
ARTICLE
J. Cent. South Univ. (2019) 26: 343-352
DOI: https://doi.org/10.1007/s11771-019-4006-x
Isolation of an acid producing Bacillus sp. EEEL02: Potential for bauxite residue neutralization
WU Hao(吴昊)1, LIAO Jia-xin(廖嘉欣)1, ZHU Feng(朱锋)1, Graeme MILLAR2,Ronan COURTNEY3, XUE Sheng-guo(薛生国)1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Institute for Future Environments, Science and Engineering Faculty, Queensland University ofTechnology (QUT), Brisbane Qld 4000, Australia;
3. Department of Life Sciences, Schrodinger Building, University of Limerick, Co. Limerick, Ireland
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
Bauxite residue deposit area (BRDA) is a typical abandoned mining wasteland representing extreme hostile environment with increased alkalinity. Microbially-driven neutralization of bauxite residue, based on the microbial acid producing metabolisms, is a novel strategy for achieving rapid pH neutralization and thus improving its environmental outcomes. The hypothesis was that these extreme conditions promote microbial communities which are capable of novel ecologically relevant functions. Several alkaliphilic acid producing bacteria were isolated in this study. One strain was selected for its superior growth pattern and acid metabolism (termed EEEL02). Based on the phylogenetic analysis, this strain was identified as Bacillus thuringiensis. The optimized fermentation conditions were as follows: pH 10; NaCl concentration 5%; temperature 25 °C; EEEL02 preferred glucose and peptone as carbon and nitrogen sources, respectively. Based on optimal fermentation conditions, EEEL02 induced a significant pH reduction from 10.26 to 5.62 in 5-day incubation test. Acetic acid, propionic acid and CO2 (g) were the major acid metabolites of fermentation, suggesting that the pH reduction in bauxite residue may be caused by acid neutralization derived from microbial metabolism. This finding provided the basis of a novel strategy for achieving rapid pH neutralization of bauxite residue.
Key words:
bauxite residue; 16S rDNA; Bacillus thuringiensis; acid production; pH neutralization;
Cite this article as:
WU Hao, LIAO Jia-xin, ZHU Feng, Graeme MILLAR, Ronan COURTNEY, XUE Sheng-guo. Isolation of an acid producing Bacillus sp. EEEL02: Potential for bauxite residue neutralization [J]. Journal of Central South University, 2019, 26(2): 343–352.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4006-x1 Introduction
Many industrial processes, such as chromium ore processing, iron and steel manufacture and alumina extraction, produce substantial amounts of alkaline tailings (residues). In particularly, the bauxite residue, which generated from the aluminium extraction process, presents the largest volumes of alkaline tailings [1]. Currently, the bauxite residue has reached 4.6 billion tons all over the world and still increases rapidly with the rate of 200 million tons per annum [2]. Commonly, the waste product is deposited in engineered impoundments forming a bauxite residue deposit areas (BRDAs) [3]. Since the extraction process involves concentrated sodium hydroxide solutions, the residues exhibit high alkalinity and salinity which dominated by sodium (Na+) ions [4, 5]. Moreover, the waste materials are compacted,which results in these sites being devoid of vegetation [6]. Without stable vegetation growth, the residue is more vulnerable to wind and water erosion, causing a series of environmental problems. Therefore, the management of bauxite residue has become a major problem which restricts the sustainable development of the alumina industry. One strategy to alleviate the outlined situation is the establishment of a methodology for formation of stable vegetation in the bauxite residue deposit area [7]. However, the excess alkalinity is the principal obstacle to the establishment of vegetation [8]. Thus, various amendments have been applied to bauxite residue prior to vegetation in order to alleviate the negative effects of alkaline substances on plant growth [9–12]. For example, application of gypsum can induce precipitation of species such as calcium hydroxide, tri-calcium aluminate, hydrocalumite and calcite. The pH of 5% gypsum treated residue decreased from an initial value of 10.5 to 8.6 with no significant difference between 5% and 8% additions which suggested that the pH was buffered [13]. Ferrous sulphate exhibited an acceptable acidifying effect on bauxite residue as it underwent rapid hydrolysis with the release of H+ ions which dissolved the alkaline substrate [14]. Acid neutralization may be more effective on bauxite residue dealkalization. KONG et al [15] compared the impacts of different types of acids (HCl, H2SO4, C6H8O7) on dealkalization of bauxite residue and found that the efficiency of neutralization only depended on the quantities of protons released. Due to the higher cost of inorganic acid, organic acids may be the most suitable substances for alkaline neutralization which were commonly obtained via microbial fermentation. Therefore, microbially- driven bioremediation appears to be a promising candidate for alkalinity neutralization in bauxite residue [16].
Currently, researches on microbially-driven bioremediation in bauxite residue deposit areas (BRDAs) mainly focused on the effects of microbial process on alkalinity neutralization in bauxite residue. A significant residual pH reduction from 13 to 7 was observed in the presence of hay [17]. Microbial communities successfully established in long-term fertilization [18]. Microbial inoculants are also applied to bauxite residue for alkalinity neutralization. KRISHNA et al [19] inoculated Aspergillus tubingensis to the residue-soil and significant pH reduction was observed in all microbial treated residues. BABU et al [20] also reported that Aspergillus tubingensis could help to neutralize residues and promoted vegetation establishment on bauxite residue. Therefore, the microbial acid metabolism seems to a promising way to induce pH down in bauxite residue.
