
Mechanism of electro-generative-leaching of chalcopyrite-MnO2 in presence of Acidithiobacillus ferrooxidans
XIAO Li(肖 利)1, 2, 3, FANG Zheng(方 正)1, QIU Guan-zhou(邱冠周)2,
WANG Shao-fen(王少芬)3, WANG Chun-xiong(王春雄)4
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China;
3. College of Chemistry and Biological Engineering, Changsha University of Science and Technology,Changsha 410004, China;
4. College of Metallurgic Engineering, Hunan University of Technology, Zhuzhou 412000, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract:
To clarify the role and mechanism of Acidithiobacillus ferrooxidans (A. ferrooxidans) in bio-electro-generative-leaching (BEGL), an experiment was made on the electro-generative leaching of chalcopyrite-MnO2 in the presence of the bacteria which grew respectively in Fe(Ⅱ) and S0 media. A dual cell system with chalcopyrite anode and MnO2 cathode was used to study the relationship between time and both of electric quantity and dissolved rate of the two minerals in BEGL. The results show that the dissolved rates for Cu2+ and Fe2+ under the action of the bacteria cultivated by S0 medium are almost 2 times faster than those by Fe(Ⅱ). And the leaching ratio for Mn2+ and the electric output increase by near 3 times. The oxidation residue of chalcopyrite was characterized by SEM and XRD, whose patterns are similar to those of raw ore in BEGL. The mechanism of anodic reaction for CuFeS2-MnO2 leaching in the presence of A. ferrooxidans cultivated by S0 medium is proposed as a successive reaction of two independent sub-processes. The first stage is the dissolution of chalcopyrite to produce Cu2+, Fe2+ and sulfur, and the second stage is bio-oxidation of sulfur, which is the control step of the process. However, dissolution of MnO2 lasts until the reaction of chalcopyrite stops or the ores exhaust in two types of leaching.
Key words:
chalcopyrite; MnO2; Acidithiobacillus ferrooxidans; bio-oxidation; electro-generative-leaching;
____________________________________________________________________________________________
1 IntroductionThere are some reports on hydrometallurgical treatment of chalcopyrite minerals[1-4], including EGL of CuFeS2-MnO2[5-6]. In this process, the Gibbs free energy could transform to applicable electrical work, simultaneously, the leaching products were acquired. The technique can not only precipitate element sulfur, but also simplify the purification for leached solution[5-7]. WANG and FANG[6] used the technology for the simultaneous leaching of chalcopyrite concentrate and MnO2. XIAO et al[5] pointed out that the accumulated sulfur covering on the surface of leached sulfides inhibits the anodic reaction going on and drops the output of electric energy in EGL greatly. As a result, it is necessary to develop a proper technique that can eliminate the accumulated sulfur on the surface of sulfides.
Acidithiobacillus ferrooxidans (A. ferrooxidans) plays an important role in the aspect of the biochemical oxidation of sulfur and ferrous ions into ferric ones[8-11]. Under the normal aerobic condition, the bacteria can utilize ions in solution as an energetic substance or obtain energy via direct oxidation of the sulfur. However, energetic metabolism of A. ferrooxidans existing in Fe(Ⅱ) medium is different from that in S0 medium. The bacteria adapted with Fe(Ⅱ) and S0 were more easy to oxidize respectively ferrous and sulfur[11-12], although, theoretically, sulfur could also be oxidized by A. ferrooxidans grown in Fe(Ⅱ) medium, provided that time is long enough (over 7 d)[11].
If A. ferrooxidans grown in S0 are added into anodic area of the electro-generative leaching cell of sulfide, the produced sulfur will be oxidized into sulfuric acid and the leaching resistance will drop largely. Similarly, A.ferrooxidans grown in Fe(Ⅱ) will be more easy to oxidize ferrous ions into ferric ones, and the leaching of iron in ores will be accelerated. TAKAMI et al[13] presented that the leaching of ZnS-MnO2 in the presence of A. ferrooxidans involved four processes: dissolution of zinc sulfide by ferric ions, bacterial oxidation of ferrous ions, and bacterial oxidation of elemental sulfur and zinc sulfide.
