文章编号:1004-0609(2010)03-0451-05
7×××铝合金退火过程中二次Al3(Sc, Zr)粒子的析出行为
戴晓元1, 2,夏长清2,彭小敏2
(1. 长沙理工大学 汽车与机械工程学院,长沙 410114;
2. 中南大学 材料科学与工程学院,长沙 410083)
摘 要:
采用透射电子显微镜,研究含钪Al-Zn-Mg-Cu-Zr系铸态合金在退火过程中二次Al3(Sc,Zr)粒子的析出形貌、尺寸及分布。结果表明:含0.20%Sc的7系铝合金铸态试样在450 ℃退火2 h后,α(Al)基体内析出呈豆瓣状的二次Al3(Sc,Zr)粒子;在450 ℃退火32 h后,Al3(Sc,Zr)粒子尺寸为16~23 nm;在450 ℃退火32 h后的二次Al3(Sc,Zr)相与α(Al)基体完全共格。
关键词:
中图法分类号:TG146.2 文献标识码:A
Precipitation behavior of Al3(Sc, Zr) secondary particles in
7××× aluminum alloys during annealing
DAI Xiao-yuan1, 2, XIA Chang-qing2, PENG Xiao-min2
(1. School of Automobile and Mechanic Engineering, Changsha University of Science and Technology,
Changsha 410114, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The microstructures, sizes and distribution of Al3(Sc, Zr) secondary particles in Al-Zn-Mg-Cu-Zr alloy containing Zr during annealing were studied by transmission electron microscopy. The results show that the bean-like Al3(Sc, Zr) secondary particles precipitate from α(Al) matrix in Al-Zn-Mg-Cu-Zr as-cast alloy with 0.20%Sc after annealing at 450 ℃ for 2 h. The size of the Al3(Sc, Zr) secondary particles is 16?23 nm after annealing at 450 ℃ for 32 h. These particles are completely coherent with the α(Al) matrix.
Key words: Al3(Sc, Zr) secondary particle; Sc; precipitation
钪(Sc)是迄今为止所发现的对优化铝合金性能最有效的合金元素。研究结果表明[1?6],在Al-Zn-Mg-Cu-Zr合金中添加微量Sc,Sc在合金的铸造组织中以2种形式存在,一是形成一次Al3(Sc,Zr)相粒子,与α(Al)基体共格,起到非均质形核核心的作用,从而大幅度细化合金铸态晶粒尺寸;另一方面,由于凝固过程中冷却速度快,形成含Sc和Zr的α(Al)过饱和固溶体,在随后的均匀化退火过程中,过饱和固溶体中的Sc和Zr将以二次Al3(Sc,Zr)相析出。已有研究[7?12]表明,添加Sc,在Al-Zn-Mg-Cu-Zr合金中形成二次Al3(Sc,Zr)相粒子阻碍位错和亚晶界的移动,提高合金抵抗变形的能力,从而提高合金的力学性能。
关于在450 ℃下均匀化退火处理对高锌的Al-Zn-Mg-Cu-Sc-Zr合金中二次Al3(Sc,Zr)粒子的析出行为的研究报道较少,为此,本文作者将研究450 ℃下退火时间对合金中二次Al3(Sc,Zr)粒子的析出行为的影响,α(Al)基体内析出二次Al3(Sc,Zr)相粒子的大小、数量和分布,二次Al3(Sc,Zr)粒子与α(Al)基体的共格性,以及二次Al3(Sc,Zr)粒子的热稳定性。
1 实验
将化学成分为Al-8.90Zn-2.42Mg-2.45Cu-0.20Sc- 0.16Zr(质量分数,%)的合金铸态样品置于箱式电阻炉中退火,退火温度为450 ℃,保温不同时间,如2、32和400 h。从退火样品上分别截取0.5~1.2 mm厚的薄片,经机械减薄至0.1 mm,冲成d3 mm的圆片,然后进行电解液双喷穿孔,制备透射电镜样品。双喷电解液为4%高氯酸酒精溶液,双喷温度约为?25 ℃(液氮冷却)。采用JEM?3010型高分辨透射电镜和TecnaiG2?20型透射电镜观察合金铸态试样在450 ℃下退火不同时间后合金中二次Al3(Sc,Zr)粒子的析出形貌、大小和分布。
