DOI: 10.11817/j.ysxb.1004.0609.2020-39452
金属离子配位调控分子组装浮选理论及其研究进展
孙文娟1,韩海生1,胡岳华1,孙 伟1,朱阳戈2,桂夏辉3,曹学锋1,邢耀文3,李成必2,卫 召1
(1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 矿冶科技集团有限公司 矿物加工科学与技术国家重点实验室,北京 102628;
3. 中国矿业大学 国家煤加工与洁净化工程技术研究中心,徐州,221116)
摘 要:
在矿物浮选过程中,金属离子发挥着重要的作用,特别是活化浮选作用。在氧化矿的金属离子活化浮选过程中,金属离子与浮选药剂形成的金属-有机/无机配合物在矿物浮选过程中发挥了极大的作用,并在捕收能力和选择性等方面体现出一定的优势。本文总结了苯甲羟肟酸铅配合物、油酸钙、盐化水玻璃、金属离子改性淀粉等在浮选领域的研究进展,并从金属离子配位调控分子组装的角度,提出金属离子在矿物浮选过程中的新作用机制。金属离子具有良好的模板效应,可以定向调控金属配合物结构实现功能组装,这一功能为新型浮选药剂的设计与开发提供了新的思路。
关键词:
文章编号:1004-0609(2020)-03-0927-15 中图分类号:TD923 文献标志码:A
金属离子在矿物浮选过程中发挥着重要的作用,特别是活化浮选作用。近期研究发现,金属离子与浮选药剂形成的金属-有机/无机配合物在氧化矿的浮选过程中也发挥着重要的作用,并在捕收能力和选择性等方面体现出一定的优势[1-2]。例如,苯甲羟肟酸铅络合配合物、盐化水玻璃、金属离子改性淀粉等[3-8]已经在浮选领域取得一系列工业化成果。然而,金属离子与表面活性剂之间的作用机理很难用经典的浮选理论解释,如何进一步拓展浮选理论面临新的挑战。事实上,金属离子具有良好的模板效应,利用“金属离子配位调控分子组装原理”设计具有特殊性能的新型材料、药剂已经成为国际研究的热点。然而,浮选领域中关于金属-有机/无机配合物的研究尚处于起步阶段,金属离子与表面活性剂配体在浮选过程中的组装机理不明,缺乏系统研究工作,未能形成系统的理论。本文从金属离子配位调控分子组装的角度,归纳总结了不同矿物浮选过程中金属离子与有机/无机表面活性剂的作用机制,尝试利用金属离子配位调控分子组装原理进一步完善金属离子与浮选药剂及矿物表面作用的机理。期望通过金属离子与捕收剂的配位组装改善传统捕收剂的捕收能力和选择性,为新型浮选药剂的设计与开发提供新的解决方案。
1 基于配位化学的经典金属离子活化浮选作用机理
自20世纪50年代起,金属离子就被发现在矿物浮选过程中发挥着十分显著的作用[9]。长期以来,已经研究了许多特定(价态)的金属离子对某种矿物浮选行为的影响,如氧化铅锌矿和镍钼矿浮选矿浆中的Zn2+、Pb2+和Ni2+等金属离子,将会活化石英等脉石矿物,对浮选分离造成不利影响;黄铁矿与白云石的浮选体系中,Ca2+、Mg2+和 Fe3+等金属离子会与抑制剂生成金属盐,强化对白云石的抑制,从而有助于浮选分离[10]。金属离子在矿物浮选中的作用效果及机理存在多样性。FUERSTENAU等[10]研究了金属离子在石英和绿柱石浮选过程中的活化作用,发现最佳活化pH值与金属离子一羟基络合物生成量最大的pH一致,提出了金属离子活化作用的主要组分是一羟基络合物的假说。然而这一假说忽略了以下3个问题:在lg c-pH图中,一羟基络合物生成量最大的pH常常对应于氢氧化物沉淀生成的pH;一羟基络合物生成的pH范围较窄且浓度很低,即使在生成最大的pH值,其浓度通常不到总浓度的1%~10%。当金属离子总浓度处于浮选有效浓度范围时,一羟基络合物组分浓度显然小于有效作用浓度。金属离子在界面区域与溶液中的性质存在较大差别。JAMES等[11]提出,矿物-水溶液界面上的金属氢氧化物沉淀溶度积比在溶液中更小。因此,在矿物-水溶液界面上容易形成金属氢氧化物,进而影响矿物与药剂的作用。研究发现[12],在矿物表面不容易形成金属羟基络合物的情况下,金属氢氧化物沉淀可活化矿物的浮选。王淀佐[13]从金属离子在氧化矿物表面的吸附、对电性和可浮性的影响等试验结果及其在界面区域的性质等方面讨论金属离子的活化作用,提出了另一种活化机理:金属氢氧化物表面沉淀物可能是金属离子在氧化矿表面吸附并起活化作用的有效组分。胡岳华等[5, 14]进一步将矿物浮选中金属离子的作用效果及机理归纳为:金属氢氧化合物与矿物表面的作用(或金属离子与矿物表面的间接作用),和金属离子与浮选药剂及矿物表面的直接作用两种。其中,经典的金属离子羟基络合物理论和金属氢氧化物沉淀理论均为金属离子与OH-作用后附着在矿物表面,属于第一种作用理论。如:油酸钠作捕收剂时,Ca2+的加入可活化石英浮选,这是由于 Ca(OH)+的存在并吸附在石英表面,增加了石英表面动电位,有利于油酸钠在石英表面的吸附;Fe2+活化闪锌矿浮选时, Fe(OH)2会沉淀在闪锌矿表面,同时会与磺酸盐形成共沉淀,从而增强闪锌矿表面的疏水性[11, 15-16]。
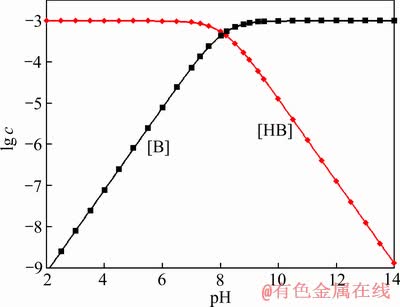
图1 苯甲羟肟酸溶液各组分浓度lg c-pH[17] (15 ℃,c(BHA)= 1×10-3 mol/L)
Fig. 1 lg c-pH relationship of BHA solution as function of pH[17] (15 ℃, c(BHA)=1×10-3 mol/L)
金属离子在溶液中是以水合金属离子形式存在,金属离子在矿物表面的吸附会导致矿物表面水化层结构的变化,从而影响捕收剂的吸附;另一方面,溶液体系中水合金属离子与捕收剂会不可避免地发生作用形成一定的配合物,这些配合物在浮选过程中的作用往往被忽略[13]。例如:苯甲羟肟酸(BHA)捕收剂和Pb2+离子,在pH 9左右,BHA电离主要以B-离子形式存在(见图1),而硝酸铅主要以Pb(OH)+ (10-4.3 mol/L)、Pb(OH)2(aq)(10-4.7 mol/L)及Pb2+(10-5.5 mol/L)形式存在(见图2),液相体系中BHA和铅离子组分将络合形成一定的配合物[17],这一组分在矿物浮选中的作用长期以来未引起重视。因此,经典的活化浮选理论是一个简化的吸附模型,对金属离子、捕收剂的吸附过程的解释并不完善,有待进一步拓展。