Various microbial species are capable of acid metabolic capabilities. The genus Aspergillus can secret various acidic metabolites under aerobic condition. Under anaerobic conditions, Acetobacter can produce acetic acid [21] and Lactobacillaceae supports lactic acid production [22]. These processes are commonly conducted under neutral or slightly acidic environments. It was scarcely reported that the acid metabolisms occur in strongly alkaline conditions. Some alkaliphiles which demonstrate the capacity of acid production have been separated from natural alkaline environments such as marine, soda lakes, and other extreme alkaline environments [23]. Microbes derived from Bacillus can produce lactic under an aerobic condition. A Halolactibacillus halophilus was isolated from a marine environment with the production of 65.8 g/L lactic acid at pH 9.0 and preferred sucrose as carbon source [24]. A new, extremely haloalkaliphilic, chemo-organotrophic, bacterium strain Z-7937T (T-type strain) can metabolize lactate, ethanol, pyruvate, and glutamate to acetate under alkaline condition (pH 8.1–10.7) [25]. A novel halotolerant, alkaliphilic, humic acid-reducing bacterium which produced lactic via a facultative anaerobic pathway was obtained from an artificial alkaline wastewater (pH 10) [26]. However, to date, minimal studies reported the acid production of isolated microbes in bauxite residue disposal area.
Therefore, the aims of this work were to: 1) investigate the acid producing capacity of isolations from bauxite residue disposal area; 2) evaluate its potential application in residue neutralization. The hypothesis was that the extreme conditions of the BRDA promoted the growth of novel microbial strains with improved acid production abilities.
2 Materials and methods
2.1 Sample collection and bacterial isolation
Residue samples were collected aseptically on a BRDA operated by the National Aluminum Company (NALCO), Guangxi Branch, China. The climate is described as humid subtropical monsoon, with an average daily temperature of 21.5 °C and mean rainfall of 1400 mm per year. Natural plant colonization was observed at several sites on the disposal area. Several samples were taken from the rhizosphere of different plants and stored in sterile bags, and then shipped to the laboratory under refrigerated (4 °C) conditions.
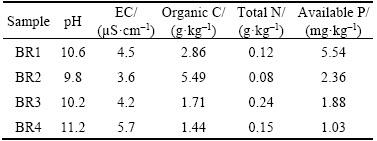
The residue samples were characterized by high pH and electrical conductivity (EC) (Table 1), representing extreme alkalinity and salinity. Due to the large supplement of sodium hydroxide, the residue was expected to be alkaline and saline. Such extreme environments often harbor considerably diverse microbial communities that exhibit significantly unusual metabolic enzymes and metabolic mechanisms.
Table 1 Characteristics of selected bauxite residue samples
Residue samples were activated in an enrichment medium which contained 2% glucose, 0.5% peptone and 0.5% NaCl to revitalize the microbial communities. After 48 h, the bacterial suspension was serially diluted and spread on acid production identification medium, containing glucose 2%, yeast extract 0.5%, peptone 0.5%, NaCl 5% and agar 1.5%–2%. Bromocresol green was added as indicator for acid production. The acid-producing bacteria were screened out according to the color changes from green to yellow of plates. These colonies were streaked onto new nutrient agar several times to obtain pure cultures and then the pure cultures were returned to the liquid fermentation medium to screen for highly acid producing bacterial strains. The strain that induced the largest pH reduction, designated as EEEL02, was chosen for further studies.
2.2 Morphology and molecular identification
The obtained pure cultures were subjected to scanning electron microscope (SEM) for morphological determination. Physiological and biochemical tests were conducted according to Bergey’s Manual of Systematic Bacteriology.
For DNA extraction and gene amplification, a TSINGKE (https://www.tsingke.net) DNA extraction kit (number: TSP101-50) was used. The 16S rDNA gene was amplified using primers as follows: forward primer (5’-AGAGTTTGAT CCTGGCTCAG-3’); reverse primer (5’-ACGGGC GGTGTGTTC-3’) and performed with GenAmp PCR system. The amplification program was as follows: preheating at 92 °C for 120 s, 36 cycles of 92 °C for 60 s, 48 °C for 30 s and 72 °C for 120 s and final extension at 72 °C for 370 s. The PCR products were gel-purified using the TSINGKE DNA purification kit. Fragments were then cloned into the DL2000 DNA Marker and positive clones were sequenced by TSINGKE Biological Technology.
The 16S rDNA gene sequence was added to the NCBI and compared with those from related bacteria using the BLASTN program and then aligned using the CLUSTALW program. The MEGA 7.0 package was used for multiple sequence alignments and phylogenetic tree construction.
2.3 Optimization of fermentation conditions
To investigate the effect of initial pH on acid production, fermentation was conducted at 25 °C in 150 mL Erlenmeyer flasks containing 100 mL of the fermentation medium which consisted of: glucose 50 g/L; yeast extract 10 g/L; peptone, 5; NaCl, 50 g/L; K2HPO4·3H2O, 1 g/L; MgSO4·7H2O, 0.2 g/L; MnSO4·H2O, 0.005 g/L; and CH3COONa, 5 g/L. The pH was adjusted to 8.0, 9.0, 10.0, 11.0 and 12.0 using appropriate amounts of 4 mol/L NaOH solution.