2 实验结果
合金经450 ℃退火2 h后,在电镜下观察到从α(Al)基体内析出呈豆瓣状、球状的粒子(见图1)。这些粒子的尺寸约为18 nm,分布比较均匀、数量多、粒径小。
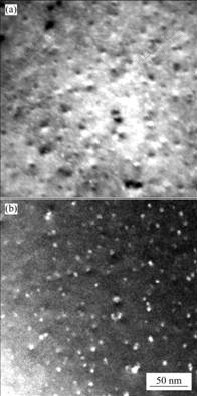
图1 含钪Al-Zn-Mg-Cu-Sc-Zr合金铸态试样在450 ℃退火2 h后的TEM像
Fig.1 TEM images of alloy after annealing at 450 ℃ for 2 h: (a) Bright field image; (b) Dark field image
合金铸态试样在450 ℃均匀化退火32 h后,在电镜下观察到呈球状的粒子尺寸为16~23 nm,粒子数目减少,如图2所示,间距为150~200 nm,比在450 ℃均匀退火2 h析出的粒子间距略有增大。图3所示为图2中α(Al)基体与析出粒子的电子衍射花样,有共格衍射斑点,表明该粒子与基体共格。参考有关文献[7?12],这些豆瓣状和球状粒子应为铸态合金均匀化退火过程中析出的二次Al3(Sc,Zr)相。
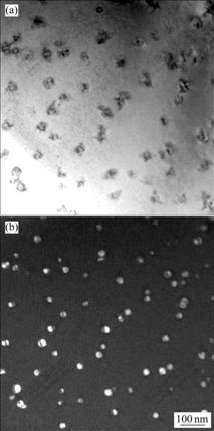
图2 含钪Al-Zn-Mg-Cu-Sc-Zr合金铸态试样在450 ℃退火32 h的TEM像
Fig.2 TEM images of alloy after annealing at 450 ℃ for 32 h: (a) Bright field image; (b) Dark field image
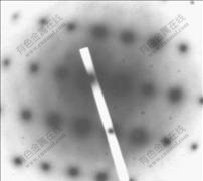
图3 含钪Al-Zn-Mg-Cu-Sc-Zr合金铸态试样在450 ℃退火32 h后Al3(Sc, Zr)粒子的电子衍射花样
Fig.3 Diffraction pattern of Al3(Sc, Zr) particles of alloy after annealing at 450 ℃ for 32 h
图4所示为铸态合金在450 ℃均匀化退火32 h后,在透射电镜下观察到的关于二次Al3(Sc,Zr)析出相的高分辨微观结构。合金铸态试样在450 ℃均匀化退火32 h的过程中,析出的二次Al3(Sc,Zr)相与α(Al)基体保持良好的共格关系。
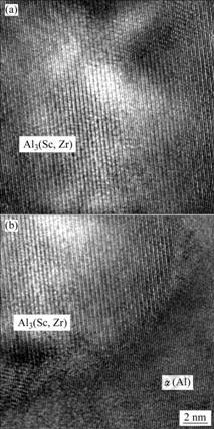
图4 Al3(Sc,Zr)以及Al3(Sc,Zr)析出粒子与α(Al)基体界面的高分辨TEM像
Fig.4 High magnification TEM images of Al3(Sc, Zr) particles (a) and interface of Al3(Sc, Zr) particles and α(Al) (b)
图5所示为合金铸态试样在450 ℃退火400 h的TEM照片。析出的Al3(Sc,Zr)粒子稍有粗化,粒径为26~40 nm,粒子分布均匀,粒子数量减少。
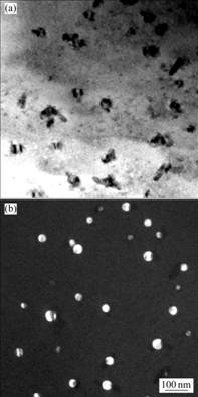
图5 含钪Al-Zn-Mg-Cu-Sc-Zr合金铸态试样在450 ℃退火400 h后的TEM像
Fig.5 TEM images of alloy after annealing at 450 ℃ for 400 h: (a) Bright field image; (b) Dark field image
图6所示为图5中Al3(Sc,Zr)粒子的选区衍射图谱,可以看到Al3(Sc,Zr)粒子的超点阵衍射斑,表明合金经450 ℃退火400 h后析出的Al3(Sc,Zr)粒子与α(Al)基体仍然保持共格关系。
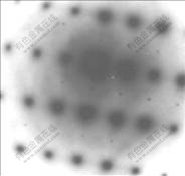
图6 Al3(Sc,Zr)粒子的电子衍射花样
Fig.6 Diffraction pattern of Al3(Sc, Zr) particles in alloy after annealing at 450 ℃ for 400 h
3 分析与讨论