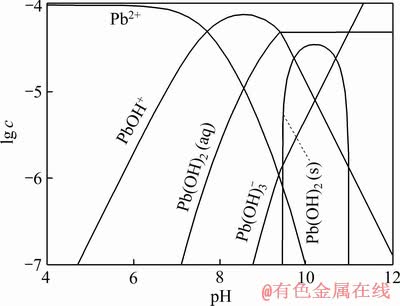
图2 Pb2+水解组分浓度的lg c-pH[17] (c(Pb)=1×10-4 mol/L)
Fig. 2 Concentration diagram of Pb ion hydrolysis in solution as function of pH[17] (c(Pb)=1×10-4 mol/L)
2 浮选过程中的金属离子配位调控分子组装行为及应用进展
2.1 铅离子与苯甲羟肟酸的配位组装及其在钨、锡浮选中的应用
2.1.1 固液界面金属离子配位调控分子组装吸附模型
铅离子与苯甲羟肟酸在工业上广泛应用于白钨矿、黑钨矿、锡石、钛铁矿等浮选。韩海生、田孟杰等[17]通过浮选实验证实了BHA体系中铅离子的活化作用并非单纯的是铅离子吸附在矿物表面上作为活性质点,捕收剂通过铅离子活性质点吸附在矿物表面。铅离子与浮选药剂形成的金属-捕收剂配合物在浮选过程中发挥了极大的作用,并在捕收能力方面体现出一定的优势,如图3。
韩海生等[1-2, 17-19]通过浮选实验发现硝酸铅与苯甲羟肟酸的加入方式对浮选结果影响显著:苯甲羟肟酸与硝酸铅预先混合明显优于顺序加药(先加入硝酸铅再加入苯甲羟肟酸),且随着硝酸铅与苯甲羟肟酸比例的变化,所形成的配合物的捕收性能和起泡性能也发生变化。不同配比下形成的Pb-BHA配合物对不同矿物的捕收能力及有效作用pH区间存在较大差异(如图4)。因此,可以通过调整Pb/BHA配比及pH控制Pb-BHA配合物的结构及捕收性能,实现白钨矿与萤石、方解石的高效浮选分离。
苯甲羟肟酸体系铅离子的活化浮选作用机制可以简单概括为以下两种原理(见图5):模型一,活化浮选模型,溶液中的Pb2+、PbOH+及Pb(OH)2胶体通过静电作用吸附在白钨矿、黑钨矿表面,并在表面发生羟桥脱水反应形成沉淀,BHA阴离子与矿物表面的铅质点反应形成“O,O”五元环,达到浮选捕收目的;模型二,金属离子界面预组装模型,溶液中的Pb2+、PbOH+及Pb(OH)2胶体与BHA阴离子配体反应形成某种或某几种配合物。这种配合物其具有类似铅离子的性质,胶体结构荷正电,通过静电作用吸附于矿物表面,在矿物表面发生羟桥缩水反应,实现捕收剂在矿物表面的吸附。在传统的活化浮选过程中,两种作用机制共存,但以模型一所示作用机制为主,而配合物体系则以模型二所示作用机制为主。虽然两种作用机制看似殊途同归,都是通过铅离子作为活性质点实现BHA在矿物表面的吸附,但是BHA在矿物表面的组分状态和结构可能存在一定的差异,例如BHA的结构、空间排布等,这些差异将导致两种作用体系下浮选效果的不同。
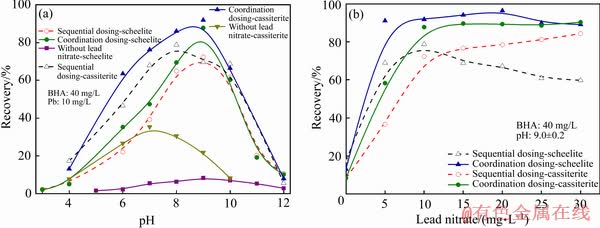
图3 不同pH(a)和硝酸铅用量(b)下白钨矿和锡石浮选回收率[17]
Fig. 3 Flotation recovery of scheelite and cassiterite at different pH (a) and lead nitrate dosage (b)
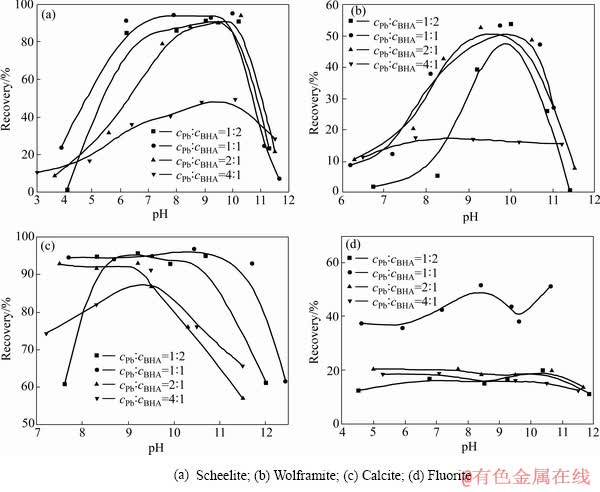
图4 不同配比的苯甲羟肟酸铅配合物对矿物的捕收能力差异(c(BHA)=1.5×10-4 mol/L) [8]
Fig. 4 Difference of recovery between different minerals using different Pb-BHA complexes (c(BHA)=1.5×10-4 mol/L) [8]
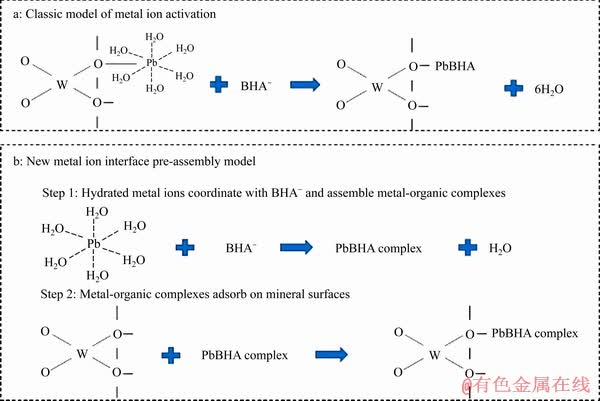
图5 经典金属离子活化模型及金属离子-有机配合物吸附模型
Fig. 5 Classic metal ion activation model(a) and new metal ion-organic complex adsorption model(b)
2.1.2 苯甲羟肟酸铅金属有机配合物及其在固液界面的吸附行为苯甲羟肟酸也称氧肟酸、异羟肟酸,是指两种互变异构体[20]:
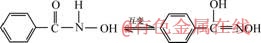
夏启斌等[20-21]用量子化学计算苯甲羟肟酸和苯甲氧肟酸分子模型,以及苯甲羟肟酸与铅离子配位结构(见图6),发现苯甲羟肟酸与铅离子形成的O—O五元环更为稳定。
何建勇[21]基于密度泛函理论 Density Function Theory(DFT)的量子化学计算了Pb-BHA 配合物(2~4配体)结构的稳定性,结果表明Pb-BHA2的两配体构型最为稳定。Pb-BHA 配合物的 XRD 分析、紫外分光光度分析及电位滴定结果表明[17, 21-22]:铅离子与 BHA 反应生成两种或两种以上相对稳定的配合物,其溶液组分相对比较复杂;在pH 9左右时,铅离子主要以配合物形式存在,游离的 Pb2+或Pb(OH)+很少,在浮选过程中起主要作用的可能是某种或某几种 Pb-BHA 配合物组分;羟基参与了 Pb-BHA 配合物的形成,且部分配合物具有 Pb2+类似的性质。通过Pb-BH配合物结晶样品热重分析和其在矿物表面吸附结构计算,韩海生等[23]推测其具体结构可能为Pb3BHA2(OH)4(PbBHA2·2Pb(OH)2) 或Pb3BHA2(OH)4- (2Pb(OH)BHA·Pb(OH)2)。
进一步的红外光谱(FTIR)分析[23]结果表明:配合物体系下,苯甲羟肟酸在白钨矿、黑钨矿表面吸附量或强度更大;动电位分析[23]结果表明:Pb-BHA配合物具有与铅离子类似的性质,在一定pH区间内其胶体表面带正电,容易通过静电作用吸附于矿物表面;光电子能谱(XPS)[1]结果表明,Pb-BHA配合物迁移至矿物表面后,与矿物表面氧质点发生化学作用。基于上述结果,建立Pb-BHA配合物在白钨矿表面的吸附模型(见图7),表面荷强正电的Pb-BHA配合物胶体在静电力的吸引作用下迁移至表面荷负电的白钨矿表面,Pb-BHA配合物铅金属基团与矿物表面氧质点发生化学作用,强化Pb-BHA配合物在白钨矿表面的吸附。
2.1.3 苯甲羟肟酸铅金属有机配合物在钨、锡浮选中的应用