To test the effect of the salinity upon acid production, fermentation was conducted in the same medium as described above, except for the addition of NaCl which ranged from 0.1% to 5 % concentration. The pH was adjusted to the optimal value obtained from above by adding sterilized 4 mol/L NaOH solution. The optimal fermentation temperature of strain EEEL02 was also tested in 150 mL Erlenmeyer flasks containing 100 mL of fermentation medium. Fermentations were conducted statically at 25, 30, 35, 40 and 45 °C. Samples were all taken after 24 h of fermentation.
2.4 Optimization of fermentation medium
The medium described above was used as the initial fermentation medium and the amount for each constituent was used in the next step for the optimization of the components. Fermentation was conducted statically at 25 g/L in 150 mL Erlenmeyer flasks containing 100 mL of medium. Samples were taken after 24 h and the pH reduction was measured in medium. The effects of glucose, lactose, sucrose, maltose, and starch on acid production were determined in medium supplemented with the carbon sources at an initial concentration of 20 g/L. The optimal nitrogen source was selected by adding 5 g/L of either beef extract (BE), yeast extract (YE), potassium nitrate (PN), ammonium nitrate (AN) and ammonium sulfate (AS).
2.5 Neutralization of bauxite residues
Experimental bioreactors were prepared in sterile 100 mL glass vials sealed with a gas-tight plastic cap containing an aperture in which a PTFE-lined silicone septum was mounted. Bauxite residue (5 g) was added to the vials, followed by 25 mL of nutrient media. Three replicates were prepared for acid quantification and qualification. Vials were sealed and placed on an orbital shaker at 150 r/min and opened once to collect residue samples at the end of the whole experiment.
2.6 Statistical methods
Data were analyzed by analysis of variance (ANOVA) and the mean values were compared by LSD at P<0.05. The statistical analyses were performed using Origin 9.1 software.
3 Results and discussion
3.1 Characterization of bacteria
In total, 48 bacteria strains were isolated from four residue samples and eight strains were capable of pH reduction ability under alkaline condition. Out of these strains, one was chosen for the further study for its superior growth pattern and pH reduction performance (termed EEEL02). Studies have improved our understanding of the biology of extreme environments and resulted in the discovery of novel microorganisms and enzymes (extremozymes) with potential for biotechnological applications, which are stable at high pH, and salt concentration.
EEEL02 exhibited irregular edges on beef extract-peptone medium, and was light yellow and shiny, slightly raised, moist and easily picked up. The SEM image shows that the strain was rod-like with flagella and the size ranged in 1–5 μm approximately (Figure 1).
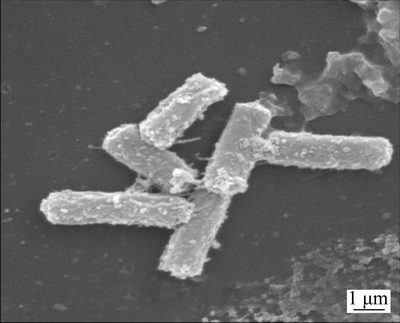
Figure 1 Morphology of EEEL02 under scanning electron microscope
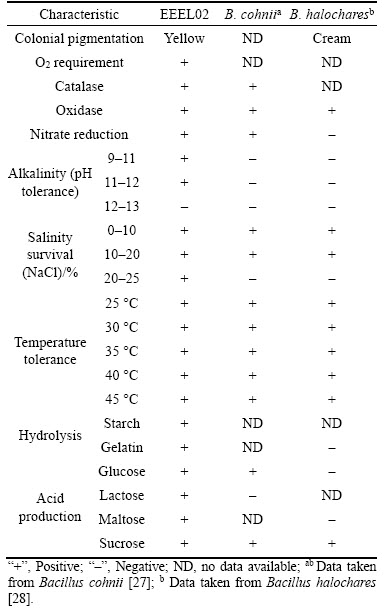
According to a series of biochemical tests, EEEL02 was: aerobic; gram variable; and catalase, oxidase and nitrate reduction positive (Table 2). Acid production was noted with maltose, glucose, lactose and sucrose. This isolate was able to grow under pH 12. When subjected to salinity and temperature tests, EEEL02 survived at 0–25% NaCl concentration and a temperature range of 25–45 °C.
The nucleotide BLAST showed that EEEL02 belonged to the phylum Firmicutes and genus Bacillus. Phylogenetic analysis revealed that EEEL02 showed 100% similarity with Bacillus thuringiensis (Figure 2). Homology analysis of 16S rRNA provides suitable genetic data that can be used to determine both close and very distant relationships. The clones that had sequence identities of over 98% to a known organism may represent the same species. In this study, the isolated Bacillus thuringiensis exhibited considerable H+ secretion capacity which unveiled the existence of H+ producing bacteria in this extreme ecosystem. Such microbial strains which are capable of surviving in such hostile environments must be more specific in terms of metabolic enzymes and metabolic mechanisms. A Paenibacillus could also grow at pH 10.5 and with a stronger saline tolerance up to 15% NaCl content [29]. An xylanolytic actinomycete strain isolated from alkaline bauxite residue was able to grow at pH 10.5 with 5% NaCl concentration [30]. Bacillus thuringiensis is a sporulating, Gram-positive facultative-aerobic soil bacterium which can produce crystal proteins [31]. Extensive researches on the application of Bacillus thuringiensis are focused on bio-pesticides formulations [32]. The H+ production of Bacillus thuringiensis however has scarcely been reported and this study has enriched the microbial application prospect for this microbe. This discovery also provides a new strategy in terms of remediation of bauxite residue disposal area.
