文章编号:1004-0609(2013)08-2290-06
德兴铜矿辉钼矿精矿的选择性电氧化浸出与分离过程
曹占芳1, 2, 3,钟 宏1, 3,姜 涛2,刘广义1, 3,王 帅1, 3
(1. 中南大学 化学化工学院,长沙 410083;
2. 中南大学 资源加工与生物工程学院,长沙 410083;
3. 中南大学 有色资源化学教育部重点实验室,长沙 410083)
摘 要:
针对德兴铜矿高铜钼精矿特性,研究开发一种选择性电氧化浸出新工艺。结果表明,辉钼矿的电氧化浸出受电解液pH值的影响显著。采用Na2CO3-NH4HCO3缓冲溶液控制电解过程pH为9左右,可以实现辉钼矿的选择性电氧化高效浸出,钼、铼的浸出率分别为99.35%和99.79%,与非缓冲体系的电氧化过程相比,钼、铼的浸出率分别提高了23.86%和26.50%。在选择性电氧化过程中,黄铜矿基本上不能浸出而留在浸出渣中,电解渣中铜品位达到10.84%,回收率达到97.93%,可作为铜冶炼原料而回收。采用N235为萃取剂,对电氧化浸出液中的钼、铼进行溶剂萃取,将溶液中HCl浓度调整为25.48 g/L时,可实现钼、铼的同时萃取,其萃取率分别为99.84%和95.19%;用17%的氨水反萃负载有机相,有机相中钼、铼的反萃率分别为99.89%和99.54%。将反萃液pH调整为8,30℃下用D201树脂吸附1 h,钼、铼的吸附率分别为3.46%和92.18%,分离因子为169.56。
关键词:
中图分类号:TF111 文献标志码:A
Selective electric-oxidation leaching and separation of Dexing molybdenite concentrates
CAO Zhan-fang1, 2, 3, ZHONG Hong1, 3, JIANG Tao2, LIU Guang-yi1, 3, WANG Shuai1, 3
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
3. Key Laboratory of Resources Chemistry of Nonferrous Metals, Central South University, Changsha 410083, China)
Abstract: A novel technique of selective electric-oxidation leaching, solvent extraction and ion exchange adsorption was studied for the recovery of Dexing molybdenite concentrates. The results show that the electric-oxidation leaching of molybdenite is impacted evidently by pH value of electrolyte. When the pH of electric-oxidation process was adjusted by buffered solutions of sodium carbonate and ammonium acid carbonate to about 9, MoS2 and ReS2 could be selectively oxidized, the dissolution rates of molybdenite and rhenium are 99.35% and 99.79%, respectively. Compared with that of the unbuffered system, the dissolution rates of molybdenite and rhenium increase by 23.86% and 26.50%, respectively. In the process of selective electric-oxidation leaching, chalcopyrite would not be leached, the grade of copper in the residue is 10.84% with a recovery rate of 97.93%, and it can be used as copper smelting materials. The separation of molybdenum and rhenium in the electrolyte was studied by solvent extraction and D201 resin adsorption. Coextraction of molybdenum and rhenium is found when N235 was used as an extractant and the concentration of HCl was adjusted to 25.48 g/L, the extraction efficiencies of Mo(Ⅵ) and Re(Ⅶ) are 99.84% and 95.19%, respectively. The stripping of molybdenum and rhenium to aqueous phase was investigated using 17% ammonia liquor, the stripping efficiencies of Mo(Ⅵ) and Re(Ⅶ) are about 99.89% and 99.54%, respectively. After stripping, rhenium was separated from molybdenum using D201 resin under pH 8 at 30 ℃, the adsorption rates of rhenium and molybdenum are 92.18%, 3.46%, respectively, and the separating factor is 169.56.