钨、锡是重要的战略矿产资源,其保障程度关系到国民经济长期稳定发展和国家安全。然而,占其主要资源储量的低品位伴生资源矿石组成日益复杂、贫细化加剧,分选难度进一步加大,对矿物分选过程中的选择性提出了更高的要求[24]。这类资源综合利用率低的本质原因在于:氧化矿体系中白钨矿、黑钨矿和锡石等有用矿物与脉石矿物具有类似的表面化学性质,而脂肪酸、螯合捕收剂等阴离子捕收剂通过含N、O、P等的有机酸官能团与矿物表面活性位点作用,选择性难以进一步提高。例如:白钨矿与萤石、方解石等均为含钙矿物,传统捕收剂通过钙质点与矿物表面同时发生作用,选择性差[25-26];锡石浮选体系中大量铜、铁、铅、钙等金属离子在矿物表面吸附,使锡石和脉石矿物表面性质趋同,传统阴离子捕收剂难以高效选择性吸附在锡石表面,导致资源利用率低、生产过程能耗和成本高、选矿过程环境问题突出[27]。苯甲羟肟酸铅金属有机配合物不同于传统阴离子捕收剂的是,其官能团为金属基,其作用位点不再是表面相似的钙质点,而是差异较大的阴离子作用基团,因此表现出极强的选择性,为含钙矿物的浮选分离提供了解决方案。
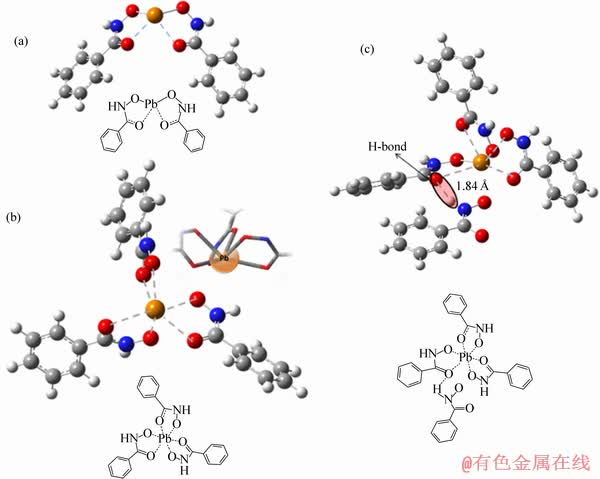
图6 基于量子化学的Pb-BHA配合物结构稳定性计算[21]
Fig. 6 Quantum chemistry calculation of structural stability of Pb-BHA complex with coordination numbers of 2(a), 3(b) and 4(c) [21]
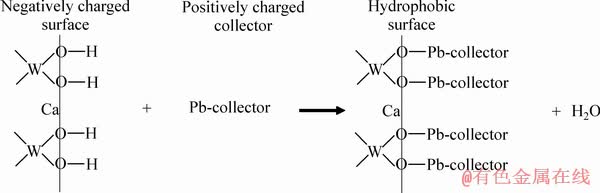
图7 Pb-BHA配合物在白钨矿表面的吸附模型[2]
Fig. 7 Adsorption model of Pb-BHA complex on scheelite surface[2]
基于金属离子配位调控分子组装的理念,Pb-BHA金属有机配合物首次作为一种新型捕收剂应用于钨矿、锡石的浮选,由此开发出黑白钨混合浮选新技术,解决了高钙、低品位、强蚀变黑白钨锡伴生资源的高效综合回收难题[8, 28]。金属-有机配合物捕收剂对黑钨矿、白钨矿和锡石具有较强的选择性捕收性能,实现对黑钨矿、白钨矿和锡石的同步浮选富集,避免萤石在钨粗精矿中的富集,钨、锡回收率得到极大提高;相对于使用浮选药剂GYR、GYB的GY法传统工艺富集比高,且极大地降低或取消了水玻璃在钨矿浮选中的应用,有利于回水处理及环境保护。同时金属-有机配合物捕收剂的选择性捕收性能使得黑白钨的常温精选成为可能,在一定程度上取代经典的“彼德洛夫法”加温浮选工艺,不仅简化了工艺流程,而且极大的提高了钨综合回收率;选钨工段水玻璃的取消为后续伴生有用矿物(萤石、锡石、铷等)的综合回收创造了条件。该工艺已经成功应用于我国最典型钨矿湖南柿竹园,钨综合回收率整体提高8%以上[19],并在行洛坑钨矿等矿山推广应用(见图8)。
2.2 油酸钙胶体在萤石表面的吸附及其应用
萤石是一种重要的非金属矿资源,化学成分为氟化钙(CaF2)。萤石用途广泛,主要作为助熔剂应用于冶金行业,同时也是氟化工业基本原料—氢氟酸的主要来源,在玻璃、陶瓷和水泥等建筑材料的生产中起着重要的作用[29-31]。脂肪酸类捕收剂因其来源广泛,价格便宜等优点,是目前萤石工业生产中最常用的捕收剂[32]。但是脂肪酸类捕收剂如油酸等在萤石浮选过程中存在选择性差,低温浮选性能较差,溶解能力差等缺点,因此,在萤石选矿厂中需要对矿浆加温再进行浮选[33],成本较高,工艺复杂。我国萤石矿平均品位偏低,矿物组成复杂,单纯用油酸很难选到高品位的萤石精矿[34-35]。近期关于油酸钙胶体颗粒在萤石浮选过程中作用机制方面的新发现为改良萤石浮选药剂提供了新的思路。
FUERSTENAU等[10, 36-38]通过原位红外光谱技术、原子力显微镜技术和溶液化学计算揭示了油酸在萤石表面吸附的本质,认为液相中生成的油酸钙胶体在萤石表面的吸附占主导作用。FA等[38-39]研究了油酸盐对方解石和萤石的吸附:在较高浓度的油酸盐和钙离子作用下,通过红外光谱分析发现了1575 cm-1和1538 cm-1附近的不对称羧酸—COO—拉伸偶联体的存在,表明了方解石、萤石表面均形成了二油酸钙沉淀。浮选实验结果表明,在较低浓度下,油酸钙胶体对萤石表现出较强的选择性捕收能力[37],有利于萤石和方解石的选择性浮选分离(见图9)。对此,FA等[37, 40]将球形二油酸钙颗粒作为AFM探针研究了二油酸钙捕收剂胶体和方解石、萤石表面之间的相互作用力(见图10),并与经典的DLVO胶体力进行了比较,结合分子动力学模拟揭示了萤石-钙水界面不同的界面水结构,发现萤石表面具有一定的疏水性,油酸钙胶体颗粒与萤石疏水表面之间存在长程疏水作用力,因此,油酸钙胶体颗粒更容易在萤石表面吸附。这一研究成果表明,对于半可溶矿物浮选,必须考虑金属阳离子与捕收剂形成的配合物胶体在浮选过程中的作用。
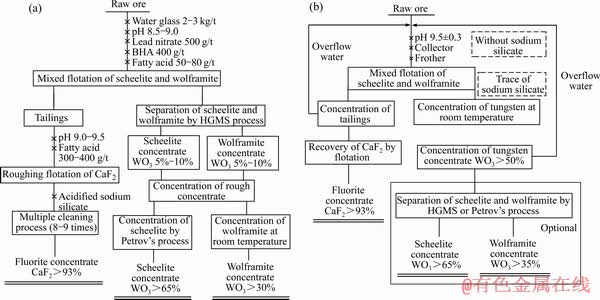
图8 采用GY系列脂肪酸药剂(GYR、GYB)的传统钨矿浮选工艺和基于金属-有机配合物的钨矿浮选新工艺[23]
Fig. 8 Traditional flotation technology using GY series fatty acid reagents (GYR, GYB) (a) and new tungsten ore flotation process based on metal-organic complex (b)[23]
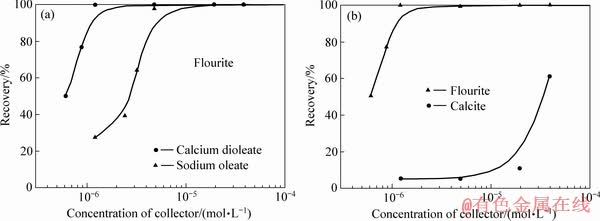
图9 油酸钙胶体颗粒对萤石和方解石的选择性捕收能力[38]
Fig. 9 Selective collecting ability of calcium oleate complex to fluorite and calcite[38]
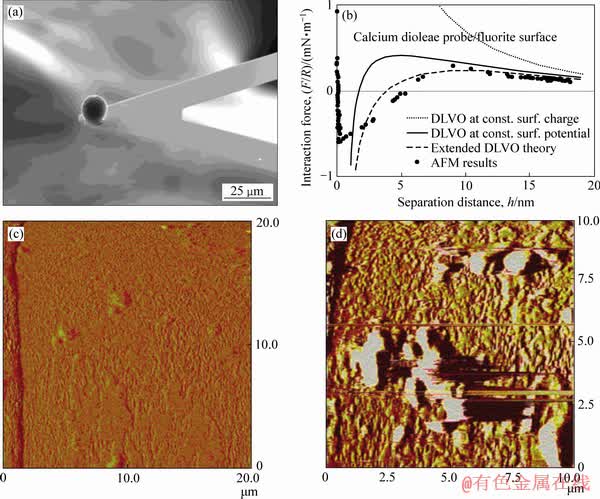
图10 油酸钙胶体颗粒与萤石(111)面的作用力及吸附形貌[38]
Fig. 10 Interaction force and adsorption morphology of calcium oleate colloidal particles and fluorite surface (111)[38]
2.3 金属离子与硅酸胶体的配位调控组装
