J. Cent. South Univ. (2016) 23: 1339-1344
DOI: 10.1007/s11771-016-3185-y
Flotation behavior and adsorption mechanism of fine wolframite with octyl hydroxamic acid
MENG Qing-you(孟庆有), FENG Qi-ming(冯其明), OU Le-ming(欧乐明)
School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
Flotation behavior and adsorption mechanism of octyl hydroxamic acid (OHA) on wolframite were investigated through flotation experiments, adsorption tests, zeta-potential measurements, infrared spectroscopy and solution chemistry calculations. Results of flotation and adsorption experiments show that the maximum values of flotation recovery and adsorption capacity occur around pH 9. In term of the solution chemistry calculations, the concentration of metal hydroxamate is greater than that of metal tungstate and metal hydroxyl, and metal hydroxamate compounds are identified to be the main species on wolframite surface at pH region of 8-10, contributing to the increase of OHA adsorption and flotation performance. Results of zeta-potential and IR spectra demonstrate that OHA adsorbs onto wolframite surface by chemisorptions. Hydroxamate ions can bond with Mn2+/Fe2+ cations of wolframite surface, forming metal hydroxamate compounds, which is a key factor in inducing the hydrophobicity of wolframite under the conditions of maximum flotation.
Key words:
wolframite; adsorption; hydroxamate; flotation;
1 Introduction
Wolframite is the most important tungsten-bearing mineral, which has been mined and processed for a century in China. Because of its crispness property, wolframite slime produced in the comminution process accounts for 20% of the mined [1]. Traditionally, wolframite is upgraded by gravity and magnetic separation, however, wolframite slime is hardly recovered through the aforementioned two methods, especially the <20 μm size fraction, resulting in significant losses [2]. Therefore, the recovery enhancement of wolframite slime becomes a key research subject.
For fine and complex minerals, flotation is an efficient method. The flotation of wolframite requires the aid of collectors to adsorb onto the mineral and form hydrophobic surfaces due to its poor hydrophobicity. Currently, some organic surfactants, such as oleic acid, phosphonic acid and arsonic acid, are usually used as collectors in wolframite flotation [3-5]. However, flotation scientists found that these reagents are provided with good flotability and poor selectivity, causing low separation efficiencies between wolframite and gangues. Therefore, some researchers pay special attentions to new chemical reagents with superior collecting ability and mineral specificity.
Hydroxamate chemicals (RCONHOH), using as collectors, have been investigated for decades and are extensively applied in the flotation of oxidized minerals [6-7]. The interactions of hydroxamic acids with minerals, such as cassiterite, rare-earth ore, titanium and iron-containing minerals, are widely investigated [8-11]. Hydroxamates are believed to have a stronger chelating capacity for transition metal and rare-earth metal ions in their mineral lattice and form more stable chelates [12-14]. It is acceptable that wolframite with hydroxamate collectors should show a good flotation performance because of the Mn2+/Fe2+ characteristics of wolframite [15-16]. More recently, alkyl hydroxamic acids have been paid much attention on fine rare-earth flotation, which can float the <20 μm size fraction [17-18], and they have been known to perform well in flotation environments imposing serious slime problems. Since alkyl hydroxamic acids have a pronounced collecting ability and selectivity, it is worthy to make an attempt to apply alkyl hydroxamic acid in fine wolframite flotation. In this work, the investigation focused on the flotation behavior of fine wolframite with octyl hydroxamic acid (OHA), more importantly, the adsorption mechanism and the resultant hydrophobicity of OHA on the mineral surface.
2 Materials and methods
2.1 Materials
High purity wolframite samples used for all experiments were obtained from Yaogangxian, Hunan Province, China. The chemical analysis result indicates that the grade of wolframite was 74.18% (mass fraction, WO3) and the purity was 98%. The sample was wet-ground and elutriated to <10 μm size fraction. The prepared wolframite material had 90% passing at 10.95 μm and 50% passing at 4.44 μm. Octyl hydroxamic acid (OHA), using as a collector, was prepared in the laboratory and was of laboratory grade. NaOH (sodium hydroxide) and HCl (hydrochloric acid) used as pH regulators were of analytical grade. Distilled water was utilized in all experimental work.