Key words: molybdenite; electric-oxidation; extraction; adsorption
现有焙烧—氨浸工艺是辉钼矿生产的主流工艺,但该工艺会产生大量的SO2气体,对环境污染严重,且生产过程中难以实现钼及伴生金属的高效经济回收[1-2]。从20世纪70年代起,钼湿法工艺研究倍受重视,相继发展了氧压煮法[3]、硝酸分解法[4]、次氯酸钠法[5]、电氧化法以及生物浸出法等[6-7]。湿法工艺过程不会产生任何烟气,且有利于综合回收多种有价元素[2]。
德兴铜矿特大型斑岩铜矿床伴生有丰富的低品位钼资源,原矿中铜含量0.42%(质量分数,下同),钼含量0.011%,钼金属工业储量达28万t。辉钼矿是该矿钼的唯一载体矿物,由于受铜钼分离效率的限制,所产钼精矿具有高铜高铼特点,铜含量一般为1%~3%,铼含量约为0.06%,其中铜主要以黄铜矿存在,铼主要以类质同像形式存在于辉钼矿中[7]。目前,采用氧化焙烧-氨浸工艺生产钼酸铵并回收铼酸铵,不仅会产生大量的SO2气体而污染环境,而且还会造成伴生铼元素的挥发损失,且铜作为杂质未能得到有效利 用[8-9]。针对德兴铜矿高铜钼精矿特性,研究开发了一种选择性电氧化浸出新工艺,通过电氧化浸出、萃取以及离子交换吸附分离,实现了高铜钼精矿中钼、铼、铜的高效分离与综合回收。电氧化工艺氧化分解辉钼矿是将辉钼矿悬浮于NaCl溶液中,在电氧化反应器中集氧化剂的生成和辉钼矿的氧化为一体,它能较大幅度地降低生产成本[6-9]。溶剂萃取法是一种利用物质在互不混溶的两相中的不同分配特性进行分离的方法,具有选择性好、生产量大、设备简单、易于实现连续化以及回收率高、成本低等特点,适合于辉钼矿浸出液中钼与杂质的分离,目前,萃取法已在钼冶金中得到广泛应用[10-15]。另外,根据金属离子与树脂离子化基团吸附能力的差异,树脂在湿法冶金领域多金属的吸附分离中应用也越来越广泛。
1 实验
实验所用矿样为江西德兴铜矿辉钼精矿(Mo 40.2%, S 26.8%, Cu 2.9%, Fe 5.0%, SiO2 4.35%,质量分数),其粒径分布在0~100 μm,粒径小于45.467 μm的占90%,体积平均粒径为20.273 μm。矿物XRD分析结果表明该矿石主要是辉钼矿,含有少量的MoO3和MoO2等,其结果如图1所示。实验所用其他试剂均为分析纯。
电氧化过程采用自制电解槽,选用形稳性阶极或尺寸稳定阳极(Dimensionally stable anode, DSA),铁阴极,电极按单极式方式固定在槽体内,极间距为10 mm,由增力电动搅拌器控制搅拌。
溶剂萃取及反萃实验是将有机相和金属离子溶液加入到125 mL带塞锥形瓶中,在水浴恒温振荡器中振荡一定时间后,移入分液漏斗中静置、分离,然后测定计算金属离子的萃取率和反萃率。
树脂吸附和洗脱实验是将树脂和金属离子溶液加入到125 mL带塞锥形瓶中,在水浴振荡器中振荡一定时间,过滤,然后测定计算金属离子的吸附率和洗脱率。
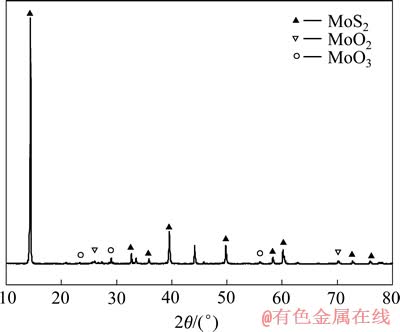
图1 钼精矿XRD谱
Fig. 1 XRD spectrum of molybdenite concentrate
2 结果与讨论
2.1 辉钼矿的选择性电氧化浸出
2.1.1 辉钼矿常规电氧化行为
在槽电压3.0 V、室温、电流密度800 A/m2、氯化钠浓度4.0 mol/L、矿浆液固比L/S 25,搅拌速度400 r/min的条件下,矿样中钼、铼和铜的浸出行为如图2所示。240 min时,钼、铼和铜的浸出率分别为75.49%、73.29%和69.41%;360 min后,则分别达到98.05%、97.64%和 96.81%。钼浸出的同时,铼和铜也一并进入了液相。