Table 2 Physiological features of EEEL02 and comparison with other bacillus species
3.2 Optimization of fermentation conditions
Initial pH of the medium is critical for microbial growth and various metabolisms such as acid production. Thus, EEEL02 was tested at an initial pH of 8.0, 9.0, 10.0, 11.0 and 12.0 to ascertain the microbial growth and acid production rate. The performance of acid production of EEEL02 showed significant different between all the treatments (Figure 3(a)). The best pH reduction was obtained at pH 10.0, with a final pH being 3.32. When the initial pH set to 11.0 or 12.0, the microbial growth and the acid production was weak and no significant difference (P>0.05) was observed, indicating that high pH was unfavorable to both microbial growth and acid formation metabolisms.
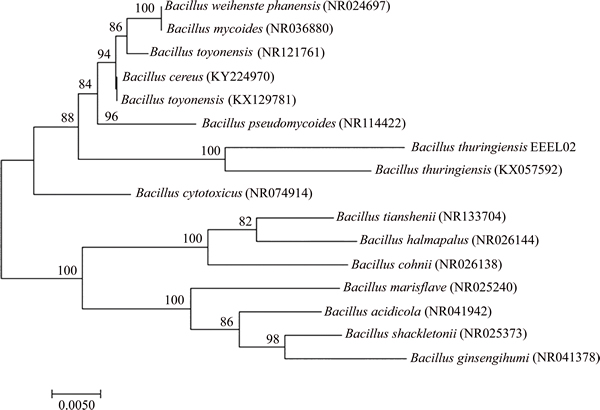
Figure 2 Neighbor-joining tree based on 16S rDNA sequences of BR1 along with sequences available in GenBank database (Numerical values indicate bootstrap percentile from 1000 replicates)
Salt content is another critical factor which can hinder microbial growth in bauxite residue. From the biochemical tests, EEEL02 was found to be capable of growth in a fluid medium with 5% NaCl. Based on this finding, the effect of NaCl concentration on acid production was further investigated under different NaCl concentrations (0.1%, 0.5%, 1.0%, 2.0% and 5.0%). Optimum acid production was observed at 2% NaCl level, and medium pH which was reduced from 10.0 to 3.51 in 48 h (Figure 3(b)). The sub-optimal condition was found at 1% NaCl level and the medium pH declined to 3.65 after 2 d fermentation. Higher salinity significantly inhibited microbial activity and its metabolic processes which were found in 2% and 5% NaCl treatments. When at the 2% and 5% NaCl levels, the medium pH declined from 10.0 to 3.81, and 4.71, respectively; and it presented significant difference (P<0.001) from alternate treatment. This observation revealed that 5% NaCl resulted in the poorest conditions for acid optimum production compared to other salinities. The condition for acid production by EEEL02 was 1% NaCl concentration.
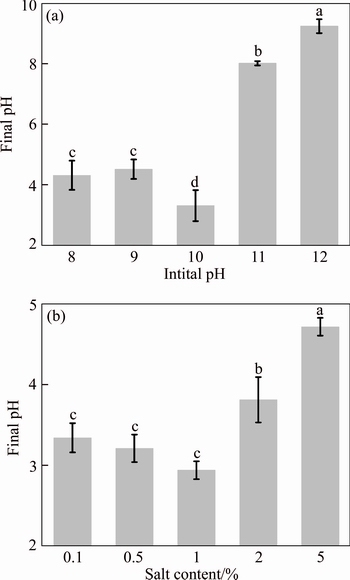
Figure 3 Final pH of culture medium with different initial pH (a) and NaCl content (b)
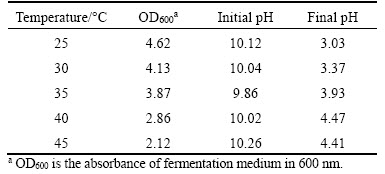
The optimum fermentation temperature was observed at 25 °C and the medium pH declined from 10.0 to 3.03 in 24 h. When the temperature increased, the microbial growth and acid metabolism suffered significantly suppression (Table 3). The pH of the culture medium decreased from 10 to 3.37, 3.93, 4.47 and 4.41, respectively. Therefore, 25 °C was chosen as the optimum temperature in the follow-up experiments.
Table 3 Microbial growth and pH reduction by Bacillus sp. EEEL02 cultured under different temperatures
3.3 Optimization of fermentation medium
In the present study, maltose, sucrose, glucose, lactose, and starch were chosen as the carbon source for the microbial growth and corresponding pH reduction. All carbon sources, except for starch, could be used by Bacillus sp. EEEL02 to produce protons. Glucose and maltose resulted in the largest pH reduction from 10.0 to 2.8 and 3.2, respectively (Figure 4(a)). Sucrose and lactose showed a weaker performance in acid production that reduced pH to around 4.5 to 4.8 (Figure 4(a)).
Different organic and inorganic nitrogen sources were also investigated for the microbial growth and acid production. Ammonium sulfate, potassium nitrate and ammonium nitrate were chosen as inorganic nitrogen sources and they showed little acid production ability. There were no significant differences between these three inorganic nitrogen sources which exhibited pH reduction from 10.0 to 7.48, 6.81 and 7.01, respectively. Organic nitrogen gave a better performance in terms of both microbial growth and acid production. When beef extract and yeast extract were used as organic nitrogen sources, the medium pH decreased from 10.0 to 3.2 and 3.08, respectively. No significant difference (P<0.05) was observed between beef extract and yeast extract and yeast extract was selected as the nitrogen source in this study.
