Trans. Nonferrous Met. Soc. China 25(2015) 590-596
Electronic structure and flotation behavior of complex mineral jamesonite
Cui-hua ZHAO1,2,3, Jian-hua CHEN 3,4, Yu-qiong LI4, Qian HE4, Bo-zeng WU2
1. College of Materials Science and Engineering, Guangxi University, Nanning 530004, China;
2. Guangxi China Tin Group Stock Co., Ltd., Liuzhou 545006, China;
3. School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China;
4. Guangxi Colleges and University Key Laboratory of Minerals Engineering, Guangxi University, Nanning 530004, China
Received 21 April 2014; accepted 13 July 2014
Abstract:
Electronic structures of complex mineral jamesonite were studied using density functional theory method together with their flotation behavior. The flotation behavior of jamesonite is similar to that of stibnite, indicating good floatability at pH below 6 and easy depression with NaOH, especially with lime. In weak alkaline condition, the flotation behavior of jamesonite is close to that of galena. The coordination structure of Pb for jamesonite is more complex than that for galena. Sb in jamesonite possesses two coordinated modes, whereas Sb of stibnite is only 3-coordinated. Pb in galena is more active than that in jamesonite. Sb (3-coordination) in jamesonite is inactive, in contrast with that in stibnite. However, 4-coordination Sb in jamesonite is more active than 3-coordination Sb. HOMO orbitals of jamesonite and stibnite contain metal atoms, which contribute to the formation of adsorption configuration of CaOH+ when there is lime; therefore, jamesonite and stibnite are easily depressed by lime.
Key words:
jamesonite; electronic structure; flotation behavior; density functional theory;
1 Introduction
Jamesonite (Pb4FeSb6S14) is a sulfosalt mineral. It is commonly found as needle crystals in a hydrothermal vein associated with other sulfides, such as pyrite (FeS2), sphalerite (ZnS), galena (PbS), stibnite (Sb2S3), and others in nature. In natural FePb4Sb6S14, the elements are partially substituted with other minor elements, such as Mn, Cu, Zn, and Se [1]. Jamesonite is found in ore bodies together with other sulfides. These sulfides are difficult to separate because their floatability performances are so similar that the presence of one sulfide seriously affects the separation of the others [2,3]. Over the last few decades, many studies have been performed on the floatability and influencing factors of jamesonite [4-7]. CHEN et al [4] investigated the jamesonite flotation using five tannin extracts from different trees. The flotation results demonstrated that the larch tannin extract is the best for improving jamesonite flotation. ZHANG et al [5] investigated the flotation behavior of jamesonite with sodium diethyldithiocarbamate (DDTC) as a collector. The results showed that jamesonite has good floatability with pH from 2 to 13. ZHAO et al [6] studied the behaviors of Sb, Sn, and As in leaching and electrowinning from jamesonite concentrate. The results showed that the separation efficiency of Sb from As and Sn in jamesonite concentrate could be improved through changing the composition of leaching solution and other conditions. HUANG and SUN [7] studied the effects of 2-aminothiophenol on the separation of jamesonite and marmatite. The jamesonite and marmatite minerals had similar surface properties and their separation using a flotation method is inefficient. The result showed that 2-aminothiophenol, as a carrier, had good selectivity for separation of jamesonite and marmatite via flotation process.
Jamesonite is a complex ore containing Pb, Sb, and S elements and one magnetic element of Fe. Some researchers assumed that the flotation of jamesonite may be related to those of pyrite, galena (PbS), and stibnite. As a result, the relationship of floatability between jamesonite and galena and stibnite was studied [8-11]. It has been shown that the characteristics of flotation for jamesonite is closer to that of galena than to stibnite [8-10]. The jamesonite that is present in chalcocite concentrates as impurities exhibits the same characteristics of flotation as stibnite, which both float upward to a certain extent only with a foaming agent (a frother). DENG and XU [11] showed that jamesonite floated out in neutral pH using butyl xanthates, which was similar to galena. However, jamesonite is very sensitive to inhibitory effects of lime, compared with galena. In addition, jamesonite, similar to stibnite, has the best floatability performance at pH of 5. In spite of that, in neutral or in weak basic media, jamesonite may float, whereas stibnite is difficult or impossible to float. Cyanide and sodium carbonate depressed the flotation of stibnite, but no effect on the flotation of jamesonite was observed [11].