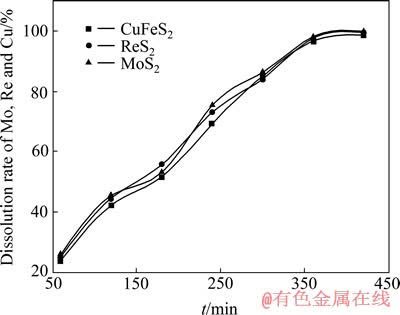
图2 矿样中钼、铼和铜的常规电氧化浸出行为
Fig. 2 Conventional electro-oxidation leaching behavior of molybdenum, rhenium and copper of molybdenite
2.1.2 pH对辉钼矿电氧化行为的影响
在槽电压3.0 V、室温、电流密度800 A/m2、氯化钠浓度4.0 mol/L、矿浆液固比L/S 25、搅拌速度400 r/min的条件下,采用滴加1 mol/L的HCl或NaOH溶液的方式控制矿浆pH,考察了pH对钼、铼和铜浸出行为的影响,结果如图3所示。从图3可以看出,pH对黄铜矿电氧化浸出的影响十分显著,酸性条件下,部分黄铜矿被氧化浸出,但在碱性条件下,黄铜矿基本不浸出。因此,在pH为7~10的范围内,能够实现辉钼矿的选择性浸出,而黄铜矿不浸出。在此条件下,不但可以节省了电能,而且有效的实现了钼、铜的分离。
浸出过程中可能发生的反应如下:
MoS2 +9NaClO+6NaOH=Na2MoO4+2Na2SO4+9NaCl+3H2O (1)
2CuFeS2+17NaClO+H2O=2CuSO4+2FeCl3+2Na2SO4+11NaCl+2NaOH (2)
从式(1)可以看出,碱性条件有利于辉钼矿分解反应向右进行,但当pH大于10时,辉钼矿浸出率明显降低,这是由于在强碱性条件下,OH-的浓度将远远大于Cl-的浓度,因此,OH-和Cl-在阳极竞争时被氧化,降低了Cl-被氧化生成NaClO的几率。同时,在强碱性条件下,ClO-的氧化性也将降低,从而降低了钼的浸出率,而式(2)则表明碱性条件将抑制黄铜矿的分解反应。
2.1.3 Na2CO3-NH4HCO3体系中辉钼矿电氧化行为
碳酸盐-酸式碳酸盐缓冲体系的pH在9左右,因此,在槽电压3.0 V、室温、电流密度800 A/m2、氯化钠浓度4.0 mol/L、矿浆液固比L/S 25、搅拌速度400 r/min,初始碳酸钠6 g/L+碳酸氢铵5 g/L,电氧化过程中加入一定量的碳酸根保持电解液pH稳定在8.5~9.5的条件下,考察了钼、铼和铜的浸出行为,结果如图4所示。从图4可以看出,240 min时,钼、铼的浸出率分别为99.35%和99.79%,与非缓冲体系的电氧化过程相比,钼、铼的浸出率分别提高了23.86%和26.50%。而电氧化过程中黄铜矿基本上不能浸出而留在浸出渣中,电解渣中铜含量达到10.84%,回收率达到97.93%,可直接作为铜冶炼原料而回收。钼、铼氧化浸出速率的加快以及铜在渣中的有效回收,不但能较大幅度地节省电能,同时也实现多金属的综合经济回收利用。
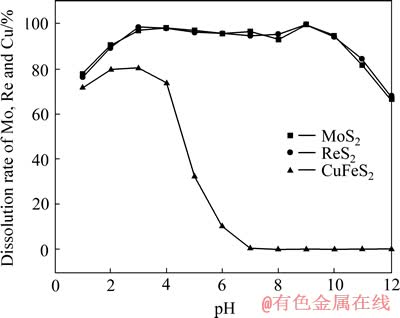
图3 pH对辉钼矿浸出行为的影响
Fig. 3 Influence of pH on electric-oxidation leaching behavior of molybdenite
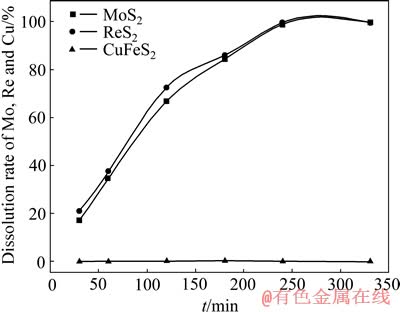
图4 Na2CO3-NH4HCO3体系中辉钼矿的浸出行为
Fig. 4 Leaching behavior of molybdenite in CO32--HCO3- buffer system