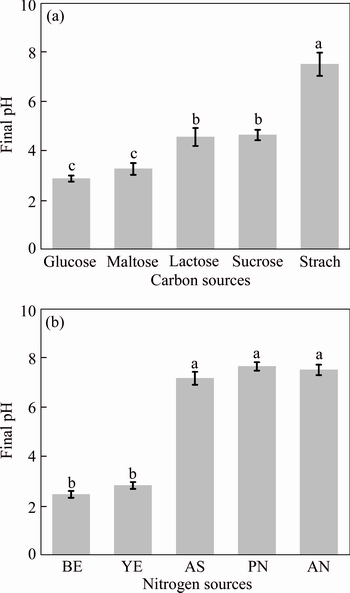
Figure 4 Final pH of culture medium with carbon sources (a) and nitrogen sources (b)
The substrate for microbial fermentation is crucial for organic acids production. The yield of acids produced (acetic, propionic, lactic, and citric acids) varies depending on the substrate provided. Glucose, which is liable to be utilized by various microorganisms, has been most widely used as a carbon source for organic acids production. For lactic acid production, glucose resulted in higher production than other glycogens, yielding up to 18 g/L L-lactic acid production [33]. H. halophilus preferred glucose as carbon source to mannose and xylose, producing 1.7 mol lactic acid per mol glucose [24]
3.4 Neutralization of bauxite residue under optimum conditions
The optimum growth medium for EEEL02 in the present study was chosen for the further neutralization study. Under these optimum conditions, a significant decrease in pH was observed along with the incubation time from an initial 10.26 to 5.62 (Figure 5(a)). This may result from acid secretion via microbial fermentation which neutralized the alkaline substrates in both the bauxite residue pore water and solid [34]. However, it was noted that the pH increased slightly in the next 3 d. This may be caused by the continuously dissolving of the alkaline minerals within bauxite residue, which made it be strong buffered [35]. The residue pH will not maintain a stable value until the solids are completely dissolved and their reaction products removed.
The pH reduction in the growth medium is necessarily due either to the formation of acids or to the removal of bases. Acetic acid, propionic acid and CO2 (g) were the major acid metabolites of fermentation, showing a significant increase in concentration of fermentation products over time (Figure 5(b)). The total acid concentration showed significant negative correlation with the pH, suggesting that the acids produced in the microbial metabolism process were the main factor driving pH reduction in bauxite residue. The metabolic products of genus Bacillus have been found majorly to be acetic acid when utilizing sucrose as a carbon source. This behavior is consistent with a functional pyruvate to acetate conversion pathway in others Bacillus [36–39]. Thus, the acid metabolism of basophilic microorganisms may be a new candidate for the technology development on alkalinity regulation of bauxite residue.
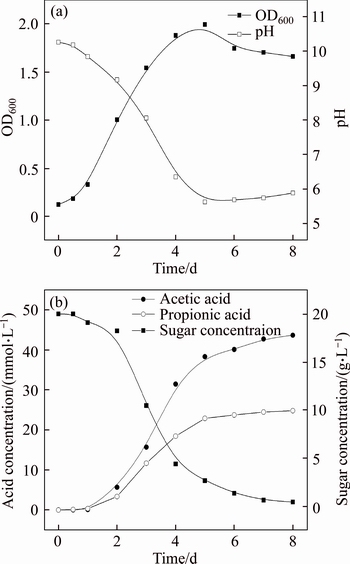
Figure 5 Time course of microbial production and pH reduction (a) and acid fermentation (b) by EEEL02
In previous studies, various alkaliphilic members have been proven to be capable of acid metabolism and applied to some alkaline waste disposal. KULSHRESHTHA et al [40] introduced several Bacillales to neutralize alkaline industrial wastewater and found that a significant pH reduction occurred. The pH of alkaline wastewater dropped from initial value of 12.0 to a final value of 7.5, which is derived from various acids from the acid secretion process. HAMDY et al [17] demonstrated that bacterial cells actively grew in the presence of essential nutrients and hay, forming organic acids and leading to a pH reduction from 13.0 to 7.0. Aspergillus tubingensis was also was applied to alkaline regulation for its excellent acid-producing ability [19, 20]. SANTINI et al [41] inoculated a diverse microbial community to bauxite residue and found that it was a novel strategy for achieving rapid pH neutralization. In this study, the final pH of bauxite residue was maintained at a relatively neutral level (pH=6.48) which was an optimum pH value for sustained vegetation growth. However, this excellent acid producing ability was expressed in the presence of glucose and yeast extract, which may not be viable when applied in the field.
4 Conclusions
In this study, an efficient acid producing bacterium was isolated from bauxite residues, which exhibited adaption to extremely alkaline conditions. Morphological and molecular analysis revealed that EEEL02 belonged to the genus Bacillus, which shared 100% sequence similarity with Bacillus thuringiensis. The optimum fermentation conditions for the acid production of EEEL02 were as follows: pH 10; NaCl 1%; carbon source, glucose; and nitrogen source, yeast extract. Under the optimal fermentation condition, the pH of bauxite residue decreased from 10.74 to 5.62 in 5-day incubation. Acetic, propionic acid and CO2, generated from microbial fermentation process may be the main reason for pH reduction in bauxite residue. This strategy provides a new technology for alkalinity regulation in bauxite residue.