2.2 Experiments
2.2.1 Flotation
Mineral flotation tests were carried out in a mechanical agitation flotation machine with a 40 mL cell. The wolframite suspension was prepared by adding 2.0 g sample to 40 mL distilled water. The slurry was adjusted to a desired pH value by adding NaOH or HCl solutions and conditioned for 2 min. Then OHA was added and conditioned for 3 min. Flotation was performed for a total of 5 min, and the flotation recovery was calculated based on the amount of floated minerals.
2.2.2 Adsorption measurements
The amount of collector adsorbed on wolframite was conducted on the difference between the initial and final concentrations, and it was assumed that the reduced amount of OHA in solution had adsorbed onto the mineral phase. The OHA concentration was analysed using UV absorbance at 507 nm. 1.0 g sample was conditioned with 40 mL OHA solution with a desired pH for 20 min at 25 °C. The sample was centrifuged at 9000 r/min for 10 min, and the supernatant was monitored by spectrophotometer (UV-2600).
2.2.3 Zeta potential measurements
The electrophoretic mobility of wolframite was determined on zeta plus meter (Brookhaven, USA). For these measurements, 30 mg wolframite sample (<5 μm) was added into 40 mL distilled water solution with/without adding OHA. The suspension was adjusted by NaOH or HCl to a pH value in the range of 2-12. An average of three measurements was calculated for each zeta-potential point.
2.2.4 Infrared spectroscopy
The infrared spectra of wolframite in the absence and the presence of OHA were recorded using a SHIMADZU infrared spectrometer (IRAffinity-1) in the range 4000-400 cm-1. In the tests, 1 mg desired wolframite sample was mixed with 100 mg spectroscopic grade KBr as diluent and pressed into pellets for recording the spectra.
3 Results and discussion
3.1 Flotation and adsorption
The effect of pH on the flotation recovery of wolframite, using 2×10-4 mol/L OHA as collector, is shown in Fig. 1. It is evidently found from Fig.1 that better flotability of wolframite is obtained over the pH range from 7.0 to 10.0, and a maximum of flotation around 67.70% is obtained at about 9.0. However, the flotation recovery is low at pH<7.0 and pH>10.0.
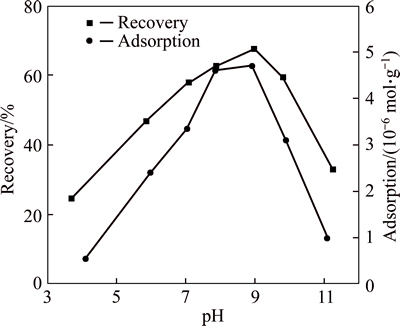
Fig. 1 Effect of pH on recovery and adsorption of wolframite (cOHA=2×10-4 mol/L)
Figure 1 also gives the adsorption of OHA on wolframite as a function of pH at a concentration of 2×10-4 mol/L OHA. According to Fig. 1, the adsorption capacity first increases and then decreases with the increase of pH, and the maximum is obtained under pH 8-9, coinciding with the floatability of wolframite. Maximum values of adsorption and flotation recovery occur at about 9.0, which is close to the pKa (the constant of dissociation equilibrium) of OHA, indicating that the interaction between octyl hydroxamic acid and wolframite can ascribe to the involvement of ionic OHA, which agrees well with earlier studies on sparingly soluble minerals [19-20].