pH是影响辉钼矿电氧化浸出效率的重要因素,因此,缓冲体系的波动范围会对浸出过程产生很大的影响。确定电解温度为室温、矿浆液固比L/S 25、槽电压3.0 V、电流密度800 A/m2、搅拌速度400 r/min的条件下,分别在不同的Na2CO3-NH4HCO3缓冲体系波动范围内,考察240 min时钼的浸出效果,结果如表1所列。
表1 pH对浸出过程辉钼矿电氧化浸出率的影响
Table 1 Influence of pH on electric-oxidation leaching rate of molybdenite

由表1可知,Na2CO3-NH4HCO3缓冲体系的波动范围越大,钼的浸出效果越差,因此,控制缓冲体系在一定的范围内成为工艺操作的关键。实验中优化选用pH为8.5~9.5作为缓冲体系的控制范围。
实验中还考察了NaCl浓度、温度、搅拌速度、液固比等因素对Na2CO3-NH4HCO3缓冲体系中辉钼矿浸出的影响,确定的优化工艺条件如下:NaCl浓度4 mol/L,初始碳酸钠6 g/L+碳酸氢铵5 g/L,pH= 9,液固比25:1,室温,搅拌速度400 r/min。电氧化完成后浸出液中钼、铼含量分别如下:15.98 g/L Mo(Ⅵ), 0.020 g/L Re(Ⅶ)。
2.1.4 Na2CO3-NH4HCO3溶液中电氧化辉钼矿渣相XRD分析
在槽电压3.0 V、室温、电流密度800 A/m2、氯化钠浓度4.0 mol/L、矿浆液固比L/S 25、搅拌速度400 r/min,初始碳酸钠6 g/L+碳酸氢铵5 g/L,过程中加入一定量的碳酸根保持电解液pH稳定在8.5~9.5的条件下,电氧化5 g辉钼矿,240 min后对渣相进行XRD分析,结果如图5所示。结果表明,此时矿渣的主要成分是CuFeS2,还含有少量没有被氧化浸出的MoS2。这也进一步验证了黄铜矿不能被电氧化浸出的结论。
2.2 钼和铼的萃取及反萃
电氧化浸出过程中钼、铼高效浸出而进入液相,伴生金属铜则不能被氧化浸出而留在浸出渣中,实现了钼、铼与铜的分离。采用组成为30%N235+20%仲辛醇+50%(体积分数)煤油的有机相萃取回收钼、铼,分别考察了相比、酸度、温度等条件对Mo(Ⅵ)、Re(Ⅶ)萃取率的影响[10-11]。优化确定的萃取条件如下:室温,HCl浓度25.48 g/L,萃取时间5 min,相比为1:2时,Mo(Ⅵ)、Re(Ⅶ)单级萃取率分别达到99.84%和95.19%。以17%的氨水为反萃剂,在相比为2:1,室温,Mo(Ⅵ)、Re(Ⅶ)的反萃率分别达到99.89%和99.54%。对反萃产物进行了XRD分析,反萃产物中钼以α-(NH4)2MoO4和β-(NH4)2MoO4的形式存在,结果如图6所示。
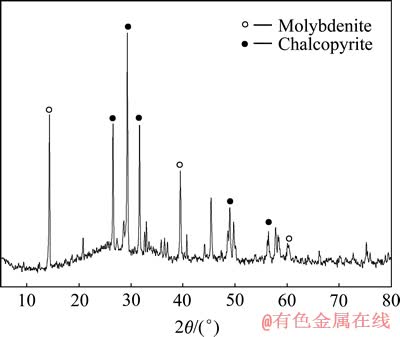
图5 Na2CO3-NH4HCO3体系条件下电氧化浸出渣的XRD谱
Fig. 5 XRD pattern of residues in Na2CO3-NH4HCO3 buffer system
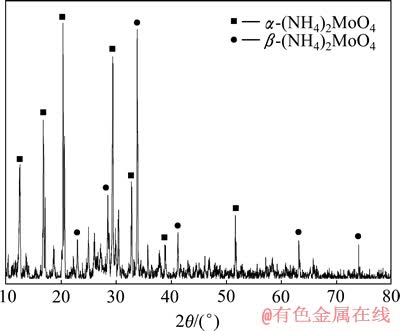
图6 反萃产物中钼的XRD谱
Fig.6 XRD pattern of Mo in stripping product
2.3 钼和铼的树脂吸附分离
上述研究表明,辉钼矿电氧化浸出液采用N235萃取-氨水反萃,可以成功实现Mo(Ⅵ)、Re(Ⅶ)的共萃取。针对反萃液中Mo(Ⅵ)、Re(Ⅶ)的分离问题,进行D201树脂吸附分离的试验研究[12]。