References
[1] XUE Sheng-guo, KONG Xiang-feng, ZHU Feng, HARTLEY W, LI Xiao-fei, LI Yi-wei. Proposal for management and alkalinity transformation of bauxite residue in China [J]. Environmental Science and Pollution Research, 2016, 23(13): 12822–12834. DOI: 10.1007/s11356-016- 6478-7.
[2] XUE Sheng-guo, WU Yu-jun, LI Yi-wei, KONG Xiang-feng, ZHU Feng, HARTLEY W, LI Xiao-fei, YE Yu-zhen. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review [J]. Journal of Central South University, 2019, 26(2): 268–288.
[3] ZHU Feng, XUE Sheng-guo, HARTLEY W, HUANG Ling, WU Chuan, LI Xiao-fei. Novel predictors of soil genesis following natural weathering processes of bauxite residues [J]. Environmental Science and Pollution Research, 2016, 23(3): 2856–2863. DOI: 10.1007/s11356-015-5537-9.
[4] KONG Xiang-feng, GUO Ying, XUE Sheng-guo, HARTLEY W, WU Chuan, YE Yu-zhen, CHENG Qing-yu. Natural evolution of alkaline characteristics in bauxite residue [J]. Journal of Cleaner Production, 2017, 143: 224–230. DOI: 10.1016/j.jclepro.2016.12.125.
[5] KONG Xiang-feng, JIANG Xing-xing, XUE Sheng-guo, HUANG Ling, HARTLEY WILLIAM, WU Chuan, LI Xiao-fei. Migration and distribution of saline ions in bauxite residue during water leaching [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 534–541. DOI: 10.1016/S1003-6326(18)64686-2.
[6] ZHU Feng, ZHOU Jia-yi, XUE Sheng-guo, HARTLEY W, WU Chuan, GUO Ying. Aging of bauxite residue in association of regeneration: A comparison of methods to determine aggregate stability & erosion resistance [J]. Ecological Engineering, 2016, 92(3): 47–54. DOI: 10.1016/j.ecoleng. 2016.03.025.
[7] XUE Sheng-guo, ZHU Feng, KONG Xiang-feng, WU Chuan, HUANG Ling, HUANG Nan, HARTLEY W. A review of the characterization and revegetation of bauxite residues (Red mud) [J]. Environmental Science and Pollution Research, 2016, 23(2): 1120–1132. DOI: 10.1007/s11356- 015-4558-8.
[8] GRAFE M, POWER G, KLAUBER C. Bauxite residue issues: III. Alkalinity and associated chemistry [J]. Hydrometallurgy, 2011, 108(1, 2): 60–79. DOI: 10.1016/ j.hydromet.2011.02.004.
[9] LI Yi-wei, JIANG Jun, XUE Sheng-guo, MILLAR G, KONG Xiang-feng, LI Xiao-fei, LI Meng, LI Chu-xuan. Effect of ammonium chloride on leaching behavior of alkaline anion and sodium ion in bauxite residue [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 2125–2134. DOI: 10.1016/S1003-6326(18)64857-5.
[10] XUE Sheng-guo, LI Meng, JIANG Jun, GRAEME J M, LI Chu-xuan, KONG Xiang-feng. Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Environmental Sciences, 2019, 77: 1–10. DOI: 10.1016/j.jes.2018.05.016.
[11] LI Xiao-fei, YE Yu-zhen, XUE Sheng-guo, JIANG Jun, WU Chuan, KONG Xiang-feng, HARTLEY W, LI Yi-wei. Leaching optimization and dissolution behavior of alkaline anions in bauxite residue [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 1248–1255. DOI: 10.1016/S1003-6326(18)64763-6.
[12] ZHU Feng, HOU Jiang-tao, XUE Sheng-guo, WU Chuan, WANG Qiong-li, HARTLEY W. Vermicompost and gypsum amendments improve aggregate formation in bauxite residue [J]. Land Degradation and Development, 2017, 28(7): 2109–2120. DOI: 10.1002/ldr.2737.
[13] WONG J. Use of waste gypsum in the revegetation on red mud deposits: A greenhouse study [J]. Waste Management & Research, 1993, 11(3): 249–256. DOI: 10.1006/wmre. 1993.1024.
[14] JONES B E H, HAYNES R J, PHILLIPS I R. Influence of amendments on acidification and leaching of Na from bauxite processing sand [J]. Ecological Engineering, 2015, 84: 435–442. DOI: 10.1016/j.ecoleng.2015.09.054.
[15] KONG Xiang-feng, LI Meng, XUE Sheng-guo, HARTLEY W, CHEN Cheng-rong, WU Chuan, LI Xiao-fei, LI Yi-wei. Acid transformation of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Hazardous Materials, 2017, 324(B): 382–390. DOI: 10.1016/j.jhazmat.2016. 10.073.
[16] SANTINI T C, KERR J L, WARREN L A. Microbially- driven strategies for bioremediation of bauxite residue [J]. Journal of Hazardous Materials, 2015, 293: 131–157. DOI: 10.1016/j.jhazmat.2015.03.024.
[17] HAMDY M K, WILLIAMS F S. Bacterial amelioration of bauxite residue waste of industrial alumina plants [J]. Journal of Industrial Microbiology & Biotechnology, 2001, 27(4): 228–233. DOI: 10.1038/sj/jim/7000181.
