.inoche.2019.107639.
[10] CHEN J W, LI X D, CAI W Q, SHI Y X, HUI X, CAI Z J, JIN W, FAN J J. High-efficiency extraction of aluminum from low-grade kaolin via a novel low-temperature activation method for the preparation of poly-aluminum- ferric-sulfate coagulant [J]. Journal of Cleaner Production, 2020, 257: 120399. DOI: 10.1016/j.jclepro.2020.120399.
[11] ILIC B, MITROVIC A, MILICIC L J, ZADUJIC M. Compressive strength and microstructure of ordinary cured and autoclaved cement-based composites with mechanically activated kaolins [J]. Construction and Building Materials, 2018, 178: 92-101. DOI: 10.1016/j.conbuildmat.2018.05. 144.
[12] SUN T, GE K Y, WANG G M, GENG H N, SHUI Z H, CHENG S K, CHEN M. Comparing pozzolanic activity from thermal-activated water-washed and coal-series kaolin in Portland cement mortar [J]. Construction and Building Materials, 2019, 227: 117092. DOI: 10.1016/j.conbuildmat. 2019.117092.
[13] ZHAO Y, ZHANG Q W, YUAN W Y, HU H M, LI Z, AI Z Q, LI Y J. High efficient coagulant simply by mechanochemically activating kaolinite with sulfuric acid to enhance removal efficiency of various pollutants for wastewater treatment [J]. Applied Clay Science, 2019, 180: 105187. DOI: 10.1016/j.clay.2019.105187.
[14] WANG J Q, HUANG Y, PAN Y X, MI J X. New hydrothermal route for the synthesis of high purity nanoparticles of zeolite Y from kaolin and quartz [J]. Microporous and Mesoporous Materials, 2016, 23277- 23285. DOI: 10.1016/j.micromeso.2016.06.010.
[15] ZHANG C, ZHANG Z, TAN Y, ZHONG M F. The effect of citric acid on the kaolin activation and mullite formation [J]. Ceramics International, 2017, 43: 1466-1471. DOI: 10.1016/j.ceramint.2016.10.115.
[16] CRISTOBAL A G S, CASTELLO R, LUENGO M A M. Vizcayno, C: Zeolites prepared from calcined and mechanically modified kaolins: A comparative study [J]. Apply Clay Science, 2010, 49: 239-246. DOI: 10.1016/ j.clay.2010.05.012.
[17] GODEK E, FELEKOGLU K T, KESKINATES M, FELEKOGLU B. Development of flaw tolerant fiber reinforced cementitious composites with calcined kaolin [J]. Applied Clay Science, 2017, 146: 423-431. DOI: 10.1016/ j.clay.2017.06.029.
[18] LEI S M, LIN M, XIA Z J, PEI Z Y, LI B. Influence of calcined coal-series kaolin fineness on properties of cement paste and mortar [J]. Construction and Building Materials, 2018, 171: 558-565.
[19] ZHANG C, LI R P, LIU J H, GUO S H, XU L, XIAO S J, SHEN Z G. Hydrogen peroxide modified polyacrylonitrile- based fibers and oxidative stabilization under microwave and conventional heating–The 1st comparative study [J]. Ceramics International, 2019, 45: 13385-13392. DOI: 10.1016/j.ceramint.2019.04.035.
[20] KOSTAS E T, BENEROSO D, ROBINSON J P. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass [J]. Renewable and Sustainable Energy Reviews, 2017, 77: 12-27. DOI: 10.1016/j.rser.2017.03.135.
[21] MURAZA O, REBROV E V, CHEN J, PUTKONEN M, NIINISTO L, CROON M H J M, SCHOUTENA J C. Microwave-assisted hydrothermal synthesis of zeolite Beta coatings on ALD-modified borosilicate glass for application in microstructured reactors [J]. Chemical Engineering Journal, 2008, 135: 117-120. DOI: 10.1016/j.cej.2007.07. 003.
[22] ZHONG S L, ZHANG M S, SU Q. Study of Mechanism of kaolin sintered by microwave heating [J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2005, 44: 71-74.
[23] ZHANG Z Y, QIAO X C, YU J G. Microwave selective heating-enhanced reaction rates for mullite preparation from kaolinite [J]. RSC Advances, 2013, 4: 2640-2647. DOI: 10.1039/C3RA43767A.
[24] YOUSSEF H, IBRAHIM D, KOMARNENI S. Microwave- assisted versus conventional synthesis of zeolite A from metakaolinite [J]. Microporous and Mesoporous Materials, 2008, 115: 527-534. DOI: 10.1016/j.micromeso.2008.02. 030.