分别配制不同组成的钼铼二元混合溶液体系,在吸附温度30 ℃、吸附时间1 h、混合溶液中Re(Ⅶ)初始浓度35 μg/mL、溶液pH=8的条件下,考察不同Mo(Ⅵ)初始浓度的二元体系混合溶液中,D201树脂对Mo(Ⅵ)、Re(Ⅶ)的吸附容量及分离因子K,结果如表2所示。
表2 D201树脂对Mo(Ⅵ)、Re(Ⅶ)的吸附选择性
Table 2 Adsorption selectivity of D201 for Mo(Ⅵ) and Re(Ⅶ)
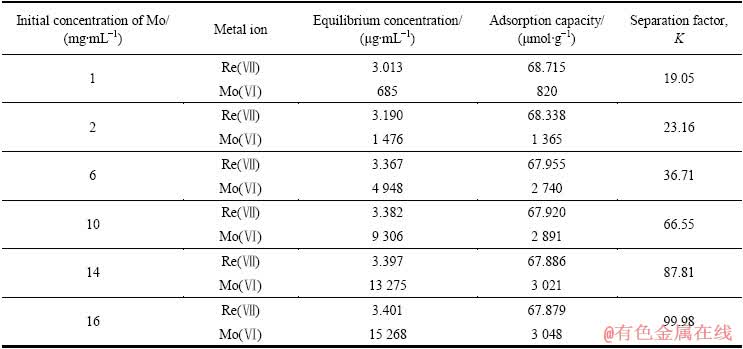
从表2可以看出,D201树脂对Mo(Ⅵ)的吸附容量随初始浓度的增加而增大,而Re(Ⅶ)的吸附容量则随Mo(Ⅵ)初始浓度的增加而轻微减小;树脂对Mo(Ⅵ)、Re(Ⅶ)的分离因子K则随Mo(Ⅵ)初始浓度的增加而增大,说明D201树脂更容易对铼进行吸附。Mo(Ⅵ)初始浓度达到16 mg/mL时,分离因子能达到99.98,说明树脂对Re(Ⅶ)具有良好的吸附选择性,能够满足分离Mo(Ⅵ)、Re(Ⅶ)的要求。
称取0.050 0 g D201干基树脂,对20 mL实际反萃液在30 ℃、pH=8的条件下静态吸附1 h,D201树脂对反萃液中的Re(Ⅶ)的吸附率分别可以达到92.18%,而对Mo(Ⅵ)的吸附率分别为3.46%,分离系数可达169.56。从铼、钼分离系数可以看出,D201树脂能够对实际反萃液中Mo(Ⅵ)、Re(Ⅶ)离子进行吸附分离。以20 mL 14%的NH4SCN溶液为洗脱液,在30 ℃,洗脱时间为1 h的条件下,对吸附了Mo(Ⅵ)、Re(Ⅶ)的D201树脂进行洗脱实验。结果表明,负载树脂Re(Ⅶ)、Mo(Ⅵ)的洗脱率分别为98.97%、15.36%。这说明14%(质量分数)NH4SCN溶液可以作为分离Re(Ⅶ)和Mo(Ⅵ)的洗脱液。
3 结论
1) 通过采用Na2CO3-NH4HCO3缓冲溶液控制电氧化过程pH值为9左右,使辉钼精矿中的钼、铼高效选择性浸出,钼、铼的浸出率分别为99.35%和99.79%,与非缓冲体系的电氧化过程相比,钼、铼的浸出率分别提高了23.86%和26.50%。在选择性电氧化过程中,黄铜矿基本上不能浸出而留在浸出渣中,电解渣中铜含量达到10.84%,回收率达到97.93%,可作为铜冶炼原料而回收。
2) 采用N235为萃取剂,对电氧化电解液中的钼、铼进行溶剂萃取,将溶液中HCl浓度调整为25.48 g/L时,钼、铼能实现同时萃取,其萃取率分别为99.84%和95.19%。用17%的氨水反萃负载有机相,钼、铼的反萃率分别为99.89%和99.54%。
3) 采用D201树脂分离铼,将反萃液pH调整为8,在30 ℃下,钼、铼的吸附率分别为3.46%和92.18%,分离因子为169.56;以14% NH4SCN溶液为洗脱液,在30 ℃下对负载树脂进行洗脱,钼、铼的洗脱率分别为15.36%和98.97%。
REFERENCES
[1] 赵中伟. 辉钼矿湿法浸出过程中某些问题之浅见[J]. 稀有金属与硬质合金, 1995, 121(6): 124-128.
ZHAO Zhong-wei. Discussion on the theoretical problems from the leaching of molybdenite[J]. Rare Metals and Cemented Carbides, 1995, 121(6): 124-128.
[2] 符剑刚, 钟 宏, 吴江丽, 卜向明. 常压条件下辉钼矿的湿法浸出[J]. 金属矿山, 2004, 342(12): 35-38.