硅酸钠的水溶液俗称水玻璃,是一种高浓度强碱性的黏稠水溶液,在传统的脂肪酸浮选工艺中,通常用于抑制矸石矿物。水玻璃作为白钨矿浮选中最常用的抑制剂,在矿石组成简单的情况下可以获得满意的指标,但当矿物组成复杂时,单一水玻璃的选择性较差,在抑制方解石和萤石等脉石矿物的同时,也对白钨矿产生了一定程度的影响,严重制约了白钨矿回收率的提高[2, 7-8, 19]。因此,在实践中为了提高硅酸钠的选择性,往往需要利用Fe2+、Pb2+、Cu2+、Mg2+、Al3+等多种金属离子对硅酸钠进行改性。由Al3+改性水玻璃得到的Al-Na2SiO3聚合物抑制剂在浮选中表现出了对方解石、硅酸盐矿物等脉石矿物的选择性抑制作用,目前广泛应用于钨矿、萤石、磷矿、重晶石等氧化矿的浮选中。
成盐理论对金属离子改性水玻璃的机理和抑制原理进行了阐述:水玻璃和金属离子之间可以发生了化学反应生成某种复合硅酸盐胶体,这种硅酸盐胶体与普通水玻璃相比胶团更大,表面羟基基团更多,活性更高,因此,在矿物表面吸附的选择性抑制作用更强[41]。卫召等[7]从金属离子配位调控分子组装的角度,对水玻璃的选择性抑制作用进行了新的阐述。Al3+改性水玻璃的红外光谱分析发现铝离子与硅酸钠之间通过化学键相联,自组装生成了更稳定的 Al-Na2SiO3聚合物(见图11)。浮选实验表明[7](见图12):与Na2SiO3相比,Al-Na2SiO3在用量达到60 mg/L以上时,白钨矿与方解石出现明显的可浮性差异,可以实现白钨矿与方解石的浮选分离;Al-Na2SiO3在100 mg/L的最佳浓度时,白钨矿的回收率达到85.58%,而37~74 μm 的粗粒级方解石回收率仅为27.59%;Al-Na2SiO3在100~150 mg/L的用量区间内,对白钨矿与方解石的混合矿具有明显的分离效果,精矿品位在65%以上,回收率在75%以上,取得良好的分选指标。
基于上述分析结果,卫召等[7]建立了Al-Na2SiO3聚合物和Pb-BHA配合物在白钨矿和方解石表面的吸附模型,如图13所示。白钨矿解理面表面主要组分是 而荷负电荷,因此,荷强正电的Pb-BHA配合物可以更容易地与白钨矿表面的O原子结合。虽然方解石的解理层暴露出更多的Ca原子,但当pH为7~9时,主要组分Ca2+会使表面荷正电,因此,Pb-BHA配合物在方解石表面上的吸附难度变大。这就是Pb-BHA配合物在白钨矿和方解石浮选中的选择性。另一方面,Al-Na2SiO3聚合物比Na2SiO3荷更多负电,所以,Al-Na2SiO3聚合物更难以静电力吸附在荷负电的白钨矿表面,化学吸附更弱,因而在白钨矿表面吸附量很低。但在方解石上的情况正好相反,Al-Na2SiO3聚合物在方解石表面的吸附量很高,使其表面具有很强的亲水性。因此,Al-Na2SiO3聚合物在白钨矿和方解石表面具有选择性。当以Al-Na2SiO3聚合物作为抑制剂,采用Pb-BHA配合物进行选择性浮选时,其选择性优势将得到充分开发和加强。白钨矿表面吸附有较多的Pb-BHA配合物和较少的Al-Na2SiO3聚合物,使得白钨矿表面强疏水性,可以浮到矿浆表面。但在方解石表面吸附的Pb-BHA配合物较少,而Al-Na2SiO3聚合物较多,使得方解石表面强亲水,沉到矿浆底部。因此,通过Pb-BHA配合物和Al-Na2SiO3聚合物的协同选择性,实现了从方解石中选择性分离白钨矿。
而荷负电荷,因此,荷强正电的Pb-BHA配合物可以更容易地与白钨矿表面的O原子结合。虽然方解石的解理层暴露出更多的Ca原子,但当pH为7~9时,主要组分Ca2+会使表面荷正电,因此,Pb-BHA配合物在方解石表面上的吸附难度变大。这就是Pb-BHA配合物在白钨矿和方解石浮选中的选择性。另一方面,Al-Na2SiO3聚合物比Na2SiO3荷更多负电,所以,Al-Na2SiO3聚合物更难以静电力吸附在荷负电的白钨矿表面,化学吸附更弱,因而在白钨矿表面吸附量很低。但在方解石上的情况正好相反,Al-Na2SiO3聚合物在方解石表面的吸附量很高,使其表面具有很强的亲水性。因此,Al-Na2SiO3聚合物在白钨矿和方解石表面具有选择性。当以Al-Na2SiO3聚合物作为抑制剂,采用Pb-BHA配合物进行选择性浮选时,其选择性优势将得到充分开发和加强。白钨矿表面吸附有较多的Pb-BHA配合物和较少的Al-Na2SiO3聚合物,使得白钨矿表面强疏水性,可以浮到矿浆表面。但在方解石表面吸附的Pb-BHA配合物较少,而Al-Na2SiO3聚合物较多,使得方解石表面强亲水,沉到矿浆底部。因此,通过Pb-BHA配合物和Al-Na2SiO3聚合物的协同选择性,实现了从方解石中选择性分离白钨矿。
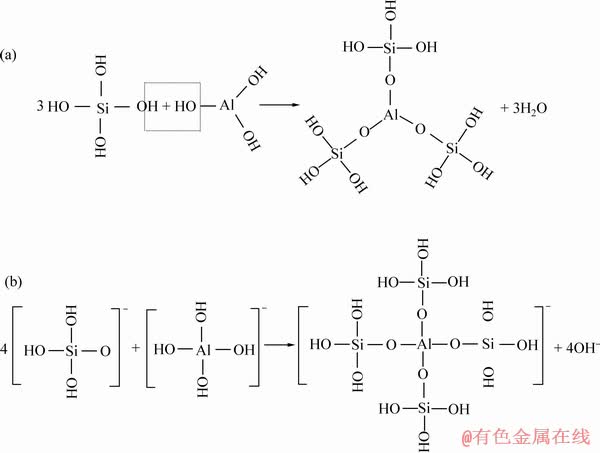
图11 溶液中硅酸根离子和铝离子优势组分自组装形成Al-Na2SiO3聚合物[7]
Fig. 11 Self-assembly of dominant species of silicate anions and aluminum ions in solution to form Al-Na2SiO3 polymer under acid or neutral conditions (a) and alkaline conditions (b)[7]
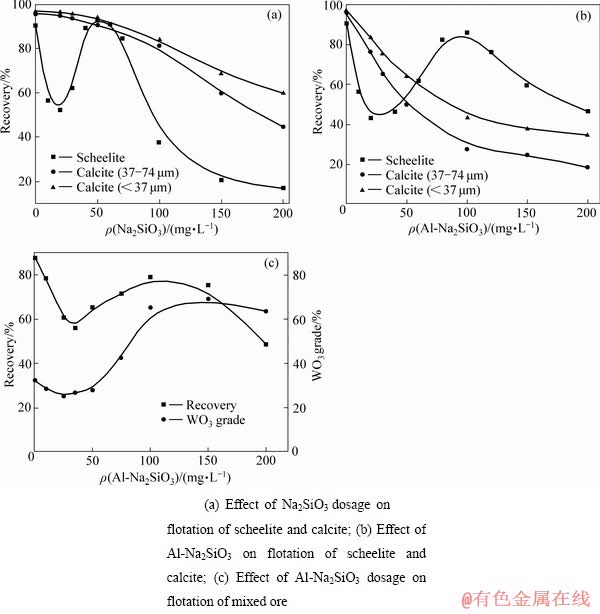
图12 Na2SiO3和Al-Na2SiO3做抑制剂的白钨矿及方解石浮选实验[7]
Fig. 12 Flotation experiments of scheelite and calcite with Na2SiO3 and Al-Na2SiO3 as inhibitors[7]
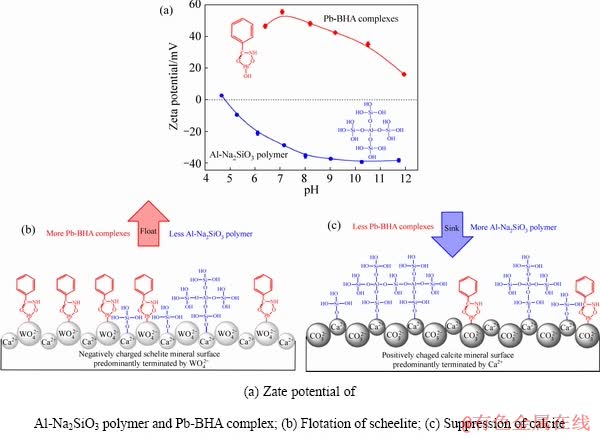
图13 Al-Na2SiO3聚合物和Pb-BHA配合物在白钨矿和方解石表面的吸附模型[7]
Fig. 13 Adsorption model of Al-Na2SiO3 polymer and Pb-BHA complex on surface of scheelite and calcite[7]
2.4 铁离子与淀粉的配位调控分子组装