To clarify the role and mechanism of A. ferrooxidans in bio-electro-generative simultaneous leaching, a comparative experiment was made on the simultaneously electro-generative leaching of chalcopyrite in the presence of bacteria cultivated, respectively by Fe(Ⅱ) and S0 media (for convenience, we called the experiments as [BEGL-Fe(Ⅱ)] and [BEGL-S0], respectively). Hydrometallurgical treatment of sphalerite minerals was reported in Refs.[1-4]. It was found that the rate of dissolution of sphalerite was proportional to the iron content in the process of ferric ion leaching of the minerals[2], and that sphalerite leaching was controlled by diffusion through a layer of sulfur formed on the surface[3].
2 Experimental
2.1 Minerals
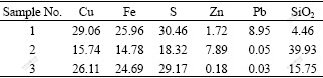
Natural hand-sorted chalcopyrite samples with 75 μm particle size were from domestic mines (Jinyan, Guangdong and Yuncheng, Shanxi, China), which were named as samples 1 and 2, respectively. The chalcopyrite concentrate was named as sample 3. The XRD analysis shows that CuFeS2 is predominant in samples, and PbS and SiO2 coexist. The results of element analysis are listed in Table 1. MnO2 was commercial reagent (Shanghai Chemical Regents Industries, Ltd., China).
Table 1 Chemical compositions of samples 1, 2, and 3 (mass fraction, %)
2.2 Set and electrode for BEGL
The cell frame placed in water bath at 303 K was made of PVC, which was divided into anolyte and catholyte compartments, each of 200 mL with 50 mL solution, connected by an anion membrane, which allowed anions to migrate freely, and blocked up cation[5, 12, 14].
Two electrodes were made of chalcopyrite and MnO2 of 2.0 g powder. Air-blowing tubes were separately inserted into anolyte and catholyte rooms to agitate and supply oxygen for bacteria. The pH value, electrode potentials (vs SCE) and output voltage of the cell were measured with a PHS-3C digital acidometer, and the output current was measured with an amperemeter and the concentration of oxygen with a Degussa oxygen meter. All of the measured instruments were calibrated before each run. The leaching time in both processes further lasted until the output current was 3-5 mA.
2.3 Solutions and bacteria
The solutions were prepared using AR reagents and distilled water. The bacteria culture medium consisted of (NH4)2SO4 3.0 g/L, KCl 0.1 g/L, K2HPO4 0.5 g/L, MgSO4?7H2SO4 0.5 g/L and Ca(NO3)2 0.01 g/L.
A pure strain of A. ferrooxidans was from Yunnan Province of China. Liquid cultures of S0 or Fe(Ⅱ) were carefully treated with the respective basal salt solution before inoculating a cell reaction.
The culture medium was acidified first to pH 1.8 by H2SO4, and the exponential growth phase bacteria, which grew in S0 and Fe(Ⅱ), were as anolytes of [BEGL-S0] and [BEGL-Fe(Ⅱ)], respectively. Oxygen in the solution was 5.9 mg/L. Normal losses due to evaporation were periodically compensated by the addition of distilled water.
3 Results and discussion3.1 Anodic and cathodic polarizability in [BEGL-S0] and [BEGL-Fe(Ⅱ)]
The Evans diagram of anodic and cathodic polarization, and interior resistance of the cell were used to study the control factor in BEGL process. Generally, the bigger the value of resistance, the greater the effect on the leaching reaction[5]. Fig.1 shows the Evans diagram of [BEGL-S0] and [BEGL-Fe(Ⅱ)] for sample 1.
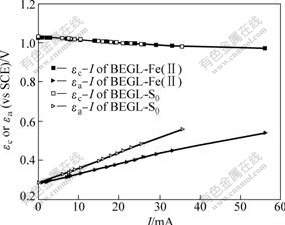
Fig.1 Evans diagram of [BEGL-S0] and [BEGL-Fe(Ⅱ)] for sample 1
As shown in Fig.1, the current increases with the over-potentials of cathode ηc and anode ηa[14]. Define polarization as:
![]() (1)
(1)
where η is over-potential, and I is galvanic current.
The polarizations of anode and cathode, Pa and Pc, are:
![]() (2)
(2)
![]() (3)
(3)
![]() (4)
(4)
![]() (5)
(5)
where R is interior resistance. Pa, Pc, R and J (density of current) are listed in Table 2. The over-potentials of anode and cathode increase with the decrease in galvanic voltage in [BEGL-S0] and [BEGL-Fe(Ⅱ)] processes.