3.2 Electrokinetics
In flotation system, surface electrical properties of mineral are mainly attributed to the dissolution of mineral surface ions and/or the adsorption of certain ions onto mineral surfaces. Therefore, electrokinetic potentials of mineral are widespread used to analyze the surface properties of mineral and explain the flotation phenomena. Figure 2 gives electrokinetic potentials of wolframite in the absence and the presence of OHA under different pH conditions. The surface of purewolframite is negatively charged in the measured pH region, which could be the difference in the solubility of potential-determining ions. The free energies of hydration of Fe2+, Mn2+ and  ions are 1952.06, 1864.28 and 836 kJ/g, respectively. Fe2+ and Mn2+ ions preferentially dissolve into the solution, and
ions are 1952.06, 1864.28 and 836 kJ/g, respectively. Fe2+ and Mn2+ ions preferentially dissolve into the solution, and  ions become dominating components of the wolframite surface. Thus, the surface potential of wolframite is negative [21]. The presence of OHA shifts the zeta potential of wolframite more negative in the pH region from 4 to 10, which is ascribed to the OHA adsorption onto wolframite surface. However, no remarkable changes of zeta-potentials are observed by adding OHA at pH>10, which may be caused by the enhanced electrostatic repulsion between negatively charged wolframite and OHA anions. In addition, the competitive adsorption between OHA- and OH- ions takes place on wolframite surface, a large number of OH- ions interacts with Mn2+/Fe2+ cations and blocks the adsorption of OHA in the strong alkaline solution. Accordingly, the adsorption and flotation recovery decrease sharply at pH>10.
ions become dominating components of the wolframite surface. Thus, the surface potential of wolframite is negative [21]. The presence of OHA shifts the zeta potential of wolframite more negative in the pH region from 4 to 10, which is ascribed to the OHA adsorption onto wolframite surface. However, no remarkable changes of zeta-potentials are observed by adding OHA at pH>10, which may be caused by the enhanced electrostatic repulsion between negatively charged wolframite and OHA anions. In addition, the competitive adsorption between OHA- and OH- ions takes place on wolframite surface, a large number of OH- ions interacts with Mn2+/Fe2+ cations and blocks the adsorption of OHA in the strong alkaline solution. Accordingly, the adsorption and flotation recovery decrease sharply at pH>10.
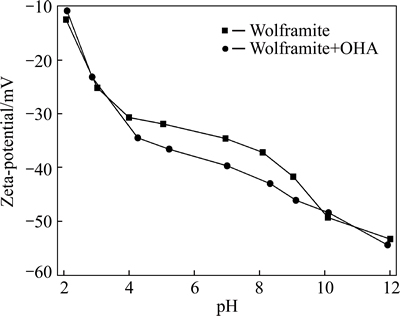
Fig. 2 Effect of pH on zeta-potential of wolframite with/ without OHA (cOHA=2×10-4 mol/L)
An interesting phenomenon is observed in Fig. 2. The presence of OHA makes wolframite more negatively charged under the flotation conditions. In the view of electrostatic properties, OHA is considered as an anionic collector, and it is incomprehensible that the ionized OHA causes the negatively charged wolframite surface more negative through electrostatic attraction. Therefore, the specific adsorption of OHA on mineral surface, likely by coordination reactions, might be involved [15, 22].
3.3 Solution chemistry
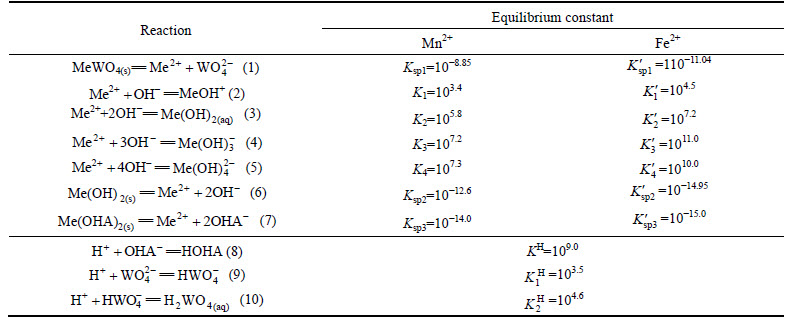
In order to understand the relationship between floatability of wolframite and adsorption mechanism of collectors, the solution chemistry calculations are conducted. In OHA-wolframite flotation system, it is reasonable that dissolution-precipitation equilibria exist between lattice cations (Fe2+/Mn2+) and anions, such as tungstate ions ( ), collector anions (OHA-) and hydroxyl ions (OH-). The various reaction equations and equilibrium constants for the present system are given in Table 1 [23].