[25] LUO Z M, WEI L D. Development and prospect of Guangxi quality kaolinclay [J]. Guangxi Geology, 2002, 15(1): 11-14. (in Chinese)
[26] MARKOVIC S, DONDUR V, DIMITRIJEVIC R. FTIR spectroscopy of framework aluminosilicate structures: carnegieite and pure sodium nepheline [J]. Journal of Molecular Structure, 2003, 654: 223-234. DOI: 10.1016/ S0022-2860(03)00249-7.
[27] JOHNSTON C, BISH D, ECKERT J, BROWN L A. Infrared and inelastic neutron scattering study of the 1.03- and 0.95-nm kaolinite-hydrazine intercalation complexes [J]. Journal Physical Chemical, 2000, 104: 8080-8088. DOI: 10.1021/jp001075s.
[28] LAPIDES I, LAHAV N, MICHAELIAN K H. Thermal intercalation of alkali halides into kaolinite [J]. Journal of Thermal Analysis and Calorimetry, 1999, 56: 865-884.
[29] CHANDRASEKHAR S. Influence of metakaolinization temperature on the formation of zeolite 4A from kaolin [J]. Clay Minerals, 1996, 31: 253-261. DOI: 10.1180/claymin. 1996.031.2.11.
[30] ALKAN M, HOPA C, YILMAZ Z, CULER H. The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite [J]. Microporous & Mesoporous Materials, 2005, 86: 176- 184. DOI: 10.1016/j.micromeso.2005.07.008.
[31] BICH C, AMBROISE J, PERA J. Influence of degree of dehydroxylation on the pozzolanic activity of metakaolin [J]. Apply Clay Science, 2009, 44: 194-200. DOI: 10.1016/ j.clay.2009.01.014.
[32] LIU Q, SPEARS D A. MAS NMR study of surface-modified calcined kaolin [J]. Apply Clay Science, 2001, 19: 89-94. DOI: 10.1016/S0169-1317(01)00057-6.
(Edited by FANG Jing-hua)
中文导读
微波加热和传统加热方式对高岭土热活化性能影响的比较
摘要:本文研究了微波加热和传统加热对高岭土直接制备沸石前驱体活化性能的影响。讨论了XRD,TG-DSC,FT-IR,SEM,粒度分析,比表面积(BET),孔径分布(BJH)和N2吸附-脱附等温线以确定最佳热活化温度。结果表明,微波场中高岭土向偏高岭土的转化在500 °C下保温30 min就能实现,这比常规煅烧温度低100 °C,时间缩短90 min,在微波与常规加热方法中高岭土相变过程相同。SEM分析表明,在微波场中,产物粒度更均匀,略有团聚。高岭土的N2吸附-解吸等温线,BET和BJH分析表明,在高岭土煅烧为偏高岭土的过程中,无论以何种方式煅烧,其孔隙性质几乎不变。以上结论表明,在微波场中活化高岭土优于常规活化。这主要是因为微波依靠物体吸收微波能量并将其转换成热能来加热,从而可以均匀地加热整个物质。
关键词:高岭土;热活化;偏高岭土;微波
Foundation item: Projects(51604135, 51504116) supported by the National Natural Science Foundational of China; Project(YNWR-QNBJ-2018-323) supported by the Yunan Ten Thousand Talents Plan Young & Elite Talents Project, China
Received date: 2020-02-05; Accepted date: 2020-06-03
Corresponding author: YIN Shao-hua, PhD, Associate Professer; Tel: +86-871-65191046, +86-871-65174756; E-mail: yinsh@kust. edu.cn; ORCID: https://orcid.org/0000-0003-2605-2442; ZHANG Li-bo, PhD, Professor; Tel: +86-871- 65174756; E-mail: zhanglibopaper@126.com; ORCID: https://orcid.org/0000-0003-3244-0142
Abstract: The effect of activation properties of the precursors of zeolite directly prepared from kaolin influenced by microwave field and conventional heating was investigated. XRD, TG-DSC, FT-IR, SEM, particle size analysis, specific surface area (BET), pore size distribution (BJH) and N2 adsorption-desorption were discussed to determine the optimal activation temperature. It is concluded that the conversion of kaolin to metakaolin in the microwave field is at 500 °C holding for 30 min, which is 100 °C lower than that in conventional calcination and 90 min shorter, and the phase transition process of kaolin under the effect of microwave field is the same as that of conventional heating method. SEM analysis indicates that the particle size is more uniform and agglomeration appears slightly in the microwave field. The N2 adsorption-desorption isotherm, BET and BJH of kaolin indicate that the pore properties are almost invariable regardless of calcination route during the process of calcining kaolin into metakaolin. It indicates that microwave calcination is superior to conventional calcination in the activation pathway of kaolin. It is attributed to microwave heating relying on objects to absorb microwave energy and convert it into thermal energy, which can simultaneously and uniformly heat the entire substance.