[18] SCHMALENBERGER A, O'SULLIVAN O, GAHAN J, COTTER P D, COURTNEY R. Bacterial communities established in bauxite residues with different restoration histories [J]. Environmental Science & Technology, 2013, 47(13): 7110–7119. DOI: 10.1021/es401124w.
[19] KRISHNA P, REDDY M S, PATNAIK S K. Aspergillus tubingensis reduces the pH of the bauxite residue (Red mud) amended soils [J]. Water, Air, & Soil Pollution, 2005, 167(1–4): 201–209. DOI: 10.1007/s11270-005-0242-9.
[20] BABU A G, REDDY M S. Aspergillus tubingensis improves the growth and native mycorrhizal colonization of bermudagrass in bauxite residue [J]. Bioremediation Journal, 2011, 15(3): 157–164. DOI: 10.1080/10889868.2011. 598486.
[21] GHOMMIDH C, NAVARRO J M, DURAND G. Acetic acid production by immobilized acetobacter cells [J]. Biotechnology Letters, 1981, 3(2): 93–98. DOI: 10.1007/ bf00145117.
[22] ROJAN P J, NAMPOOTHIRI K M, NAIR A S, PANDEY A. L (+)-lactic acid production using Lactobacillus casei in solid-state fermentation [J]. Biotechnology Letters, 2005, 27(21): 1685–1688. DOI: 10.1007/s10529-005-2731-8.
[23] SARETHY I P, SAXENA Y, KAPOOR A, SHARMA M, SHARMA S K, GUPTA V, GUPTA S. Alkaliphilic bacteria: Applications in industrial biotechnology [J]. Journal of Industrial Microbiology & Biotechnology, 2011, 38(7): 769–790. DOI: 10.1007/s10295-011-0968-x.
[24] CALABIA B P, TOKIWA Y, AIBA S. Fermentative production of L: -(+)-lactic acid by an alkaliphilic marine microorganism [J]. Biotechnology Letters, 2011, 33(7): 1429–1433. DOI: 10.1007/s10529-011-0573-0.
[25] ZHILINA T N, KEVBRIN V V, TUROVA T P, LYSENKO A M, KOSTRIKINA N A, ZAVARZIN G A. Clostridium alkalicellum sp. nov., an obligately alkaliphilic cellulolytic bacterium from a soda lake in the Baikal region [J]. Microbiology, 2005, 74(5): 642–653. DOI: 10.1007/s11021- 005-0103-y.
[26] WU Chun-yuan, ZHUANG Li, ZHOU Shun-gui, LI Fang-bai, HE Jian. Corynebacterium humireducens sp. nov., an alkaliphilic, humic acid-reducing bacterium isolated from a microbial fuel cell [J]. International Journal of Systematic and Evolutionary Microbiology, 2011, 61(Pt4): 882–887. DOI: 10.1099/ijs.0.020909-0.
[27] NOGUEIRA E W, HAYASH E A, ALVES E, LIMA C A D, ADORNO M T, BRUCHA G. Characterization of alkaliphilic bacteria isolated from bauxite residue in the southern region of minas gerais, Brazil [J]. Brazilian Archives of Biology and Technology, 2017, 60. DOI: 10.1590/1678-4324- 2017160215.
[28] PAPPA A, SáNCHEZ-PORRO C, LAZOURA P, KALLIMANIS A, PERISYNAKIS A, VENTOSA A, DRAINAS C, KOUKKOU A I. Bacillus halochares sp. nov., a halophilic bacterium isolated from a solar saltern [J]. International Journal of Systematic and Evolutionary Microbiology, 2010, 60(6): 1432–1436. DOI: 10.1099/ ijs.0.014233-0.
[29] ARORA A, KRISHNA P, MALIK V, REDDY M S. Alkalistable xylanase production by alkalitolerant Paenibacillus montaniterrae RMV1 isolated from red mud [J]. Journal of Basic Microbiology, 2014, 54(10): 1023–1029. DOI: 10.1002/jobm.201300357.
[30] KRISHNA P, ARORA A, REDDY M S. An alkaliphilic and xylanolytic strain of Actinomycetes Kocuria sp RM1 isolated from extremely alkaline bauxite residue sites [J]. World Journal of Microbiology & Biotechnology, 2008, 24(12): 3079–3085. DOI: 10.1007/s11274-008-9801-8.
[31] SANAHUJA G, BANAKAR R, TWYMAN R M, CAPELL T, CHRISTOU P. Bacillus thuringiensis: A century of research, development and commercial applications [J]. Plant Biotechnology Journal, 2011, 9(3): 283–300. DOI: 10.1111/ j.1467-7652.2011.00595.
[32] SANSINENEA E. Bacillus thuringiensis biotechnology [M]. Springer, 2012: 201–214.
[33] MENG Ying, XUE Yan-fen, YU Bo, GAO Cheng-hua, MA Yan-he. Efficient production of L-lactic acid with high optical purity by alkaliphilic Bacillus sp. WL-S20 [J]. Bioresource Technology, 2012, 116(4): 334–339. DOI: 10.1016/j.biortech.2012.03.103.
[34] LIAO Jia-xin, JIANG Jun, XUE Sheng-guo, CHENG Qing-yu, WU Hao, RAJENDRAN M, HARTLEY W, HUANG Long-bin. A novel acid-producing fungus isolated from bauxite residue: The potential to reduce the alkalinity [J]. Geomicrobiology Journal, 2018, 35(10): 840–847. DOI: 10.1080/01490451.2018.1479807.