), collector anions (OHA-) and hydroxyl ions (OH-). The various reaction equations and equilibrium constants for the present system are given in Table 1 [23].
The [Me] species mainly exist in forms of Me2+, MeWO4(s), MeOH+, Me(OH)2(aq), 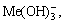
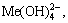 Me(OH)2(s) and Me(OHA)2(s). As a consequence, the concentrations of these species are calculated based on formulas (1)-(10), respectively.
Me(OH)2(s) and Me(OHA)2(s). As a consequence, the concentrations of these species are calculated based on formulas (1)-(10), respectively.

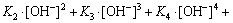
 (11)
(11)
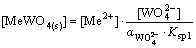 (12)
(12)
Table 1 Reaction equations and equilibrium constants
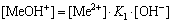 (13)
(13)
 (14)
(14)
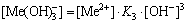 (15)
(15)
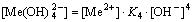 (16)
(16)
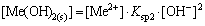 (17)
(17)
 (18)
(18)
where 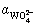 and
and  are respectively the protonation constants of
are respectively the protonation constants of 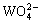 and OHA- given by
and OHA- given by
 (19)
(19)
 (20)
(20)
From the results above, lgC-pH diagrams of the [Me] species in OHA-wolframite flotation system under varying pH are shown in Fig. 3. When pH<8.0 and pH>10.0, MeWO4 and Me(OH)2 play a dominant role on wolframite surface, respectively. However, Me(OHA)2 preponderates over the formers at the region of pH 8-10.
By comparing Fig.1 with Fig. 3, the flotation recovery of wolframite and the adsorption of OHA on mineral surface are related to the concentration of metalhydroxamate precipitates in OHA-wolframite flotation system. Under acidic conditions, the concentration of metal hydroxamate precipitates is low, accordingly, the recovery and adsorption of wolframite decrease. The main reasons are the low solubility of wolframite and the protonation of OHA in acidic conditions. OHA in the form of ions is so rare that it can not form sufficient metal hydroxamate precipitates on wolframite surface, causing a decrease in adsorption and flotability of wolframite. Such plateaus of the metal hydroxamate compounds occur over the pH range from 8 to 10 for Mn2+ and Fe2+, and the concentration of metal hydroxamate precipitates is higher than that of metal tungstate and metal hydroxyl, suggesting that metal hydroxamates are the dominant species on mineral surface. Hydroxamate anions adsorb onto wolframite surface by exchanging OH- ions of MeOH- and 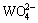 ions of MeWO4 [24], which accordingly enhances the flotability of wolframite. When the pH surpasses 10, the concentration of metal hydroxamate precipitates decreases, but the concentration of metal hydroxyl precipitates increases. The concentration of metal hydroxyl is higher than that of metal hydroxamate at pH>11, suggesting that metal hydroxyl precipitates have occupied most of the wolframite surface. Metal hydroxyl precipitates are hydrophilic and reduce the floatability of wolframite.
ions of MeWO4 [24], which accordingly enhances the flotability of wolframite. When the pH surpasses 10, the concentration of metal hydroxamate precipitates decreases, but the concentration of metal hydroxyl precipitates increases. The concentration of metal hydroxyl is higher than that of metal hydroxamate at pH>11, suggesting that metal hydroxyl precipitates have occupied most of the wolframite surface. Metal hydroxyl precipitates are hydrophilic and reduce the floatability of wolframite.