Although some works were done on the flotation of jamesonite galena and stibnite, the relationships between the electronic structure and the floatability of minerals, and the main causes for the similarities and differences in their floatability have not been reported. In recent years, the first-principle pseudopotential method based on density functional theory (DFT) has become a very useful tool for carrying out theoretical studies of the electronic and structural properties of materials. A great deal of research works have been done on electronic structure and property of mineral materials by the method, and some significant conclusions were obtained [12-14]. In this work, electronic structures of jamesonite, galena, and stibnite were studied using DFT method, together with their flotation behavior. The emphasis was put on the flotation behaviors of jamesonite, galena, and stibnite.
2 Experimental
2.1 Materials and flotation examination
Jamesonite samples were obtained from the Dachang Tongkeng Mine in Nandan, China. The jamesonite sample with a size of <0.074 nm has a purity of 94.5%. Galena and stibnite samples were obtained from Dali, Yunnan Provinces, China, respectively. The purities of galena and stibnite were 94.7% and 96.5%, respectively. Chemical grade ethyl xanthate was provided by Zhuzhou Flotation Reagents Factory of Hunnan Province, China.
The flotation experiments were carried out in a 50 mL flotation cell. First, 2.0 g mineral sample was added to the flotation cell after ultrasonic cleaning for 5 min, and then the supernatant was removed. The mineral sample was firstly conditioned with NaOH solution for 1 min, and then butyl xanthate solution (5×10-5 mol/L) was added with 3 min of conditioning, followed by 40 g/t pine oil with 1 min of conditioning. The flotation time was 5 min.
2.2 Computational methods
Jamesonite is a sulfosalt mineral containing Pb, Fe, and antimony sulfide with formula Pb4FeSb6S14. It is a dark grey metallic mineral that forms acicular prismatic monoclinic crystals with space group P21/a. The cell parameters are a=1.557 nm, b=1.898 nm, c=0.403 nm, and β=91°48′ [15]. Each Sb atom coordinates with adjacent three or four S atoms, as shown in Fig. 1(a). Jamesonite is one of the few sulfide minerals to form fibrous or needle like crystals. It can also form large prismatic crystals similar to stibnite with which it can be associated. It is usually found at low to moderate temperature hydrothermal deposits. Stibnite crystallizes in an orthorhombic space group (Pnma). Each S atom coordinates with three Sb atoms, as shown in the model of Fig. 1(b). Galena belongs to cubic crystal structure with a space group of Fm3m. Each Pb atom coordinates with six S atoms (Fig. 1(c)).