我国铁矿资源较为丰富, 总量位居世界第三, 在已探明的储量中排世界第五位[42-43]。赤铁矿在我国铁矿石中所占比例最大, 但是其利用率却不高。我国已探明的100多亿吨(以金属铁计)复杂难选铁矿中难选赤铁矿比例最大[44]。因为赤铁矿正浮选工艺一直存在着药剂用量大、精矿过滤脱水困难等问题,目前主要采取阴离子反浮选工艺:先用钙离子活化石英,再用脂肪酸类捕收剂使脉石矿物石英上浮,浮选槽中产品就是铁精矿。为得到高品质铁精矿,常常用含羟基的有机化合物使含铁矿物表面亲水,以提高石英等其他硅酸盐矿物的浮选选择。淀粉分子中的单葡萄糖结构中的羟基使淀粉聚合物具有很强的亲水性,使其成为浮选当中常用的抑制剂。淀粉还可以在溶液中通过“桥联”的作用成为矿物尤其是细颗粒矿物的有效絮凝剂。
对于一些复杂难选铁矿石,普通淀粉常常存在用量大、选择性抑制效果差等问题。WU等[4]研制了以金属氢氧根胶体和苛性淀粉为基础的一系列新型抑制剂,称为金属-淀粉复合物(MSC)。MSC是一种纳米级胶体,可用于铁矿物的浮选抑制剂,能够有效抑制目的矿物,淀粉消耗较低[45-48]。YUE等[49]研究了金属淀粉复合物(MSC)对赤铁矿的抑制作用,并提出了分子模型:淀粉分子最初吸附在胶体核(β-FeOOH)上,由胶体核、覆盖的亲水金属羟基络合物以及淀粉分子形成稳定的“化合物分子”这种“大淀粉分子”借助淀粉的桥接吸附和氢键吸附对铁颗粒和亲水性金属羟基配合物具有特殊吸附作用。
关于淀粉在赤铁矿上选择性吸附的机理,文献中的观点各不相同[50],有静电相互作用和氢键作用[47, 51-52]、酸碱相互作用[53-54]、疏水相互作用[55-56]、化学络合作用[48, 57-58]、氢键作用和化学相互作用作用、以及淀粉分子与矿物表面金属离子位点分布的结构相容性等等。伍喜庆等[4]从金属离子配位组装理论出发对铁离子淀粉配合物在铁矿石反浮选中的抑制作用和抑制机理进行了研究。通过红外光谱分析发现,淀粉中的羟基与铁离子存在一定的氢键作用,进而推测铁离子淀粉可能是以β-FeOOH为胶核,淀粉分子依靠配位键作用吸附在铁核表面形成的复合物,结构与右旋糖酐铁类似(见图14)。这种铁离子淀粉复合物相对苛性淀粉具有更大的分子量,对十二胺在铁矿表面吸附具有更强的空间阻碍作用,抑制性能更好。
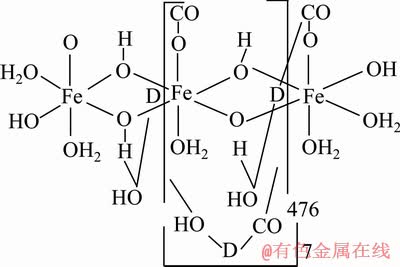
图14 铁离子淀粉的可能结构[4]
Fig. 14 Possible structure of iron ion starch[4]
反浮选实验显示铁离子淀粉的抑制能力和选择性皆优于苛性淀粉(见图15)。在矿浆pH 为中性、铁离子淀粉用量为1000 g/t、十二胺用量为400 g/t 情况下,采用一次粗选流程处理铁品位为20.50%的巴西某矿石,可得到铁品位为46.79%、铁回收率为79.36%的铁精矿,该指标明显优于苛性淀粉的指标[4]。
3 基于金属配位调控分子组装原理的新型浮选药剂设计理念与发展方向
油酸钙胶体、Al-Na2SiO3、铁离子淀粉复合物以及Pb-BHA配合物捕收剂,都是由金属离子与有机/无机物配位组装而成,在浮选过程中显示出了更好的效果与作用,并得到了广泛的应用。基于上述研究进展,韩海生等据此提出了浮选领域中的“金属离子配位调控分子组装”理念,并开展了进行一系列实验与研究,实现工业化应用,从理论和实践上证明了这一发现[1, 18-19]。基于金属离子配位调控分子组装的理念,将Pb-BHA金属-有机配合物作为一种新型捕收剂应用于白钨矿和黑钨矿等的浮选,开发了黑白钨混合浮选新技术,解决了高钙、低品位、强蚀变黑白钨伴生资源的高效综合回收难题[2, 19]。这一新的认识及工业化成果给新型捕收剂的开发提供了重要的启示:可以通过金属离子与捕收剂的配位组装改善传统捕收剂的捕收能力和选择性。在众多的非共价键中,金属离子配位键具有相对强的键能(60~200 kJ/mol)和高的取向性。将金属离子引入到超分子或聚合物体系是构建杂化超分子聚合物的一种重要方法[59-61]。目前,在高分子材料、医药、化工等领域,金属离子配位调控分子组装应用广泛,已形成相对成熟的配位调控分子组装理论,并取得一系列的成就。近年来,研究发现了大量结构新颖、多样化、具有各种功能的配位聚合物,应用领域日趋广泛,例如气体分子与小分子有机蒸汽的吸附与分离、分子与离子交换、手性识别与分离、多相催化、多相分离、分子磁性质、发光与非线性光学性质,以及电学等性质。过去20年中,定向组装一直是配位聚合物领域中的重要研究课题[62]。通过精确地设计超分子或聚合物嵌段、配体结构或者是选择不同的金属离子为金属超分子聚合物组装体带来了丰富的拓扑结构多样性;另一方面,结构上巧妙设计的金属掺杂团簇体,如含多种离子组分的组装体,相比单离子作用更加全面。例如:2018年初,JACS期刊封面报道中南大学高稳定金属有机Bucky球的研究进展,王平山团队[63]首先合成了一种新型的含有六个未配位的枝状星形三联吡啶有机配体,将其与Zn2+进行配位自组装,一步反应定量得到一个Bucky球状超分子“纳米容器”,在主客体识别、药物释放、纳米材料和催化化学等领域具有良好的应用前景(见图16)。
配位聚合物领域中金属离子配位调控分子设计组装对于浮选领域新型药剂的开发设计具有一定的借鉴意义。尽管通过金属离子配位调控分子组装设计新型浮选药剂具有极大地可行性,但是矿物浮选不同于化工、医药、材料等领域,它是一个关于固/液/气三相界面的复杂体系,现有的金属离子配位调控分子组装理论和技术难以直接应用于浮选体系,有待进一步完善和扩展。
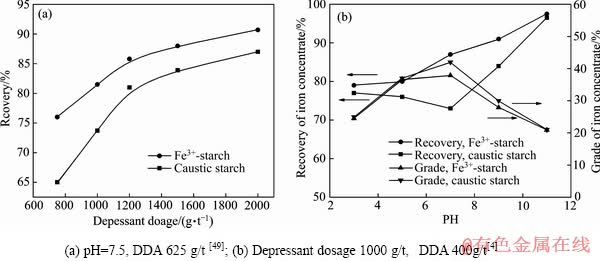
图15 铁离子淀粉与苛性淀粉的反浮选实验
Fig. 15 Reverse flotation experiment of iron-starch and caustic starch
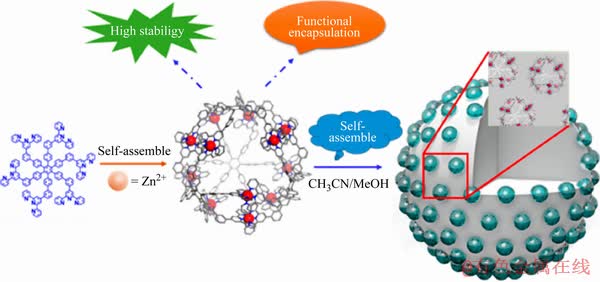
图16 基于三联吡啶配位系统作为分子容器的分层自组装模型[63]
Fig. 16 Layered self-assembly model based on terpyridine coordination system as molecular container [63]
1) 浮选体系中捕收剂和金属离子在水溶液体系中的真实组分状态存在一定的争议,其组装行为尚不清楚,难以实现定向调控;
2) 捕收剂和金属离子在水溶液中形成有效配合物组分的组装条件尚不清楚,难以实现定向组装;