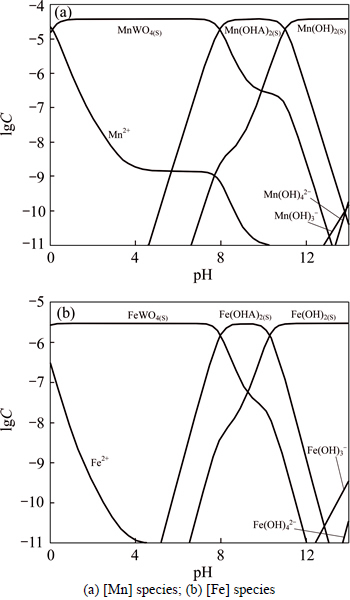
Fig. 3 Relationship between lgC and pH in OHA-wolframite system (cOHA=2×10-4 mol/L):
3.4 Infrared spectroscopy
To further investigate the adsorption mechanism of OHA with wolframite, IR spectra of OHA, wolframite mineral and wolframite after OHA adsorption are presented in Fig. 4. The IR spectrum of OHA (Fig. 4(a)) exhibits the adsorption band at 3260 cm-1 due to the overlap peak of N—H stretching vibration and O—H stretching vibration, and the adsorption bands of 2918 cm-1 and 2849 cm-1 are associated with stretching vibration of C—H. The peaks at 1662 cm-l, 1623 cm-1 and 1571cm-1 corresponding to the characteristic peaks of C=O stretching vibration, C=N stretching vibration and N—H bending vibration, respectively [25-26]. The IR spectrum of wolframite (Fig. 4(b)) gives two prominent bands at 810 cm-1 and 617cm-1 which belong to characteristic peaks of [WO6] and [(Mn,Fe)O6] [27]. The IR spectrum of wolframite treated with OHA at pH 9.0 is shown in Fig. 4(b). It is found that the two new bands at 2918 cm-1 and 2846 cm-1 precisely assign to the stretching vibration C—H group of OHA, which indicates that OHA has adsorbed onto the wolframite surface. The remarkable absorption band at 1655cm-1 is assigned to the overlap peak of original OHA bands at 1662 cm-l (C=O stretching) and 1623 cm-1 (C=N stretching), and the bending vibration of N-H shifts from 1571 to 1532 cm-1, while the adsorption band of OHA at 3260 cm-1 disappears, which is contributed to the possible formation of metal coordination bonds. The oxygen atoms from carbonyl (C=O) and hydroxyl (N—OH) of OHA can easily bond with the Fe2+/Mn2+ ions on the wolframite surface and form stable metal chelate compounds [15, 28]. Moreover, the characteristic peak of [(Mn, Fe)O6] shifts from 617cm-1 to 605 cm-1, which further indicates the chelation of the special functional groups of OHA with Fe2+/Mn2+ ions. Therefore, there are sufficient evidences to demonstrate the chemisorption of OHA onto wolframite surface and form surface precipitates of metal-OHA compounds.
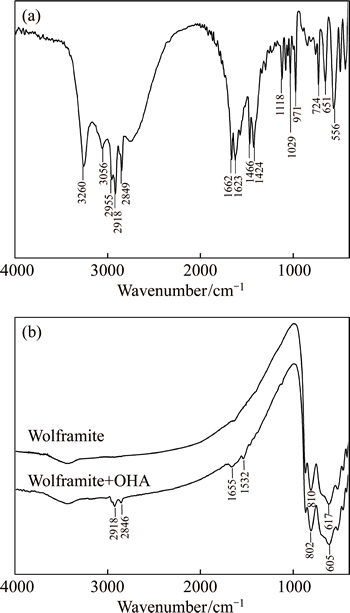
Fig. 4 Infrared spectra of OHA (a), wolframite and wolframite treated with OHA (b) after treatment with OHA
4 Conclusions
1) Wolframite could be readily floated with OHA over the pH range from 7 to 10, and the flotation maximum occurs at about pH 9.0. The adsorption of OHA on wolframite surface coincides with the flotation performance, and accordingly promotes the flotability of wolframite.
2) Based on solution chemistry analyses, metal hydroxamate precipitates become dominant components on mineral surface under weak alkaline conditions, in which flotation recovery and OHA adsorption are higher. This result indicates that flotation response and OHA adsorption correlate well with the concentrations of metal hydroxamate precipitates.