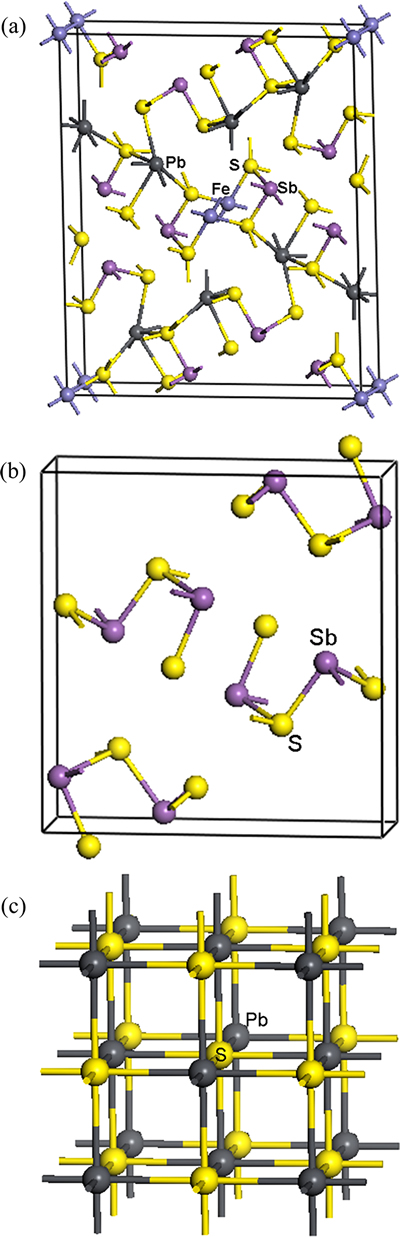
Fig. 1 Models of Pb4FeSb6S14 (a), Sb2S3 (b) and PbS (c)
All calculations were performed using the programs CASTEP [16,17] and Dmol3 [18,19], which are the first principles pseudopotential methods based on DFT. The calculations of geometry optimization and electronic structures on jamesonite, galena, and stibnite were performed using CASTEP and GGA-PW91 [20]. Based on the test results, the plane wave cut-off energies of jamesonite, galena, and stibnite were found to be 270, 280, and 300 eV, respectively, which are the most stable conditions. The valence electrons configuration considered in this work include Fe 3d64s2, Pb 5d106s26p2, Sb 5s25p3 and S 3s23p4 states.
The convergence tolerances for geometry optimization calculations were set to the maximum displacement of 0.005 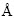 , the maximum force of 0.04 eV/
, the maximum force of 0.04 eV/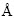 , and the maximum energy change of 2.0×10-5 eV/atom-1. The SCF convergence tolerance was set to 1.0×10-5 eV/atom with a global cutoff of 4.4
, and the maximum energy change of 2.0×10-5 eV/atom-1. The SCF convergence tolerance was set to 1.0×10-5 eV/atom with a global cutoff of 4.4  . The calculated lattice parameters of perfect jamesonite were a=1.592 nm, b=1.998 nm, and c=0.398 nm, which were close to the experimental values. The values indicate that the calculated results agree well with the experimental data.
. The calculated lattice parameters of perfect jamesonite were a=1.592 nm, b=1.998 nm, and c=0.398 nm, which were close to the experimental values. The values indicate that the calculated results agree well with the experimental data.
The atomic orbital coefficients of the highest occupied molecular orbital (HOMO), the lowest unoccupied molecular orbital (LUMO), and the energies of the frontier orbitals of jamesonite, galena, pyrite, and stibnite were calculated using Dmol3 program, followed by optimization with CASTEP program. The calculations were performed using GGA-PW91 with fine quality, effective core potentials, an atomic orbital basis set of DNP, and SCF convergence threshold of 1.0×10-6 eV/atom.
3 Results and discussion
3.1 Flotation test results
Figure 2 shows the flotation recovery of jamesonite, galena, and stibnite at various pH in the presence of xanthate. The concentration of xanthate is 5×10-5 mol/L. Galena exhibits the highest flotation recovery and then jamesonite and stibnite are followed. The flotation recoveries of jamesonite at various pH are observed close to that of stibnite, indicating good floatability at pH below 6. But the flotation recoveries are greatly reduced with increasing pH to above 6. As for galena, pH has little effect on its flotation recovery compared with those for jamesonite and stibnite.
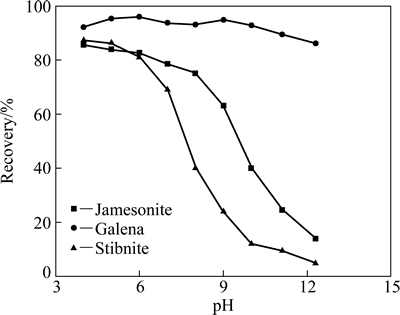
Fig. 2 Flotation recovery of jamesonite, galena and stibnite at various pH in presence of xanthate
3.2 Crystal structures
Although Pb atoms in jamesonite and galena are 6-coordinated, their structures are completely different, as shown in Fig. 3. The Pb atoms in galena coordinate with six symmetrical S atoms, whereas six S atoms coordinated with Pb atoms of jamesonite have different structures (Figs. 3(a) and (b)). In jamesonite, two S atoms (S1 and S4) are connected with two Sb atoms, two other S atoms (S2 and S6) are connected with three Pb atoms and one Sb atom, and the left two S atoms (S3 and S5) are connected with one Sb atom, one Fe and another Pb atom. Therefore, the coordination structure of Pb atoms in jamesonite is more complex than that in galena.