3) 不同于传统阴离子捕收剂,金属-有机配合物捕收剂的有效官能团为金属基,其在固/液界面的微观吸附结构和吸附机理尚不完善;
4) 矿物的晶体化学因素和捕收剂的结构是决定矿物可浮性差异性的根本原因,二者之间的匹配关系有待进一步研究。
高性能计算和现代分析检测技术的飞速发展为上述问题的解决奠定了良好的基础。计算机模拟、和频振动光谱(SFG)和原子力显微镜(AFM)技术可直观地观察到界面聚集体的三维结构,提供分子层次上聚集体的微观行为和物化性质,有利于揭示配合物体系的自组装过程及各组分之间的相互作用机理及规律[64-66]。
4 结语
油酸钙胶体、Al-Na2SiO3、铁离子淀粉复合物以及Pb-BHA配合物捕收剂,都是由金属离子与有机/无机物配位反应或组装而成,在浮选过程中表现出了更好的效果与作用,并得到了广泛的应用。由此可知,通过金属离子配位调控分子组装设计新型捕收剂或药剂具有极大地可行性,其关键在于建立矿物表面特性与金属-有机配合物结构的匹配关系,实现金属-有机配合物结构的定向调控,形成适合于浮选体系的配位调控分子组装理论。配位聚合物领域中金属离子配位调控分子组装将为新型金属-有机配合物浮选药剂的开发提供了一定的理论指导。
REFERENCES
[1] HAN Hai-sheng, HU Yue-hua, SUN Wei, LI Xiao-dong, CHEN Ke-feng, ZHU Yang-ge, NGUYEN A V, TIAN Meng-jie, WANG Li, YUE Tong, LIU Run-qing, GAO Zhi-yong, CHEN Pan, ZHANG Chen-yang, WANG Jian-jun, WEI Zhao, WANG Ruo-lin. Novel catalysis catalysis mechanisms of benzohydroxamic acid adsorption by lead ions and changes in the-surface ofscheelite particles[J]. Minerals Engineering, 2018, 119(4): 11-22.
[2] HAN Hai-sheng, HU Yue-hua, SUN Wei, LI Xiao-dong, CAO Chong-gao, LIU Run-qing, YUE Tong, MENG Xiang-song, GUO Yan-zhe, WANG Jian-jun, GAO Zhi-yong, CHEN Pan, HUANG Wei-sheng, LIU Jie, XIE Jia-wen, CHEN Yu-lin. Fatty acid flotation versus BHA flotation of tungsten minerals and their performance in flotation practice[J]. International Journal of Mineral Processing, 2017, 159(2): 22-29.
[3] XU Xiao-jun, LIU Bang-guo. Study on activation of non-floating copper oxide minerals with ethylenediamine phosphate and D-2 agents[J]. Nonferrous Metals(Mineral Processing Section), 1991, 43(3): 28-33.
[4] WU Xi-qing, WANG Zhi-xi, YUE Tao. Study on depressing effect and mechanism of Ferric-starch complex in reverse flotation of an iron mine[J]. Metal Mine, 2017, 52(11): 70-74.
[5] SUN Wei, WANG Ruo-lin, HU Yue-hua, HAN Hai-sheng. Activation and new theory of lead ion in minerals flotation process[J]. Nonferrous Metals(Mineral Processing Section), 2018, 70(2): 91-98.
[6] YANG Rui-ying. Application of mixed regulators[J]. Nonferrous Metals(Mineral Processing Section), 1982, 34(3): 18-19, 10.
[7] WEI Zhao, HU Yue-hua, HAN Hai-sheng, SUN Wei, WANG Ruo-lin, WANG Jian-jun. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector[J]. Minerals Engineering, 2018, 120(5): 29-34.
[8] WEI Zhao, HAN Hai-sheng, HU Yue-hua, ZHU Yang-ge, SUN Wei, WANG Jian-jun, LIN Shang-yong, CHENG Peng-fei, LI Cheng-wei, CHEN Yu-feng. Flotation of wolframite and scheelite at the room temperature based on Pb-BHA coordination collector[J]. Nonferrous Metals Engineering, 2017, 69(6): 70-75.
[9] HU Yue-hua, LIU X, XU Zheng-he. Role of crystal structure in flotation separation of diaspore from kaolinite, pyrophyllite and illite[J]. Minerals Engineering, 2003, 16(3): 219-227.