3) The presence of OHA makes the zeta potential of wolframite more negative. Moreover, infrared spectroscopy results indicate that the adsorption mechanism can be fixed as a consequence of the chemisorption of OHA onto wolframite surface. Hydroxamate ions interact with metal ions and form stable metal-hydroxamate compounds on wolframite surface, which are responsible for inducing hydrophobicity of wolframite.
References
[1] FU Guang-qin, HE Xiao-juan, ZHOU Xiao-tong. The research progress of wolframite slime flotation [J]. China Tungsten Industry, 2010, 25(1): 22-25. (in Chinese)
[2] SRIVASTAVA J P, PATHAK P N. Pre-concentration: A necessary step for upgrading tungsten ore [J]. International Journal of Mineral Processing, 2000, 60(1): 1-8.
[3] WEI Da-wei, WEI Ke-wu, QIU Ji-cun. Hydrophobic agglomeration and spherical agglomeration of wolframite fines [J]. International Journal of Mineral Processing, 1986, 17(3/4): 261-271.
[4] SRINIVAS K, SREENIVAS T, PADMANABHAN N P H, VENUGOPAL R. Studies on the application of alkyl phosphoric acid ester in the flotation of wolframite [J]. Mineral Processing and Extractive Metallurgy Review: An International Journal, 2004, 25(4): 253-267.
[5] LIU De-quan, ZHOU Jun-shan, WANG Dian-zuo. Flotation interaction of wolframite with benzylarsonic acid and sodium butyl xanthate [J]. The Chinese Journal of Nonferrous Metals, 1992, 2(3): 25-29. (in Chinese)
[6] MARABINIA A M, CIRIACHIB M, PLESCIAC P, BARBAROB M. Chelating reagents for flotation [J]. Minerals Engineering, 2007, 20(10): 1014-1025.
[7] PRADIP. Applications of chelating agents in mineral processing [J]. Minerals and Metallurgical Processing, 1988, 5: 80-89.
[8] QIN Wen-qing, REN Liu-yi, XU Yang-bao, WANG Pei-pei, MA Xi-hong. Adsorption mechanism of mixed salicyhydroxamic acid and tributyl phosphate collectors in fine cassiterite electro-flotation system [J]. Journal of Central South University, 2012, 19(6): 1711-1717.
[9] ADAM J, CHRISTOPHER M, OLGA K, KRISTIAN E. Surface chemistry considerations in the flotation of  [J]. Minerals Engineering, 2014, 66–68: 119-129.
[J]. Minerals Engineering, 2014, 66–68: 119-129.
[10] REN Jun, WANG Wen-mei, LUO Jia-ke, ZHOU Gao-yun, TANG Fang-qiong. Progress of flotation reagents of rare earth minerals in China [J]. Journal of Rare Earths, 2003, 21(1): 1-8.
[11] LIU Jin-wei, HU Hui-ping, WANG Meng, CHEN Xiang-pan, CHEN Qi-yuan, DING Zhi-ying. Synthesis of modified polyacrylamide with high content of hydroxamate groups and settling performance of red mud [J]. Journal of Central South University, 2015, 22: 2073-2080.
[12] SREENIVAS T, PADMANABHAN N P H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 205(1/2): 47-59.
[13] PRADIP P, FUERSTENAU D W. Design and development of novel flotation reagents for the beneficiation of mountain pass rare-earth ore [J]. Minerals and Metallurgical Processing, 2013, 30(1): 1-9.
[14] BASILIO C, LOWE R A, GORKEN A, MAGLIOCCO L, HAGY R. Modified hydroxamate collectors for kaolin flotation [J]. Developments in Mineral Processing, 2000, 13: C8b-51-C8b-55.
[15] HU Yue-hua, WANG Dian-zuo, XU Zheng-he. A study of interaction and flotation of wolframite with octyl hydroxamate [J]. Minerals Engineering, 1997, 10(6): 623-633.