According to crystal field theory, the different ligand fields have different effects on central atoms. Figs. 3(c,d) show ligand field model of Pb atoms in jamesonite and galena. It is indicated that Pb atoms and corresponding coordination S atoms in jamesonite and galena form the octahedron crystal field, but their structures have a big difference. The octahedron crystal field formed by ligand field of Pb atom for jamesonite has an asymmetric structure, while that for galena possesses a symmetrical structure. As a result, Pb atoms in jamesonite and in galena are different concerning their stability, electronic property, activity, and other properties.
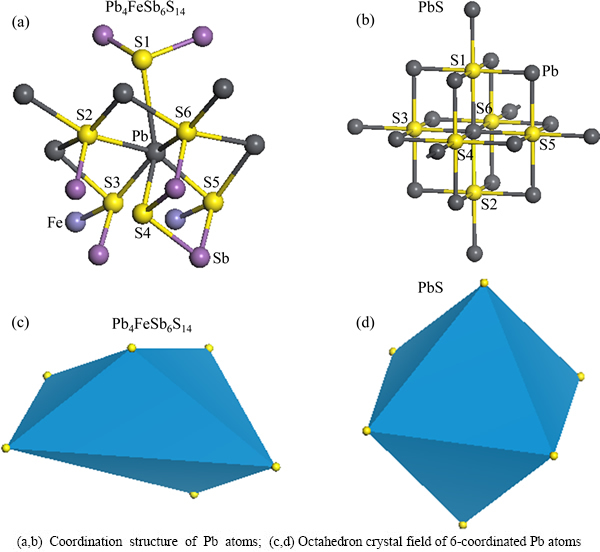
Fig. 3 Coordination structures of Pb atoms for jamesonite (a,c) and galena (b,d)
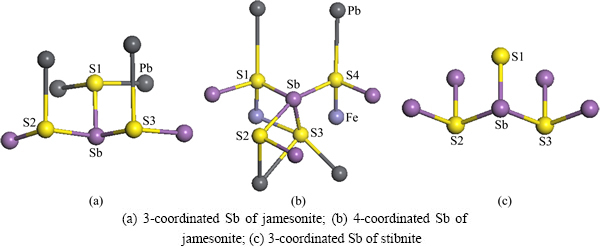
Fig. 4 Coordinated modes of Sb atoms for jamesonite and stibnite
Sb atoms of jamesonite have two coordinated modes: one is 3-coordinated and the other one is 4-coordinated. However, Sb atoms of stibnite are only 3-coordinated, as shown in Fig. 4. Compared with 3-coordinated Sb atoms of stibnite and jamesonite, the structures of their coordination S atoms are largely different. In stibnite, one S atom (S1) coordinated by Sb atom is a single atom; the other two S atoms (S2 and S3) are connected with two Sb atoms (Fig. 4(c)); whereas three S atoms coordinated by Sb atom for jamesonite (Fig. 4(a)) are different with those of stibnite. S1 atom is connected with two Pb atoms, S2 and S3 atoms are connected with one Pb and one Sb atoms. The structure of 4-coordinated Sb atom of jamesonite (Fig. 4(b)) is more complex than that of 3-coordinated Sb atom. There are two states for four S atoms coordinated by Sb atom. S1 and S4 are connected with one Pb, one Fe, and the other Sb atoms. S2 atom is only connected with one Pb and the other Sb atoms. S3 atom is connected with two Pb and one Fe atoms. Therefore, the properties and activities between 3-coordinated Sb atoms and 4-coordinated Sb atoms are different.