FU Jian-gang, ZHONG Hong, WU Jiang-li, PU Xiang-ming. Wet leaching of molybdenite at atmospheric temperature and pressure[J]. Metal Mine, 2004, 342(12): 35-38.
[3] VICLOR J. Pressure oxidation process for the production of molybdenum trioxide from molybdnite. US6149883[P]. 2000-11-21.
[4] MANOJ K, MANKHAND T R, MURTHY D S R, MUKHOPADHYAY R, PRASAD P M. Refining of a low-grade molybdenite concentrate[J]. Hydrometallurgy, 2007, 86(3): 56-62.
[5] ROMANO P. Comparative study on the selective chalcopyrite bioleaching of a molybdenite concentrate with mesophilic and thermophilic bacteria[J]. FZMS Microbiology Letters, 2001, 196(1): 71-75.
[6] FU Jian-gang, ZHONG Hong. Electro-oxidation process for molybdenum concentrates[J]. Journal of Central South University of Technology, 2005, 12(2): 134-140.
[7] CAO Zhan-fang, ZHONG Hong, LIU Guang-yi, FU Jian-gang, WANG Shuai, QIU Tun-ren. Electric-oxidation kinetics of molybdenite concentrate in acidic NaCl solution[J]. The Canadian Journal of Chemical Engineering, 2009, 87(6): 939-944.
[8] 曹占芳, 钟 宏, 闻振乾, 符剑刚, 丁 超. 超声电氧化辉钼矿精矿研究[J]. 中国矿业大学学报, 2009, 38(2): 229-234.
CAO Zhan-fang, ZHONG Hong, WEN Zhen-qian, FU Jian-gang, DING Chao. Research on ultrasonic electro-oxidation process of MoS2 concentrate[J]. Journal of China University of Mining & Technology, 2009, 38(2): 229-234.
[9] CAO Zhan-fang, ZHONG Hong, WEN Zhen-qian, LIU Guang-yi, WANG Shuai. Electric-oxidation extraction of molybdenite concentrate in alkaline NaCl electrolyte[J]. Journal of Central South University of Technology, 2010, 17(3): 23-26.