由合金化原理可知,合金元素Sc在铝合金中主要以2种形式存在:固溶在α(Al)基体或形成金属间化合物以及金属化合物如Al3Sc、Al3(Sc,Zr)相。Sc原子在α(Al)和Al3(Sc,Zr)两相中的分配主要取决于溶质元素Sc和溶剂元素Al本身的性质。当Sc元素加入量低于在α(Al)中的固溶度时,Sc固溶于α(Al)中;当Sc元素加入量高于在α(Al)中的固溶度时,在动力学允许的条件下,因热力学驱动力作用,将形成饱和α(Al)固溶体和Al3(Sc,Zr)相。当向α(Al)中单独添加微量Sc元素时,决定溶质Sc元素在α(Al)中的固溶度主要取决于原子尺寸和化学亲和力(用电负性差表示)。根据Hume-Rothery规则[13],当溶质和溶剂的原子尺寸差超过14%~15%时,合金体系只能形成固溶度很小的固溶体;当二者电负性差超过0.4~0.5时,有利于形成化合物而固溶度减小。Al与Sc的原子半径和电负性如表1所列。
表1 Al和Sc的原子半径和电负性
Table 1 Atom radii and electronegativities of Al and Sc
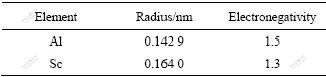
由表1可知,Sc和Al的原子半径相差0.021 1 nm (14.7%),电负性相差0.2,介于形成固溶体或化合物之间。由此可以初步判断,Sc在Al中除以Al3Sc化合物形式存在外,还有少量Sc固溶在α(Al)中。在实际的非平衡凝固中,即使在结晶冷却速度不快的情况下,Sc也倾向于固溶在α(Al)中形成非平衡的过饱和固溶体;而Sc的过饱和固溶体极不稳定,其分解速度非常快(如在250 ℃下时效,其孕育期仅500 s)[14]。所以,对于非平衡凝固的Al-Sc合金,在铸态下,绝大部分Sc都是过饱和地固溶在α(Al)基体中,仅有微量的Sc以中间化合物初生Al3Sc的形式存在;在随后的均匀化退火过程中,过饱和固溶体中的绝大部分Sc又以二次Al3Sc相的形式析出,只有极少量的Sc留在α(Al)固溶体中。
当向α(Al)中单独添加Sc时,由于Sc在铝合金的溶液和固溶体中的溶解度相当(平衡分配系数k=cS/cL≈1),Sc在α(Al)基体中分布均匀,微观偏聚小。由于Sc原子在α(Al)中扩散速率快,在铸锭均匀化退火过程中,过饱和固溶体分解,迅速生成与α(Al)基体共格的二次Al3Sc粒子在α(Al)基体中分布均匀、弥散。Al3Sc与基体铝有极小的界面能和共格应变能以及比容应变能和相变形核势垒很小,有利于析出相大量弥散均质形核。Sc在铝合金中快的扩散速率使得Al3Sc粒子粗化速度相对较快,Al3Sc粒子热稳定性受到限 制[15?18]。
从Al-8.0Zn-2.0Cu-0.3Sc-0.3Zr-0~8.0Mg六元变温截面图[19]可知,在铝合金中当其他合金元素溶入α(Al)基体后,Sc在铝中的最大固溶度降低。Al-8.90Zn- 2.42Mg-2.45Cu-0.20Sc-0.16Zr合金中含有Zn、Mg、Cu、Zr元素使得Sc在合金中的最大固溶度降低,在合金凝固过程中析出初生Al3(Sc,Zr)粒子,同时,Sc在铸态的α(Al)基体中处于过饱和状态,因此,在随后的均匀化以及热加工过程中有二次Al3(Sc,Zr)粒子析出。图1、2、4和5表明,合金在450 ℃退火,保温不同时间后均析出二次Al3(Sc,Zr)粒子。
Sc与空位的结合能为0.35 eV[16],高于Zr与空位的结合能,所以,Sc在α(Al)基体中的扩散速率远大于Zr在α(Al)基体中的扩散速率,在二次Al3(Sc,Zr)粒子形核过程中首先消耗基体中的Sc。Sc在铝合金基体中分布均匀,因此,二次Al3(Sc,Zr)粒子的形核也是均匀分布的。文献[14]报道,在铝合金中二次Al3(Sc,Zr)粒子的孕育期与二次Al3Sc的形核孕育期基本相同,可以认为二次Al3(Sc,Zr)粒子的形核速率与二次Al3Sc粒子的形核速率基本一致。由于析出粒子内Sc与Zr原子之间几乎不发生相互扩散,粒子内部富Sc外部富锆[20],这也表明二次Al3(Sc,Zr)粒子的形核实际与二次Al3Sc粒子的形核一样,或者可以把二次Al3(Sc,Zr)粒子的形核看作只有Al和Sc 2种元素析出过程中的形核[21]。
在粒子长大的过程中,常常是粒径大于临界粒径的粒子通过元素的扩散长大,而粒径较小的粒子会在保温时间延长的过程中消失,重新溶入基体中。析出粒子外层富锆,由于锆在铝基体中的扩散比钪慢得多,因而,Al3(Sc,Zr)粒子的长大速率比Al3Sc慢得多[22],具有比Al3Sc相更高的热稳定性。另外,α(Al)、富Sc的Al3(Sc,Zr)、富Zr的Al3(Sc,Zr)层之间的界面能存在如下关系[23]:
γ1, 3>γ1, 2+γ2, 3 (1)
式中:γ为单位表面积的界面能;下标1、2和3分别表示α(Al)基体、富Zr的Al3(Sc,Zr)层和富Sc的Al3(Sc,Zr)层。Al3(Sc,Zr)粒子的长大受阻有利于析出粒子的细化。经均匀化处理后可以得到细小弥散的二相粒子。
4 结论