3.3 DOS analysis
To understand the relationships and differences of electronic structures among jamesonite, galena, and stibnite, the partial density of states (PDOS) of Pb and Sb atoms of jamesonite and corresponding atoms of galena and stibnite were studied.
Figure 5 shows the DOS of Pb atom for Pb4FeSb6S14 and PbS. The conduction band is mainly formed from Pb 6p with few contributions from Pb 6s. The valence band is formed between Pb 6s and Pb 6p. DOS of Pb 6s for jamesonite is located in the range from -10 to -7 eV, which shifts to low energy level compared with that of galena with strong peak localization. Compared with galena, DOS of Pb 6s and Pb 6p at Fermi level is small. These results indicate that Pb of jamesonite is inactive. On the other hand, the DOS curve of jamesonite shifts to lower energy in contrast to that of galena, indicating that Pb in jamesonite is more stable than that in galena. According to the electronic structure, it is very easy for PbS to lose electrons (S-2-S0), therefore, PbS reveals good floatability. As a result, PbS flotation is far easier than that of jamesonite and can perform even in collectorless conditions, which is in well agreement with the flotation recovery of jamesonite and galena (Fig. 2).
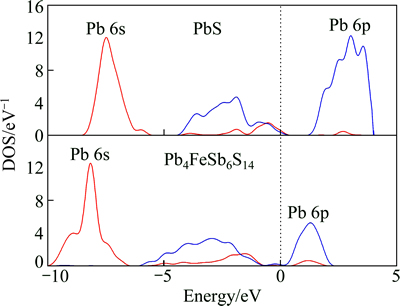
Fig. 5 DOS of Pb atoms for Pb4FeSb6S14 and PbS
Figure 6 shows the DOS of Sb atom for Pb4FeSb6S14 and Sb2S3. DOS curve of 3-coordination Sb atoms for jamesonite is similar to that of Sb atoms (3-coordination) for stibnite. However, Sb 5s and Sb 5p peaks of jamesonite are narrower than those of stibnite, and DOS of Sb 6s and Sb 6p at Fermi level is small, indicating that Sb (3-coordination) of jamesonite is inactive in contrast to that of stibnite. The DOS curve of 4-coordination Sb atoms for jamesonite is different from that of 3-coordination Sb. Peaks of Sb 5s and Sb 5p are lower than those of 3-coordination; however, DOS of Sb 5p for 4-coordination Sb atoms near the Fermi energy is large. Hence, 4-coordination Sb is more active than 3-coordination Sb. On the other hand, DOS curves of jamesonite also shift to lower energy in contrast to that of stibnite. As a result, jamesonite is more stable than stibnite.
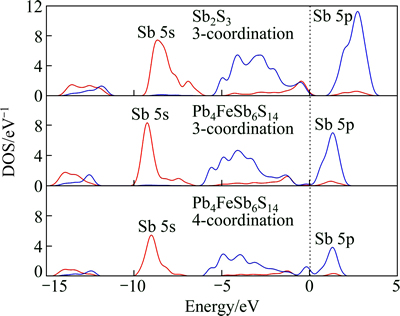
Fig. 6 DOS of Sb atoms for Pb4FeSb6S14 and Sb2S3
3.4 Analysis of frontier orbital
Table 1 presents the atomic orbital coefficients of HOMO and LUMO of Pb4FeSb6S14, PbS, Sb2S3, and FeS2. A high value of coefficient (absolute value) indicates a large contribution of atom to the frontier orbitals, whereas a low value (absolute value) indicates a small contribution of atom. Moreover, the same signs of coefficient denote the bonding between atoms, while the opposite signs of coefficient denote the anti-bonding state between atoms. Here, only the absolute and maximum values are concerned. According to the data in Table 1, the content of Fe in jamesonite is low, but it is very active. The HOMO orbital of jamesonite consists of Fe, S, Sb, and Pb atoms, but the main contributing atoms are Fe and S with small contributions of Sb and Pb. The main contribution of LUMO orbital for jamesonite is from Fe, S, and Sb atoms with small contributions from Pb.