[16] YANG Si-yuan, FENG Qi-ming, QIU Xian-yang, GAO Yu-de, XIE Zhen-fu. Relationship between flotation and Fe/Mn ratio of wolftamite with benzohydroxamic acid and sodium oleate as collectors [J]. Physicochemical Problems of Mineral Processing, 2014, 50(2): 747-758.
[17] GIBSON C E, KELEBEK S, AGHAMITIAN M, YU B. Flotation of pyrochlore from low grade carbonatite gravity tailings with benzohydroxamic acid [J]. Minerals Engineering, 2015, 71: 97-104.
[18] XIAO Ni, QI Liu. The adsorption and configuration of octyl hydroxamic acid on pyrochlore and calcite [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 411: 80-86.
[19] WANG Pei-pei, QIN Wen-qing, REN Liu-yi, WEI Qian, LIU Rui-zeng, YANG Cong-ren, ZHONG Shui-ping. Solution chemistry and utilization of alkyl hydroxamic acid in flotation of fine cassiterite [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(6): 1789-1796.
[20] FUERSTENAU D W, PRADIP. Mineral flotation with hydroxamate collectors [M]// Reagents in the Minerals Industry. London: IMM, 1984: 161-168.
[21] YE Zhi-ping. Study on the flotation mechanism of wolframite with benzohydroxamic acid [J]. Nonferrous Metals, 2000(5): 35-39. (in Chinese)
[22] SREENIVAS T, PADMANABHAN N P H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 205(1/2): 47-59.
[23] SMITH R M, MARTELL A E. Critical stability constants: Inorganic complexes [M]. New York: Plenum Press, 1976: 5-19.
[24] MARINAKI K I, KELSALL G H. Adsorption of decyl sulphate and decyl phosphate on wolframite, (Fe,Mn)WO4, and their use in the two-liquid flotation of fine wolframite particles [J]. Journal of Colloid and Interface Science, 1985, 106(2): 517-531.
[25] HOPE G A, WOODS R, PARKER G K, BUCKLEY A N, MCLEAN J. A vibrational spectroscopy and XPS investigation of the interaction of hydroxamate reagents on copper oxide minerals [J]. Minerals Engineering, 2010, 23(11/12/13): 952-959.
[26] CUI J, HOPE G A, BUCKLEY A N. Spectroscopic investigation of the interaction of hydroxamate with bastnaesite (cerium) and rare earth oxides [J]. Minerals Engineering, 2012, 36-38: 91-99.
[27] MOENKE H. Mineral spektren [M]. Berlin: Akademie-Verlag, 1966: 33.
[28] LU Yong-quan, DENG Zhen-hua. Analysis of practical infrared spectrum [M]. Beijing: Electronic Industry Press, 1989: 132. (in Chinese)
(Edited by FANG Jing-hua)
Foundation item: Project(2014CB643402) supported by the National Basic Research Program of China; Project(CX2013B082) supported by the Hunan Provincial Innovation Foundation for Postgraduate, China
Received date: 2015-03-02; Accepted date: 2015-11-19
Corresponding author: FENG Qi-ming, Professor, PhD; Tel/Fax: +86-731-88836817; E-mail: feng_309@csu.edu.cn
Abstract: Flotation behavior and adsorption mechanism of octyl hydroxamic acid (OHA) on wolframite were investigated through flotation experiments, adsorption tests, zeta-potential measurements, infrared spectroscopy and solution chemistry calculations. Results of flotation and adsorption experiments show that the maximum values of flotation recovery and adsorption capacity occur around pH 9. In term of the solution chemistry calculations, the concentration of metal hydroxamate is greater than that of metal tungstate and metal hydroxyl, and metal hydroxamate compounds are identified to be the main species on wolframite surface at pH region of 8-10, contributing to the increase of OHA adsorption and flotation performance. Results of zeta-potential and IR spectra demonstrate that OHA adsorbs onto wolframite surface by chemisorptions. Hydroxamate ions can bond with Mn2+/Fe2+ cations of wolframite surface, forming metal hydroxamate compounds, which is a key factor in inducing the hydrophobicity of wolframite under the conditions of maximum flotation.