Table 2 Pc, Pa, R and J of BEGL for sample 1
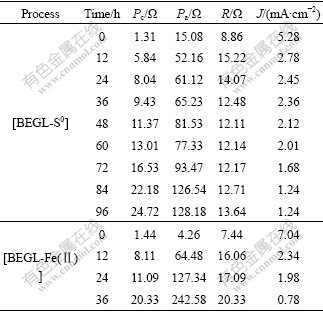
The increase of anodic potential with the decrease of output potential and cathodic potential can be observed in [BEGL-S0] and [BEGL-Fe(Ⅱ)] processes. It is seen from Table 2 that Pa and R are small at the first time due to the electric catalytic activity of acetylene black[14] mixed in both electrodes. Another important feature of polarization reflects the data of different stages (see columns 3 and 4 in Table 2). The increase in Pa in [BEGL-Fe(Ⅱ)] is faster than that in [BEGL-S0] with the decrease in J. This can be attributed to the fact that elemental sulfur accumulated in the anode hinders the reaction in both processes. Similarly, Pc comes to steady-value of about 20-25 Ω with the decrease in the galvanic current in [BEGL-Fe(Ⅱ)] as the reactions continue. The decrease in the solution acidity in both processes might be the reason for the increase in Pc.
3.2 Relations between electric quantity and time in both processes in the first stage
Each run started under open-circuit, and the measure of electric output was under closed-circuit with 8 Ω load, which was corresponding to the maximum power output[5]. As shown in Fig.2, the measured electric quantity (MEQ) is almost linearly related to time in [BEGL-S0] and [BEGL-Fe(Ⅱ)] for sample 1. The fitted equations and correlation coefficient (r) are in Table 3.
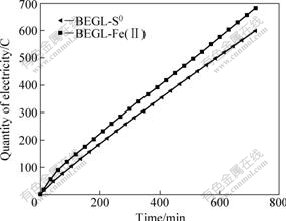
Fig.2 Relations between MEQ and time for 12 h in BEGL-S0 and BEGL-Fe(Ⅱ) for sample 1
Table 3 Fitted equations and correlation coefficient r of sample 1 in [BEGL-S0] and [BEGL-Fe(Ⅱ)] in the first stage

From the slopes of straight lines it can be seen that A. ferrooxidans accelerates the oxidation of ores in 12 h, resulting in an increase of MEQ in [BEGL-Fe(Ⅱ)] for sample 1. But, the advantage in 12 h is unremarkable.
3.3 Relations between electric quantity and dissolved rate of metal ion and time in both processes in second stage
Theoretically, 1 mol CuFeS2 produces 1 mol Cu2+, 1 mol Fe2+ and 2 mol S0 in disorganization. Accordingly, it is reasonable to consider that the total generated amount of element sulfur ![]() can be determined by the dissolved
can be determined by the dissolved ![]() and
and ![]() . Assume that the generated
. Assume that the generated
current is from S2- to S0, which is taken as the theoretic electric quantity (TEQ) and can be calculated based on
![]() ,
, ![]() and Faraday’s law. MEQ can be obtained
and Faraday’s law. MEQ can be obtained
by measured current and time. Both TEQ and MEQ in BEGL should be equal. However, MEQ in [BEGL-S0] for 96 h is about 1 200 C larger than that in TEQ for sample1. This means that the part of S0 in [BEGL-S0] forms the sulfate group, which is called as biologic electric quantity (BEQ). The ratio of BEQ to MEQ (RBTM) can be used to predict the progress of [BEGL-S0].
Table 4 lists the dissolved rates of Cu2+, Fe2+ and Mn2+, TEQ and MEQ in both processes for 12 h, and RBTM in [BEGL-S0] of sample 1.