Table 1 Frontier orbital coefficients of Pb4FeSb6S14, PbS, Sb2S3 and FeS2.

The frontier orbitals represent the materials properties. Comparing the frontier orbital coefficients of jamesonite, galena, stibnite, and pyrite, the LUMO orbital of jamesonite is similar to that of stibnite, which is easy to react with collectors and has good floatability. However, the HOMO orbital of jamesonite is similar to those of pyrite and stibnite, which means that it is easy to be depressed in alkaline medium, especially solution containing lime. Figure 7 shows the flotation behavior of jamesonite, galena, stibnite, and pyrite with various lime contents.
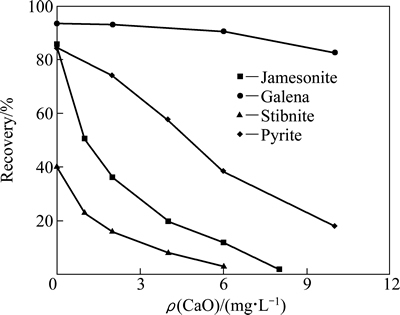
Fig. 7 Flotation behavior of jamesonite, galena, stibnite, and pyrite with various lime contents
Generally, the highest occupied orbitals of electron easily lose electron, whereas the lowest unoccupied orbitals gain easily electron. The frontier orbitals can be simply summarized as nucleophilic HOMO and electrophilic LUMO orbitals, for instance, the frontier orbitals of galena (Table 1). The main contribution of HOMO orbital is from S atoms, but that of LUMO orbital depends on Pb atoms. Therefore, it is easy for S atoms of galena to lose electron and enter in the oxidation reaction. The Pb ion reacts easily with xanthate anions to form lead xanthate. The HOMO orbitals of jamesonite, stibnite, and pyrite contain metal atoms. The configurations of metal atoms in HOMO orbitals are due to the low valence of Fe atoms of jamesonite (+2), Sb atoms of stibnite (+3), and Fe atoms of pyrite (+2), which have both electron and empty orbitals and can lose electrons to become high valence. As a result, these metal atoms appear in HOMO orbitals as the electron occupants. Now, let us discuss how the appearances of metal atoms in HOMO orbitals relate to the inhibiting effect of lime.
According to Fig. 7, the flotation of galena is insensitive to lime, although those of jamesonite, pyrite, and stibnite are easily inhibited by lime. The inhibitive component in lime is CaOH+ that easily reacts with nucleophilic HOMO orbitals. The inhibition model of pyrite [21] shows that Ca atom adsorption on S site is the most stable adsorption configurations of CaOH+ on the surface of pyrite and oxygen of hydroxyl is adsorbed on Fe site. Therefore, the appearance of metal atoms in HOMO orbitals contributes to the formation of adsorption configuration of CaOH+ like in the flotation of jamesonite, stibnite, and pyrite with various lime concentrations. If no metal atom appears in HOMO, such as in galena, it acts against the formation of the adsorption configuration of CaOH+. In these observations, lime has less inhibitory effect on the flotation of galena.
4 Conclusions
1) The properties and flotation behaviors of jamesonite depend on its chemical composition (Sb and Pb) and crystal structure. The flotation behavior of jamesonite is similar to that of stibnite, which shows good floatability at pH below 6 and is easily depressed by NaOH, especially by lime. In weak alkaline condition, the flotation behavior of jamesonite is close to that of galena, which has good floatability at high pH values.
2) The coordination structure of Pb atoms of jamesonite is more complex than that of galena. The octahedron crystal field formed by ligand field of Pb atom of jamesonite has an asymmetric structure, but that of galena possesses a symmetrical structure. The Sb atoms in jamesonite have two coordinated modes: 3-coordinated and 4-coordinated, whereas Sb atoms of stibnite are only 3-coordinated. The structures of three S atoms coordinated by Sb in stibnite and jamesonite are different. The structure of 4-coordinated Sb atoms of jamesonite is more complex than that of 3-coordinated Sb atoms. These results lead to different flotation behaviors.