[35] KONG Xiang-feng, TIAN Tao, XUE Sheng-guo, HARTLEY W, HUANG Long-bin, WU Chuan, LI Chu-xuan. Development of alkaline electrochemical characteristics demonstrates soil formation in bauxite residue undergoing natural rehabilitation [J]. Land Degradation & Development, 2018, 29(1): 58–67. DOI: 10.1002/ldr.2836.
[36] KANSO S, GREENE A C, PATEL B K. Bacillus subterraneus sp. nov., an iron- and manganese-reducing bacterium from a deep subsurface Australian thermal aquifer [J]. International Journal of Systematic and Evolutionary Microbiology, 2002, 52(Pt3): 869–874. DOI: 10.1099/ ijs.0.01842-0.
[37] TAKAMI H, TAKAKI Y, UCHIYAMA I. Genome sequence of Oceanobacillus iheyensis isolated from the Iheya Ridge and its unexpected adaptive capabilities to extreme environments [J]. Nucleic Acids Research, 2002, 30(18): 3927–3935. DOI: 10.1093/nar/gkf526.
[38] ISHIKAWA M, NAKAJIMA K, ITAMIYA Y, FURUKAWA S, YAMAMOTO Y, YAMASATO K. Halolactibacillus halophilus gen. nov., sp. nov. and Halolactibacillus miurensis sp. nov., halophilic and alkaliphilic marine lactic acid bacteria constituting a phylogenetic lineage in Bacillus rRNA group 1 [J]. International Journal of Systematic and Evolutionary Microbiology, 2005, 55(Pt6): 2427–2439. DOI: 10.1099/ijs.0.63713-0.
[39] SARKAR P K, HASENACK B, NOUT M J. Diversity and functionality of Bacillus and related genera isolated from spontaneously fermented soybeans (Indian Kinema) and locust beans (African Soumbala) [J]. International Journal of Food Microbiology, 2002, 77(3): 175–186. DOI: 10.1016/s0168-1605(02)00124-1.
[40] KULSHRESHTHA N M, KUMAR A, BISHT G, PASHA S, KUMAR R. Usefulness of organic acid produced by Exiguobacterium sp. 12/1 on neutralization of alkaline wastewater [J]. The Scientific World Journal, 2012, 2012(4): 345101. DOI: 10.1100/2012/345101.
[41] SANTINI T C, MALCOLM L I, TYSON G W, WARREN L A. pH and organic carbon dose rates control microbially driven bioremediation efficacy in alkaline bauxite residue [J]. Environmental Science & Technology, 2016, 50(20): 11164–11173. DOI: 10.1021/acs.est.6b01973.
(Edited by YANG Hua)
中文导读
耐碱产酸菌筛选及其在赤泥碱性调控中的应用
摘要:赤泥堆场是一种典型矿业废弃地,盐度高,碱性强,对植物生长十分不利。酸碱中和是降低赤泥碱性的主要方法,对堆场植被重建具有重要意义。本研究从赤泥堆场中筛选出1株耐碱产酸细菌EEEL02,经鉴定,该菌株为苏云金芽孢杆菌,从属于芽孢杆菌门。通过单因素试验确定该菌株最佳产酸条件:初始pH为10,盐浓度5%,培养温度25 °C;最优发酵培养基组成为葡萄糖2%,蛋白胨0.5%。将EEEL02接种于赤泥中,在最佳培养条件下培养5 d后,赤泥pH从10.26降低至5.62。EEEL02在发酵过程中主要代谢产物为乙酸、丙酸和二氧化碳。微生物发酵产酸过程能有效降低赤泥碱度,为赤泥碱性调控提出一种新思路。
关键词:赤泥;16S rDNA;苏云金芽孢杆菌;产酸;碱性调控
Foundation item: Projects(41877511, 41842020) supported by the National Natural Science Foundation of China; Project(502221703) supported by the Innovative Project of Independent Exploration of Central South University, China
Received date: 2018-10-15; Accepted date: 2018-11-27
Corresponding author: XUE Sheng-guo, PhD, Professor; Tel: +86-13787148441; E-mail: sgxue70@hotmail.com, sgxue@csu.edu.cn; ORCID: 0000-0002-4163-9383
Abstract: Bauxite residue deposit area (BRDA) is a typical abandoned mining wasteland representing extreme hostile environment with increased alkalinity. Microbially-driven neutralization of bauxite residue, based on the microbial acid producing metabolisms, is a novel strategy for achieving rapid pH neutralization and thus improving its environmental outcomes. The hypothesis was that these extreme conditions promote microbial communities which are capable of novel ecologically relevant functions. Several alkaliphilic acid producing bacteria were isolated in this study. One strain was selected for its superior growth pattern and acid metabolism (termed EEEL02). Based on the phylogenetic analysis, this strain was identified as Bacillus thuringiensis. The optimized fermentation conditions were as follows: pH 10; NaCl concentration 5%; temperature 25 °C; EEEL02 preferred glucose and peptone as carbon and nitrogen sources, respectively. Based on optimal fermentation conditions, EEEL02 induced a significant pH reduction from 10.26 to 5.62 in 5-day incubation test. Acetic acid, propionic acid and CO2 (g) were the major acid metabolites of fermentation, suggesting that the pH reduction in bauxite residue may be caused by acid neutralization derived from microbial metabolism. This finding provided the basis of a novel strategy for achieving rapid pH neutralization of bauxite residue.