Table 4 Dissolved rates of valuable metals, TEQ and MEQ in both processes in the second stage for 12 h, and RBTM in [BEGL-S0] of sample 1
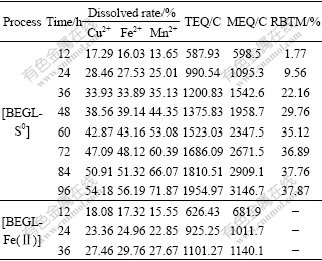
As observed from Table 4, very small difference between TEQ and MEQ in [BEGL-Fe(Ⅱ)] indicates that the anodic dissolution of chalcopyrite accords with the theoretical analysis, and the reaction is as follows:
CuFeS2(s)→Cu2+(aq)+Fe2+(aq)+2S(s) (6)
As well known, A. ferrooxidans grown in Fe(Ⅱ) can be more easy to oxidize Fe(Ⅱ) to Fe(Ⅲ) in anolyte in a few hours [11], and Fe(Ⅲ) enhances the dissociation of CuFeS2[3]. As a result, more sulfur covering on the surface of leached sulfides inhibits the anodic reaction going on and therefore, reducing the output of electric energy.
It can also be seen from Table 4 that the dissolved rates for Cu2+ and Fe2+ in [BEGL-S0] are almost 2 times faster than those in [BEGL-Fe(Ⅱ)]. And the leaching ratio for Mn2+ and the electric output increase by nearly 3 times. After 12 h of [BEGL-S0], RBTM is 1.77%, showing that oxidation of sulfur on the surface of chalcopyrite initiates. Subsequently, the increase in the ratio is up to about 37.87% as time lasts for 96 h, indicating that part of S0 is continuously discharged to form sulfate group.
The dissolved rate of Mn2+ is 27.67% in [BEGL-Fe(Ⅱ)], whereas up to 71.87% in [BEGL-S0]. Therefore, dissolution of MnO2 in this system is dependent on behavior of chalcopyrite, and progress is along until the reaction on the ores stops.
The SEM images of the oxidation debris in [BEGL-Fe(Ⅱ)] and [BEGL-S0] are shown in Fig.3. It can be seen that a large quantity of sulfur floccules form on the surface of ores after [BEGL-Fe(Ⅱ)] and the clear surface of ores after [BEGL-S0]. The results of the element analysis by AAS are listed in Table 5. It can also be seen that with the increase of element sulfur in the oxidation debris, metallic elements decrease. Compared with [BEGL-Fe(Ⅱ)], the decrease of element S with the increase of Cu and Fe in [BEGL-S0] indicates that the produced sulfur is partly oxidized in the process.
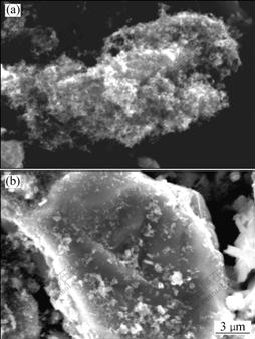
Fig.3 SEM images of oxidation debris of sample 1: (a) Treated by [BEGL-Fe(Ⅱ)]; (b) Treated by [BEGL-S0]
Table 5 Main elements in sample 1 before and after leaching (mole fraction, %)
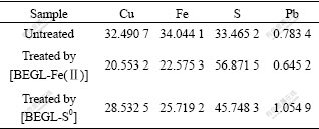
Fig.4 shows the XRD patterns of untreated and treated chalcopyrite. The phases untreated and treated by [BEGL-Fe(Ⅱ)] are similar, and impurity PbS exists in residue. However, PbS in [BEGL-S0] is oxidized into insoluble PbSO4, covering the surface of unreacted ores. The XRD pattern of leached residue still has the peak of chalcopyrite. Accordingly, chalcopyrite in oxidation debris contains less insoluble impurity to make the dissolved rate of valuable metals increase much.
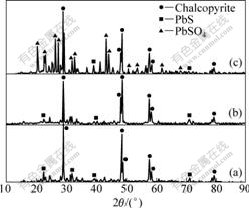
Fig.4 XRD patterns of different samples: (a) Untreated sample; (b) Treated by [BEGL-Fe(Ⅱ)]; (c) Treated by [BEGL-S0]
The behavior of chalcopyrite in [BEGL-S0] in 12 h is similar to that in [BEGL-Fe(Ⅱ)] regarding dissolved rate of Cu2+, Fe2+ and S, and RBTM for the former is lower. The XRD pattern and SEM image of the residue for long-time leaching indicate that sulfur is partly oxidized in [BEGL-S0]. Accordingly, the reaction mechanism in [BEGL-S0] can be proposed as a successive reaction of two independent sub-processes. The first is that chalcopyrite dissolves and Cu2+, Fe2+ and elemental sulfur produce, as shown by reaction (6), and the second is elemental sulfur is subsequently oxidized to sulfate group by oxygen via promotion of A. ferrooxidans according to reaction (7):
S(s)+H2O(l)+O2(g)→SO42-(aq)+H+(aq) (7)
The rate of reaction (6) is faster than that of reaction (7) in [BEGL-S0] based on RBTM. In the first 12 h in [BEGL-S0], the ratio is only 1.77%, showing that discharge is principally based on reaction (6). As the ratio is up to about 40%, the oxidation of sulfur is still not complete, and becomes leaching resistance except insoluble PbSO4. The second stage is the control step of [BEGL-S0], because the oxidation by a promotion of bacteria is generally very slow.