3) Jamesonite is more stable than galena and stibnite. Pb in galena is more active than that in jamesonite, but Sb (3-coordination) of jamesonite is inactive with that of stibnite. 4-coordination Sb for jamesonite is more active than 3-coordination Sb.
4) The main contribution of HOMO orbital for galena is from S atoms, whilst that of LUMO orbital comes from Pb atoms. Pb ion reacts easily with xanthate anions to form lead xanthate. HOMO orbitals of jamesonite, stibnite, and pyrite contain metal atoms. The appearance of metal atoms in HOMO orbitals contributes to the formation of adsorption configuration of CaOH+ in presence of lime, making an inhibitory effect on the flotation of jamesonite, stibnite, and pyrite.
References
[1] ANTHONY J W, BIDEAUX R A, BLADH K W, NICHOLS M C. Handbook of mineralogy [M]. Tucson, AZ: Mineral Data Publishing, 1990.
[2] WARK I W, COOK A B. An experimental study of the effect of xanthates on contact angles at mineral surfaces [J]. Transactions of the American Institution of Mining and Metallurgy Engineering, 1934, 112: 189-244.
[3] GAUDIN A M, FINKELSTEIN N P. Interactions in the system galena-potassium ethyl xanthate—oxygen [J]. Nature, 1956, 207: 389-391.
[4] CHEN J H, LI Y Q, LONG Q R, WEI Z W, CHEN Y. Improving the selective flotation of jamesonite using tannin extract [J]. International Journal of Mineral Processing, 2011, 100: 54-56.
[5] ZHANG Qin, HU Yue-hua, XU Jing, CHEN Jing, CHEN Tie-jun. FTIR spectroscopic study of electrochemical flotation of jamesonite- diethyldithiocarbamate system [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(2): 493-496.
[6] ZHAO Rui-rong, JIANG Han-ying, ZENG Zhen-ou, QIN Yi-hong, XU Shun. Behaviors of Sb, Sn, and As in leaching and electrowinning from jamesonite concentrate [J]. Acta Metallurgica Sinica, 1990, 26: B48-B54. (in Chinese)
[7] HUANG H, SUN W. Effect of 2-aminothiophenol on the separation of jamesonite and marmatite [J]. Mining Science and Technology, 2010, 20: 0425–0427.
[8] LAGER T. The present of stibnite-bearing ore [J]. Metallic Ore Dressing Abroad, 1990, 8: 1-9.
[9] LAGER T, FORSSBERG K S E. Comparative study of the flotation properties of jamesonite and stibnite [J]. Scandinavian Journal of Metallurgy, 1989, 18(3): 122-130.
[10] LAGER T, FORSSBERG K S E. Benefication characteristics of antimony mineral: A review—Part 1 [J]. Minerals Engineering, 1989, 2(3): 321-336.
[11] DENG H B, XU S. Mechanism of flotation of jamesonite and separation of marmatite [J]. Nonferrous Metals (Mineral Processing Section), 1990, 6: 15-18. (in Chinese).
[12] LI Y Q, CHEN J H, CHEN Y, GUO J. Density functional theory study of influence of impurity on electronic properties and reactivity of pyrite [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1887-1895.
[13] ZHAO C H, CHEN J H, LONG X H, GUO J. Density functional theory study on natural hydrophobicity of sulfide surfaces [J], Transactions of Nonferrous Metals Society of China, 2014, 24: 491-498.
[14] CHEN J H, LAN L H,LIAO X J. Depression effect of pseudo glycolythiourea acid in flotation separation of copper-molybdenum [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(3): 824-831.
[15] NIIZEKI N, BUERGER M J. The crystal structure of jamesonite, FePb4Sb6S14 [J]. Zeitschrift Fuer Kristallographie, 1957, 109: 161-183.
[16] CLARK S J, SEGALL M D, PICKARD C J, HASNIP P J, PROBERT M J, REFSON K, PAYNE M C. First principles methods using CASTEP [J]. Zeitischrift fuer Kristallographie, 2005, 220(5/6): 567-570.