[10] FUERSTENAU M C, MILLER J D, PRAY R E, PERINNE B F. Metal iron activation in xanthate flotation of quartz[J]. Trans AIME, 1965, 232(1): 359-365.
[11] JAMES R O, HEALY T W. Adsorption of hydrolyzable metal iron at the oxide-water interface. Ⅰ. Co(Ⅱ) adsorption on SiO2 and TiO2 as model systems[J]. Journal of Colloid and Interface Science, 1972, 40(1): 42-52.
[12] HU Yue-hua, WANG Dian-zuo. Mechanism of adsorption and activation flotation of metallic ions on oxide mineral-water interface[J]. Journal of Central South University(Science and Technology), 1987, 32(5): 501-508.
[13] WANG Dian-zuo, HU Yue-hua. Flotation solution chemistry[M]. Changsha: Hunan science and Technology Press, 1988: 200-289.
[14] GAO Yue-sheng, GAO Zhi-yong, SUN Wei. Research progress of influence of metal ions on mineral flotation behavior and underlying mechanism[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(4): 859-868.
[15] CHEN Jin, CHEN Wan-xiong, SUN Zhong-xi. Critera and laws of metal ion activated quartz[J]. Metal Mine, 1982,17(6): 30-33, 56.
[16] ZHANG Qing-song. Sphalerite activation in the presence of iron ions[D]. Montreal: McGill Unversity, 1994: 69-82.
[17] HU Yue-hua, HAN Hai-sheng, TIAN Meng-jie, SUN Wei, WANG Jian-jun, WEI Zhao, WANG Ruo-lin. The application of metal-coordinated complexes in the flotation of oxide minerals and fundamental research of the adsorption mechanism[J]. Conservation and Utilization of Mineral Resources, 2018, 38(1): 42-47, 53.
[18] TIAN Meng-jie, HU Yue-hua, SUN Wei, LIU Run-qing, Study on the mechanism and application of a novel collector-complexes in cassiterite flotation[J]. Colloids and Surfaces A—Physicochemical and Engineering Aspects, 2017, 522(6): 635-641.
[19] HAN Hai-sheng, LIU Wen-li, HU Yue-hua, SUN Wei, LI Xiao-dong. A novel flotation scheme: Selective flotation of tungsten minerals from calcium minerals using Pb-BHA complexes in Shizhuyuan[J]. Rare Metals, 2017, 36(6): 533-540.
[20] XIA Qi-bin, LI Xian, QIU Xian-yang, DAI Zi-lin. Quantum chemical study on benzyhydroximic acid flotation agent[J]. Mining and Metallurgical Engineering, 2004, 24(1): 30-33.
[21] HE Jian-yong, HAN Hai-sheng, ZHANG Chen-yang, HU Yue-hua, YUAN Dan-dan, TIAN Meng-jie, CHEN Dai-xiong, SUN Wei. New insights into the configurations of lead(Ⅱ)-benzohydroxamic acid coordination compounds in aqueous solution: A combined experimental and computational study[J]. Minerals, 2018, 8(9): 368-384.
[22] GAO Yu-de, ZHONG Chuan-gang, QIU Xian-yang, FENG Qi-ming, WAN Li. Activation mechanism of Pb2+ in flotation of wolframite with benzohydroxamic acid as collector[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(9): 1999-2006.
[23] HAN Hai-sheng. Application and mechanism of new metal complex collector in flotation of tungsten ore[D]. Changsha: Central SouthUniversity, 2017: 70-86.
[24] ANONYMITY.
[25] LIU Qing-gao, HAN Zhao-yuan, GUAN Ze-gao. Research progress on scheelite flotation technology[J]. China Tungsten Industry, 2009, 24(4): 23-27.
[26] YANG Fan, SUN Wei, HU Yue-hua, LONG Si-si. Cationic flotation of scheelite from calcite using quaternary ammonium salts as collector: Adsorption behavior and mechanism[J]. Minerals Engineering, 2015, 81(10): 18-28.
[27] Lü Jin-fang, TONG Xiong, ZHOU Yong-cheng. Research status on flotation reagents for fine cassiterite[J]. Hydrometallurgy of China, 2010, 29(2): 71-74.
[28] ZHANG Ye, HAN Hai-sheng, SUN Wei, HU Yue-hua, LI Xiao-dong, MENG Xiang-song. New concentration technology of wolframite and scheelite at the room temperature based on new ligand collector[J]. China Tungsten Industry, 2016, 31(3): 19-26.
[29] CHEN Shi-yi, ZHANG Shou-ting. Approaches to use and development of fluorite resource in China’s fluorite chemical industry[J]. Resources & Industries, 2013, 15(2): 79-83.
[30] ZHENG Shui-lin. Processing and application of nonmetallic mineral materials[J]. China Non-Metallic Mining Industry Herald, 2002, 23(4): 3-7.
[31] HE Tian-yu, REN Zi-jie, GAO Hui-min. Development of the investigation on the progressing of fluorite-calcite-barite ore[J]. Multipurpose Utilization of Mineral Resources, 2017, 38(6): 1-4.
[32] PENG Wen-sheng, LUO Li-qun, DENG Xiang-xiang. Exploitations & utilizations of fluorite resource and developments of flotation techniques on fluorite[J]. Hunan Nonferrous Metals, 2010, 26(2): 13-18, 61.
[33] CHEN Yuan-dao, LU Yi-ping, WANG Feng-ling, OU Le-ming, ZHANG Guo-fan, FENG Qi-mimg. Method for improving flotation performance of carboxylic acid collectors[J]. Metallic Ore Dressing Abroad, 2003, 41(4): 4-8.
[34] DONG Feng-zhi, REN Jing-cheng, LIU Xin-zhong, YANG Xin-chun. Floatation of fluorite and separation from barite[J]. Non-metallic Mines, 2001, 29(3): 36-37.
[35] DU Fei-fei, Lü Xian-jun, SUN Li-jun. Overview on low temperature flotation technology of oleic acid[J]. Modern Mining, 2010, 26(1): 31-34.
[36] FREE M L, MILLER J D. The significance of collector colloid adsorption phenomena in the fluorite/oleate flotation system as revealed by FTIR/IRS and solution chemistry analysis[J]. International Journal of Mineral Processing, 1996, 48(3): 197-216.
[37] FA Ke-qing, NGUYEN A V, MILLER J D. Interaction of calcium dioleate collector colloids with calcite and fluorite surfaces as revealed by AFM force measurements and molecular dynamics simulation[J]. International Journal of Mineral Processing, 2006, 81(3): 166-177.
[38] FA K Q, TAO J A, NALASKOWSKI J, MILLER J D. Interaction forces between a calcium dioleate sphere and calcite/fluorite surfaces and their significance in flotation[J]. Langmuir, 2003, 19(25): 10523-10530.
[39] YOUNG C A, MILLER J D. Effect of temperature on oleate adsorption at a calcite surface: An FT-NIR/IRS study and review[J]. International Journal of Mineral Processing, 2000, 58(1): 331-350.
[40] FA Ke-qing, NGUYEN A V, MILLER J D. Hydrophobic attraction as revealed by AFM force measurements and molecular dynamics simulation[J]. Journal of Physical Chemistry B, 2005, 109(27): 13112-13118.
[41] CHEN Chen. Effect of inorganic anions on flotation behavior and mechanism of three typical calcium-bearing salts[D]. Changsha: Central SouthUniversity, 2011: 123-157.
[42] HAN Yue-xin, GAO Peng, LI Yan-jun, SUN Yong-sheng. Development strategies of available use of inferior quality and optimal use of high quality for domestic iron ore resources[J]. Metal Mine, 2016, 51(12): 2-8.
[43] ZHANG Liang, YANG Hui-fan, FENG An-sheng, TAN Xiu-min. Study on utilization and analysis of supply and demand of global iron ore resources[J]. Conservation and Utilization of Mineral Resources, 2016, 36(6): 57-63.