3.4 Effect of impurity in chalcopyrite on bio-electro- generative leaching
Sample 2 contained 39.93% SiO2, and sample 3 contained 15.75% SiO2. They were used to study the effect of SiO2 in BEGL. The fitted equations and correlation coefficients of samples 2 and 3 in the first stage in both processes are shown in Table 6. Tables 7 and 8 show the dissolved rates of valuable metals, TEQ and MEQ in both processes in the second stage for 12 h, and RBTM in [BEGL-S0] for samples 2 and 3, respectively.
Table 6 Fitted equations and correlation coefficient r of samples 2 and 3 in [BEGL-S0] and [BEGL-Fe(Ⅱ)] in the first stage
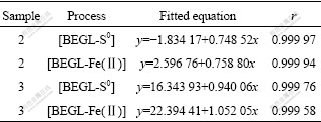
Table 7 Dissolved rates of valuable metals, TEQ and MEQ in both processes in second stage for 12 h, and RBTM in [BEGL-S0] for sample 2
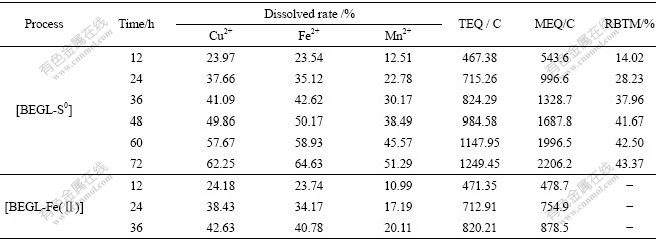
Table 8 Dissolved rates of valuable metals, TEQ and MEQ in both processes for 12 h, and RBTM in [BEGL-S0] for sample 3

It can be seen from Tables 6-8 that the fitted equations, dissolved rates of valuable metals, TEQ, MEQ in both processes and RBTM in [BEGL-S0] for samples 2 and 3 are similar to those of sample 1. This may be attributed to the insulation SiO2 in anodic powder, which has little effect on dissociation reaction on the surface of chalcopyrite[9, 15]. But impurity PbS in sample 1 is oxidized to insoluble PbSO4 in [BEGL-S0] on the surface of chalcopyrite, resulting in lower dissolved rate of valuable metals.
For the MEQ and dissolved rate of Mn2+ among three samples, sample 2 is the lowest because CuFeS2 for discharge reaction in the sample is the least. The RBTM of 43.37% in 72 h for sample 2 is the highest due to chalcopyrite containing more SiO2, which is optimum for the attachment of bacteria, accelerating the oxidation of S0 by oxygen via promotion of A. ferrooxidans[9, 15-16].
4 Conclusions1) The simultaneous electro-generative leaching of CuFeS2 and MnO2 in the presence of A. ferrooxidans is studied.
2) The difference between [BEGL-S0] and [BEGL-Fe(Ⅱ)] for leaching of chalcopyrite is studied by a comparison of electric quantity and dissolved metal ion rate. The results show that the SEM images and XRD patterns of oxidized residue of chalcopyrite in [BEGL-Fe(Ⅱ)] are similar to those of the raw ore, and a large quantity of sulfur floccules deposit on surface of ores. While in [BEGL-S0], the intermediate product sulfur is partly oxidized in the presence of A. ferrooxidans.