[17] SEGALL M D, LINDAN P J D, PROBERT M J, PICKARD C J, HASNIP P J, CLARK S J, PAYNE M C. First-principles simulation: ideas, illustrations and the CASTEP code [J]. Journal of Physics: Condensed Matter, 2002, 14(11): 2717-2744.
[18] DELLEY B. An all-electron numerical method for solving the local density functional for polyatomic molecules [J]. Journal of Chemical Physics, 1990, 92(1): 508-517.
[19] DELLEY B. From molecules to solids with the DMol3 approach [J]. Journal of Chemical Physics, 2000, 113(18): 7756-7764.
[20] PERDEW J P, WANG Y. Accurate and simple analytic representation of the electron-gas correlation energy [J]. Physical Review B, 1992, 45(23): 13244-13249.
[21] CHEN J H, LI Y Q, CHEN Y. Cu–S flotation separation via the combination of sodium humate and lime in a low pH medium [J]. Minerals Engineering, 2011, 24: 58-63.
复杂矿物脆硫锑铅矿的电子结构与浮选行为
赵翠华1,2,3, 陈建华3,4 李玉琼4, 何 茜4, 吴伯增2
1. 广西大学 材料科学与工程学院,南宁530004;
2. 广西华锡集团股份有限公司,柳州,545006;
3. 广西大学 化学化工学院,南宁 530004;
4. 广西大学 广西高校矿物工程重点实验室,南宁 530004
摘 要:采用密度泛函理论研究复杂矿物脆硫锑铅矿的电子结构及其浮选行为。脆硫锑铅矿的浮选行为与辉锑矿相似,pH值小于6时具有较好的可浮性,并且容易被NaOH抑制,尤其是在有石灰存在时。在弱碱性条件下,脆硫锑铅矿的浮选行为接近于方铅矿的浮选行为。脆硫锑铅矿中Pb的配位结构比方铅矿中Pb的配位结构复杂。脆硫锑铅矿中的Sb存在2种配位模式,而辉锑矿中的Sb仅存在3-配位。方铅矿中的Pb比脆硫锑铅矿中的Pb活跃。与辉锑矿中的Sb相比,脆硫锑铅矿中的Sb(3-配位)不活跃,但是4-配位的Sb比3-配位的Sb活跃得多。脆硫锑铅矿和辉锑矿的HOMO轨道含有金属原子,石灰的存在有助于CaOH+吸附构型的形成。因此石灰对脆硫锑铅矿和辉锑矿都具有抑制作用。
关键词:脆硫锑铅矿;电子结构;浮选行为;密度泛函理论
(Edited by Yun-bin HE)
Foundation item: Project (NCET-11-0925) supported by the New Century Excellent Talents in University, China; Project (51164001) supported by the National Natural Science Foundation of China; Project supported by Open Foundation of Guangxi Key Laboratory for Advanced Materials and Manufacturing Technology, China
Corresponding author: Jian-hua CHEN; Tel: +86-771-3232200; E-mail: jhchen@gxu.edu.cn
DOI: 10.1016/S1003-6326(15)63641-X
Abstract: Electronic structures of complex mineral jamesonite were studied using density functional theory method together with their flotation behavior. The flotation behavior of jamesonite is similar to that of stibnite, indicating good floatability at pH below 6 and easy depression with NaOH, especially with lime. In weak alkaline condition, the flotation behavior of jamesonite is close to that of galena. The coordination structure of Pb for jamesonite is more complex than that for galena. Sb in jamesonite possesses two coordinated modes, whereas Sb of stibnite is only 3-coordinated. Pb in galena is more active than that in jamesonite. Sb (3-coordination) in jamesonite is inactive, in contrast with that in stibnite. However, 4-coordination Sb in jamesonite is more active than 3-coordination Sb. HOMO orbitals of jamesonite and stibnite contain metal atoms, which contribute to the formation of adsorption configuration of CaOH+ when there is lime; therefore, jamesonite and stibnite are easily depressed by lime.