1) 铸态Al-8.90Zn-2.42Mg-2.45Cu-0.20Sc-0.16Zr合金在450 ℃退火2 h后,α(Al)基体内析出呈豆瓣状的二次Al3(Sc,Zr)粒子,粒径约为18 nm。
2) 在450 ℃退火32 h后,Al3(Sc,Zr)粒子尺寸为16~23 nm,粒子粗化速度慢。
3) 在透射电镜下观察到的二次Al3(Sc,Zr)析出相的微观结构表明,在450 ℃退火32 h后的二次Al3(Sc,Zr)相与α(Al)基体完全共格。
[1] MILMAN Y V, SIRKO A I, LOTSKO D V, SENKOY O N, MIRACLE D B. Microstructure and mechanical properties of cast and wrought Al-Zn-Mg-Cu alloys modified with Zr and Sc[J]. Materials Science Forum, 2002, 396/402: 1127?1132.
[2] 戴晓元, 夏长清, 马 科, 刘 娟. Sc对Al-Zn-Mg-Cu-Zr合金铸态组织和力学性能的影响[J]. 中国有色金属学报, 2007, 17(8): 1324?1329.
DAI Xiao-yuan, XIA Chang-qing, MA Ke, LIU Juan. Effect of Sc on as-cast microstructures and mechanical properties of Al-Zn-Mg-Cu-Zr alloys[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(8): 1324?1329.
[3] SENKOY O N, MIRACLE D B, MILMAN Y V, SCOTT J M, LOTSKO D V, SIRKO A I. Low temperature mechanical properties of scandium-modified Al-Zn-Mg-Cu alloys[J]. Materials Science Forum, 2002, 396/402: 664?670.
[4] MORMAN A F, HYDE K, COSTELLO F, THOMPSON S, BIRLEY S, PRANGNELL P B. Examination of the effect of Sc on 2000 and 7000 series aluminum alloy castings: For improvements in fusion welding[J]. Mater Sci Eng A, 2003, 354: 188?198.
[5] YIN Zhi-min, JIANG Feng, PAN Qing-lin, GUO Fei-yue, ZHU Da-peng, SONG Lian-peng, ZENG Yu, WANG Tao. Microstructures and mechanical properties of Al-Mg and Al-Zn-Mg based alloys containing minor scandium and zirconium[J]. Trans Nonferrous Met Soc China, 2003, 13(3): 515?520.
[6] 李 海, 杨迎新, 郑子樵, 王芝秀. 少量Sc对7055铝合金组织与性能的影响[J]. 材料科学与工艺, 2006, 14(1): 46?49.
LI Hai, YANG Ying-xin, ZHENG Zi-qiao, WANG Zhi-xiu. Effect of minor addition of scandium on microstructures and mechanical properties of 7055 aluminum alloy[J]. Materials Science and Technology, 2006, 14(1): 46?49.
[7] IWAMURA S, MIURA Y. Loss in coherency and coarsening behavior of Al3Sc precipitates[J]. Acta Materialia, 2004, 52: 591?600.
[8] GABRIEL M, NOVOTNY, ALAN J, ARDEL L. Precipitation of Al3Sc in binary Al-Sc alloys[J]. Mater Sci Eng A, 2001, 318: 144?154.