[44] ZHAO Yi-ming. Main genetic types and geological characteristics of iron-rich ore deposits in China[J]. Mineral Deposits, 2013, 32(4): 686-705.
[45] LIU Ruo-hua. Mechanism and technology of different starch inhibiting hematite[D]. Changsha: Central South University, 2012: 87-106.
[46] DOGU I, AROL A I. Separation of dark-colored minerals from feldspar by selective flocculation using starch[J]. Powder Technology, 2004, 139(3): 258-263.
[47] WEISSENBORN P K, WARREN L J, DUNN J G. Selective flocculation of ultrafine iron ore 2, Mechanism of selective flocculation[J]. Colloids and Surfaces A—Physicochemical and Engineering Aspects, 1995, 99(1): 29-43.
[48] KHOSLA N K, BHAGAT R P, GANDHI K S, BISWAS A K. Calorimetric and other interaction studies on mineral-starch adsorption systems[J]. Colloids and Surfaces, 1984, 8(4): 321-336.
[49] YUE Tong, WU Xi-qing. Depressing iron mineral by metallic-starch complex (MSC) in reverse flotation and its mechanism[J]. Minerals, 2018, 8(3): 85-96.
[50] ABRO M I, PATHAN A G, MEMON A R, SIRAJUDDIN. Dual polymer flocculation approach to overcome activation of gangue minerals during beneficiation of complex iron ore[J]. Powder Technology, 2013, 245(18): 281-291.
[51] AROL A I. The effect of clay minerals on selective flocculation of iron ores[D]. Turkey: University of Minnesota, 1984: 23-28.
[52] BALAJEE S R, IWASAKI I. Adsorption mechanism of starches in flotation and flocculation of iron ores[J]. Trans AIME, 1969, 244(1): 401-406.
[53] WHISTLER R L, BEMILLER J N, PASCHALL E F. Starch chemistry and technology[M]. New York: Academic Press, 1984: 153-183
[54] NAKATANI J, OZAWA S, OGINO Y. Adsorptive interactions of glucose and carbon dioxide with basic sites over alumina[J]. Journal of the Chemical Society, Faraday Transactions, 1990, 86(10): 1885-1888.
[55] WIE J M, FUERSTENAU D W. The effect of dextrin on surface properties and the flotation of molybdenite[J]. International Journal of Mineral Processing, 1974, 1(1): 17-32.
[56] MILLER J D, LIN C L, CHANG S S. Coadsorption phenomena in the separation of pyrite from coal by reserve flotation[J]. Coal Preparation, 1984, 1(1): 21-38.
[57] KHOSAL N K, BISWAS A K. Mineral-collector-starch constituent interactions[J]. Colloids and Surfaces, 1984, 9(3): 219-235.
[58] SOMASUNDARAN P. Adsorption of starch and oleate and interaction between them on calcite in aqueous solutions[J]. Journal of Colloid and Interface Science, 1969, 31(4): 557-565.
[59] CHEN Li-jun, ZHAO Guang-zhen, JIANG Bo, SUN Bin, WANG Ming, XU Lin, HE Jiu-ming, ABLIZ Zeper, TAN Hong-wei, LI Xiao-peng, YANG Hai-Bo. Smart stimuli-responsive spherical nanostructures constructed from supramolecular metallodendrimers via hierarchical self- assembly[J]. Journal of the American Chemical Society, 2014, 136(16): 5993-6001.
[60] ZHOU J, WHITTEL G R, MANNERS I. Metalloblock copolymers: New functional nanomaterials[J]. Macromolecules, 2014, 47(11): 3529-3543.
[61] ZHANG Jiu-yang, MILLER Y P, KRISTEN P G, MITRA S B, MARPE Y, YI N, MITZI D, ALAN W, TANG Chuan-bin. Antimicrobial metallopolymers and their bioconjugates with conventional antibiotics against multidrug-resistant bacteria[J]. Journal of the American Chemical Society, 2014,136(13): 4873-4876.
[62] LIU Yu-qi. Study on coordination assembly, structure and properties of N and O organic ligands and transition metals[D]. Kuming: Kunming University of Science and Technology, 2014: 5-33.
[63] CHEN Ming-zhao , WANG Jun, LIU Die, JIANG Zhi-long, LIU Qian-qian, WU Tun, LIU Hai-sheng, YU Wei-dong, YAN Jun, WANG Ping-shan. Highly stable spherical metallo-capsule from a branched hexapodal terpyridine and its self-assembled berry-type nanostructure[J]. Journal of the American Chemical Society, 2018, 140(7): 2555-2561.
[64] WANG Xu-ming, LIU Jin, DU Hao, MILLER J D. States of adsorbed dodecyl amine and water at a silica surface as revealed by vibrational spectroscopy[J]. Langmuir, 2010, 26(5): 3407-3414.
[65] WANG Li, HU Yue-hua, SUN Wei, SUN Yong-sheng. Molecular dynamics simulation study of the interaction of mixed cationic/anionic surfactants with muscovite[J]. Applied Surface Science, 2015, 327(2): 364-370.
[66] XU Yao, LIU Yue-long, HE Dan-dan, LIU Gou-sheng. Adsorption of cationic collectors and water on muscovite (001) surface: A molecular dynamics simulation study[J]. Minerals Engineering, 2013, 53(11): 101-107.
Flotation theory and research progress of metal ion coordination regulation molecule assembly
SUN Wen-juan1, HAN Hai-sheng1, HU Yue-hua1, SUN Wei1, ZHU Yang-ge2, GUI Xia-hui3, CAO Xue-feng1, XING Yao-wen3, LI Cheng-bi2, WEI Zhao1
(1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083;
2. State Key Laboratory of Mineral Processing Science and Technology, BGRIMM Technology Group, Beijing 102628;
3. China University of Mining and Technology, Chinese National Engineering Research Center of Coal Preparation and Purification, Xuzhou 221116)
Abstract: Metal ions play an important role in mineral flotation, especially in activation flotation. Recently, some studies show that metal-organic/inorganic complexes form by metal ions and flotation agents play important role in mineral flotation. The metal-organic/inorganic complexes show certain advantages over the traditional flotation reagents in terms of collecting capacity and selectivity. This paper summarizes the application of lead benzohydroxamic acid complex, calcium oleate, water glass modified by metal ions, and modified starch-in mineral flotation separation. Metal ions are good templates for molecular assembly and can be used to control the structure of metal complexes and get some special properties. This function provides a new idea for the design and development of new flotation agents.
Key words: metal ion; coordination compound; coordinate assembly; activation theory; flotation reagents
Foundation item: Projects (51804340) supported by the National Natural Science Foundation of China; Project (BGRIMM-KJSKL-2019) supported by the State Key Laboratory of Mineral Processing Science and Technology, China
Received date: 2019-01-10; Accepted date: 2019-05-23
Corresponding author: HAN Hai-sheng; Tel: +86-15111046402; E-mail: hanhaishengjingji@126.com
(编辑 龙怀中)
基金项目:国家自然科学基金青年项目(51804340);矿物加工科学与技术国家重点实验室开放基金资助项目(BGRIMM-KJSKL-2019)
收稿日期:2019-01-10;修订日期:2019-05-23
通信作者:韩海生,副教授,博士;电话:15111046402;E-mail:hanhaishengjingji@126.com
摘 要:在矿物浮选过程中,金属离子发挥着重要的作用,特别是活化浮选作用。在氧化矿的金属离子活化浮选过程中,金属离子与浮选药剂形成的金属-有机/无机配合物在矿物浮选过程中发挥了极大的作用,并在捕收能力和选择性等方面体现出一定的优势。本文总结了苯甲羟肟酸铅配合物、油酸钙、盐化水玻璃、金属离子改性淀粉等在浮选领域的研究进展,并从金属离子配位调控分子组装的角度,提出金属离子在矿物浮选过程中的新作用机制。金属离子具有良好的模板效应,可以定向调控金属配合物结构实现功能组装,这一功能为新型浮选药剂的设计与开发提供了新的思路。