[10] CAO Zhan-fang, ZHONG Hong, QIU Zhao-hui. Solvent extraction of rhenium from molybdenum in alkaline solution[J]. Hydrometallurgy, 2009, 97(3/4): 153-157.
[11] 钟 宏, 符剑刚, 刘凌波. 采用N235从含Mo, Mn酸浸液中萃取回收Mo[J]. 过程工程学报, 2006, 6(1): 28-31.
ZHONG Hong, FU Jian-gang, LIU Ling-bo. Recovery of Mo from acid leaching solution containing Mo and Mn by solvent extraction of N235[J]. The Chinese Journal of Process Engineering, 2006, 6(1): 28-31.
[12] 林春生. 萃取法从钼、铼溶液中回收铼[J]. 中国钼业, 2005, 29(1): 41-43.
LIN Chun-sheng. Recovery of rhenium from the mixed solution of molybdennum and rhenium by extraction[J]. China Molybdenum Industry, 2005, 29(1): 41-43.
[13] MA H Z, LAN X Z. Study on the sorption of rhenium from the leaching solution of the fume of molybdenite calcinations by anion exchange resin[J]. Journal of Xi’an University of Architecture and Technology, 2005, 31: 41-47.
[14] WANG S, ZHONG H, LIU G Y, ZHANG Q, LI T. Synthesis and adsorption properties for Au(Ⅲ) of alkoxycarbonyl thiourea resin[J]. Journal of Central South University of Technology, 2008, 15(4): 463-468.
[15] MA H M, ZHU Z L, ZHANG R H, LIN J W, ZHAO J F. Kinetics of adsorption of copper from water by weak base epoxy anion-exchange resin[J]. Ion Exchange and Adsorption, 2006, 22: 519-526.
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(21106188);湖南省自然科学基金项目(12JJ4013);中国博士后科学基金资助项目(2011M501299, 2012T50709);中南大学中央高校基本科研业务费专项资金资助项目(2011QNZT050)
收稿日期:2012-02-11;修订日期:2013-05-20
通信作者:钟 宏,教授,博士;电话:0731-88830603;传真:+86 731-88879616;E-mail: zhongh@mail.csu.edu.cn
摘 要:针对德兴铜矿高铜钼精矿特性,研究开发一种选择性电氧化浸出新工艺。结果表明,辉钼矿的电氧化浸出受电解液pH值的影响显著。采用Na2CO3-NH4HCO3缓冲溶液控制电解过程pH为9左右,可以实现辉钼矿的选择性电氧化高效浸出,钼、铼的浸出率分别为99.35%和99.79%,与非缓冲体系的电氧化过程相比,钼、铼的浸出率分别提高了23.86%和26.50%。在选择性电氧化过程中,黄铜矿基本上不能浸出而留在浸出渣中,电解渣中铜品位达到10.84%,回收率达到97.93%,可作为铜冶炼原料而回收。采用N235为萃取剂,对电氧化浸出液中的钼、铼进行溶剂萃取,将溶液中HCl浓度调整为25.48 g/L时,可实现钼、铼的同时萃取,其萃取率分别为99.84%和95.19%;用17%的氨水反萃负载有机相,有机相中钼、铼的反萃率分别为99.89%和99.54%。将反萃液pH调整为8,30℃下用D201树脂吸附1 h,钼、铼的吸附率分别为3.46%和92.18%,分离因子为169.56。
[1] 赵中伟. 辉钼矿湿法浸出过程中某些问题之浅见[J]. 稀有金属与硬质合金, 1995, 121(6): 124-128.
[2] 符剑刚, 钟 宏, 吴江丽, 卜向明. 常压条件下辉钼矿的湿法浸出[J]. 金属矿山, 2004, 342(12): 35-38.
[8] 曹占芳, 钟 宏, 闻振乾, 符剑刚, 丁 超. 超声电氧化辉钼矿精矿研究[J]. 中国矿业大学学报, 2009, 38(2): 229-234.
[11] 钟 宏, 符剑刚, 刘凌波. 采用N235从含Mo, Mn酸浸液中萃取回收Mo[J]. 过程工程学报, 2006, 6(1): 28-31.