[9] CHEN Zhi-guo, RINGER S P, ZHENG Zi-qiao, ZHONG Jue. Microstructural evolution in Al-Sc and Al-Sc-Zr alloys[J]. Materials Science Forum, 2007, 546/549: 629?632.
[10] NAGA R P, SRINIVASA R K, REDDY G M, KAMARAJ M, PRASAD R K. Microstructure and high temperature stability of age hardenable AA2219 aluminium alloy modified by Sc, Mg and Zr additions[J]. Mater Sci Eng A, 2007, 464: 192?201.
[11] FORBORD B, AURAN L, LEFEBVRE W, HALLEM H, MARTHINSEN K. Rapid precipitation of dispersoids during extrusion of an Al–0.91wt.% Mn–0.13wt.% Zr–0.17wt.% Sc-alloy[J]. Mater Sci Eng A, 2006, 424: 174?180.
[12] LIU Zhong-xia, LI Zi-jiong, WANG Ming-xing, WENG Yong-gang. Effect of complex alloying of Sc, Zr and Ti on the microstructure and mechanical properties of Al-5Mg alloys[J]. Mater Sci Eng A, 2008, 483/484: 120?122.
[13] HUME-ROTHERY W, RAYNOR G V. The structure of metals and alloys[M]. London: The Institute of Metals, 1954: 197?203.
[14] DAVYDOV V G, ROSTOVA T D, ZAKHAROV V V, FILATOV YU A, YELAGIN V I. Scientific principles of making an alloying addition of scandium to aluminium alloys[J]. Mater Sci Eng A, 2000, 280: 30?36.
[15] HARADA Y, DUNAND D C. Thermal expansion of Al3Sc and Al3(Sc0.75X0.25)[J]. Scripta Materialia, 2003, 48: 219?222.
[16] MIURA Y, JOH C H, KATSUBE T. Determination of vacancy-Sc interaction energy by electrical resistivity measurements[J]. Materials Science Forum, 2000, 331/337: 1031?1036.
[17] ROYSET J, RYUM N. Kinetics and mechanisms of precipitation in an Al-0.2wt.% Sc alloy[J]. Mater Sci Eng A, 2005, 396: 409?422.
[18] FORBORD B, HALLEM H, ROYSET J, MARTHINSEN K. Thermal stability of Al3(Scx, Zr1?x)-dispersoids in extruded aluminium alloys[J]. Mater Sci Eng A, 2008, 475: 241–248.
[19] ROKHLIN L L, DOBATKINA T V, BOCHVAR N R, LYSOVA E V. Investigation of phase equilibria in alloys of the Al-Zn-Mg-Cu-Zr-Sc system[J]. Journal of Alloys and Compounds, 2004, 367: 10?16.
[20] DESCHAMPS A, LAE L, GUYOT P. In situ small-angle scattering study of the precipitation kinetics in an Al-Sc-Zr alloy[J]. Acta Materialia, 2007, 55: 2775?2783.
[21] FORBORD B, LEFEBVE W, DANOIX F, HALLEM H, MARTHINSEN K. Three dimensional atom probe investigation on the formation of Al3(Sc, Zr)-dispersoids in aluminium alloys[J]. Scripta Materialia, 2004, 51: 333?337.
[22] CLOUET E, LAE L, EPLAIER T, LEFEBVRE W, NASTAR M, DESCAAMPS A. Complex precipitation pathways in multicomponent alloys[J]. Nature Materials, 2006, 5: 482?488.
[23] TOYLLEY A, RADMILOVIC V, DAHMEN U. Segregation in Al3(Sc, Zr) precipitates in Al-Sc-Zr alloys[J]. Scripta Materialia, 2005, 52: 621?625.
基金项目:湖南省教育厅资助项目(08C093);国家重点基础研究发展规划资助项目(2005CB623706)
收稿日期:2009-03-06;修订日期:2009-12-29
通信作者:戴晓元,副教授,博士;电话:0731-85258644;E-mail: dxy0124@126.com
摘 要:采用透射电子显微镜,研究含钪Al-Zn-Mg-Cu-Zr系铸态合金在退火过程中二次Al3(Sc,Zr)粒子的析出形貌、尺寸及分布。结果表明:含0.20%Sc的7系铝合金铸态试样在450 ℃退火2 h后,α(Al)基体内析出呈豆瓣状的二次Al3(Sc,Zr)粒子;在450 ℃退火32 h后,Al3(Sc,Zr)粒子尺寸为16~23 nm;在450 ℃退火32 h后的二次Al3(Sc,Zr)相与α(Al)基体完全共格。