3) The mechanism is proposed for leaching of CuFeS2-MnO2 in the presence of the bacteria as a successive reaction of two independent sub-processes for anode. The first stage, common to both processes, is dissolution of chalcopyrite on the surface to produce Cu2+, Fe2+ and sulfur. The second stage is subsequent oxidation of sulfur in [BEGL-S0], which is control step of the process. However, dissolution of MnO2 lasts until the dissolution reaction of chalcopyrite stops or the ores exhaust. Chalcopyrite containing more SiO2, which is optimum for attachment of bacteria, accelerates the oxidation of S0 by oxygen via promotion of A. ferrooxidans in [BEGL-S0].
References[1] RODR?GUEZ Y, BALLESTER A, BL?ZQUEZ M L, GONZ?LEZ F, MU?OZ J A. New information on the chalcopyrite bioleaching mechanism at low and high temperature [J]. Hydrometallurgy, 2003, 71(1): 47-56.
[2] ZHOU H B, ZENG W M, YANG Z F. Bioleaching of chalcopyrite concentrate by a moderately thermophilic culture in a stirred tank reactor [J]. Bioresource Technology, 2009, 100(2): 515-520.
[3] C?RDOBA E M, MU?OZ J A, BL?ZQUEZ M L. Leaching of chalcopyrite with ferric ion. Part I: General aspects [J]. Hydrometallurgy, 2008, 93(1): 81-87.
[4] QIU M Q, XIONG S Y, ZHANG W M. Efficacy of chalcopyrite bioleaching using a pure and a mixed bacterium [J]. Mineral, 2006, 13(1): 7-10.
[5] XIAO L, LIU J S, FANG Z, QIU G Z. Factors affecting output power in electro-generative leaching system of chalcopyrite [J]. The Chinese Journal of Process Engineering, 2006, 6(4): 576-579. (in Chinese)
[6] WANG S F, FANG Z. Simultaneous electrogenerative leaching of chalcopyrite concentrate and MnO2 [J]. J Cent South Univ Technol, 2006, 13(1): 49-52.
[7] FANG Z, ZHANG Q R. Thermoelectrochemistry and its application to metallurgical research [J]. J Mater Sci Technol, 2001, 17(Suppl 1): 20-24.
[8] SILVEMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields [J]. Journal of Bacteriology, 1959, 77(3): 642-647.
[9] XIA L X, LIU X X, ZENG J. Mechanism of enhanced bioleaching efficiency of Acidithiobacillus ferrooxidans after adaptation with chalcopyrite [J]. Hydrometallurgy, 2008, 92(1): 95-101.
[10] SHARMA P K, DAS B A, HANUMANTHA R K, FORSSBERG K S E. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions [J]. Hydrometallurgy, 2003, 71(1): 285-292.
[11] ANDRE? S Y B, CORINNE A A, JEANINE R, VIOLAINE B. Regulation of the expression of the Acidithiobacillus ferrooxidans rus operon encoding two cytochromes c, a cytochrome oxidase and rusticyanin [J]. Microbiology, 2004, 150(10): 2113-2123.
[12] HECTOR O, VER?NICA M. Iron homestasis strategies in acidophilic iron oxidizers: Studies on Acidithiobacillus and Leptospirillum [J]. Hydrometallurgy, 2008, 94(1): 175-179.
[13] TAKAMI K, SUENAGA Y I, MIGITA A, TAKAHASHI T. Kinetic model for simultaneous leaching of zinc sulfide and manganese dioxide in the presence of iron-oxidizing bacteria [J]. Chem Eng Sci, 2000, 55(17): 3429-3436.
[14] XIAO L, FANG Z, QIU G Z, LIU J S. Electro-generative mechanism for simultaneous leaching of pyrite and MnO2 in presence of A. ferrooxidans [J]. Trans Nonferrous Met Soc China, 2007, 17(6): 1373-1378.
[15] RODR?GUEZ Y, BALLESTER A, BL?ZQUEZ M L. Study of bacterial attachment during the bioleaching of pyrite, chalcopyrite, and sphalerite [J]. Geomicrobiology Journal, 2003, 20(1): 131-141.
[16] SHARMA P K, HANUMANTHA R K. Surface characterization of bacterial cells relevant to the mineral industry [J]. Minerals and Metallurgical Processing, 2005, 22(1): 31-37.
_______________________________
Foundation item: Project(50874119) supported by the National Natural Science Foundation of China; Project supported by the Post-doctoral Program of Central South University, China
Corresponding author: XIAO Li; Tel: +86-13637338663; E-mail: xiaoli_csu@163.com


