DOI:10.19476/j.ysxb.1004.0609.2018.11.21
镓精矿中镓的提取工艺
朱茂兰1,黄中省2,衷水平3,陈 杭3,胡志彪1
(1. 龙岩学院 化学与材料学院,龙岩 364012;
2. 中南大学 冶金与环境学院,长沙 410083;
3. 福州大学 紫金矿业学院,福州 350108)
摘 要:
采用“焙烧-硫酸浸出-中和沉淀”工艺提取明矾石精矿中的镓,获得含Ga 0.1%以上的镓精矿。为了实现镓精矿的高值化利用,提出“碱浸-离子交换-溶液净化-电积”回收镓精矿中金属镓的工艺,对最佳工艺条件进行研究。在最佳工艺条件下,镓精矿中Ga的碱浸浸出率达98.46%;离子交换实验表明,采用D5240树脂可以有效地富集镓,但杂质钒也得到富集,树脂解吸液中含镓2.1g/L,钒0.23g/L;在pH=12的条件下,可通过钙盐法除钒,当解吸液中Ca2+添加量为0.02 mol/L时,钒浓度降至1.45 mg/L;镓电积过程中电流效率呈现先增大后减小的趋势,当电积时间为2h时,电流效率达到峰值4.3%;随着电积液中NaOH浓度的升高,阴极表面形貌由粗糙变得光滑。当NaOH浓度为200g/L时,阴极表面平整光滑,可获得99.94%金属镓。
关键词:
文章编号:1004-0609(2018)-11-2351-07 中图分类号:TF843.1 文献标志码:A
镓是元素周期表IIIA族中的稀散金属元素,其氧化态为+1和+3价,它能形成砷化物[1]、溴化物、氮化物、硫化物和碲化物等多种化合物[2],这些化合物是半导体和芯片行业的重要材料[3-4]。自然条件下很难形成镓的矿物,地壳中镓含量仅为1×10-5 [5]。由于镓和铝的地球化学性质相似,镓主要分布在水云母,霞石、铝土矿、磷灰石和明矾石矿物中[6-7]。目前,镓主要通过在锌、铝和粉煤灰工业中作为副产品进行回收[8-9]。镓回收的方法主要有[10]:置换法、电化学法、萃取法、沉淀法和离子交换法。目前,在铝工艺回收镓过程中,电化学法和置换法基本被淘汰,国内外大部分企业都开始采用树脂法[11-12];从锌冶炼渣等副产物回收镓,主要为酸性体系,早期采用沉淀法,近年来以萃取法为主[13-14]。
明矾石中镓的含量为0.002%~0.006%[15],明矾石主要通过酸法[16],碱法[17-18]和酸碱联合法[19-20]回收其中的铝、钾资源,关于明矾石中镓回收的相关报道很少。福建某铜矿山,明矾石含量为20%,镓含量为35 g/t。经浮选后,明矾石精矿中镓含量超过50 g/t。课题组通过“焙烧-硫酸选择性浸出-中和沉淀”的方法,获得了镓含量大于0.1%的镓精矿。为了实现镓的高值化回收,本文作者采用“浸出-离子交换-电积”的方法提取镓精矿中的金属镓,并考察这些过程的最佳参数,为明矾石矿中镓的提取提供数据参考和技术支持。
1 实验
1.1 实验原料
实验原料是由明矾石精矿经焙烧-酸浸-中和沉镓后获得,其主要成分如表1所示。由表1可知,镓精矿产品中铝含量最高为11.95%,钠含量为6.92%,钾含量和铁含量相近分别为3.67%和4.78%,钒含量较低,为0.02%,其中镓含量为0.11%。图1所示为镓精矿原料的XRD分析结果。由图1可知,镓精矿主要物相为2Al2O3·4SO3·8H2O和KAl3(SO4)2(OH)6,未能发现含镓物相特征峰。其他实验原料主要有镓吸附树脂(Purolite D5240),以及分析纯的硫酸、氢氧化钠和氧化钙。
表1 镓精矿多元素分析结果
Table 1 Analysis result of gallium concentrate (mass fraction, %)
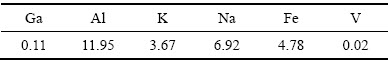
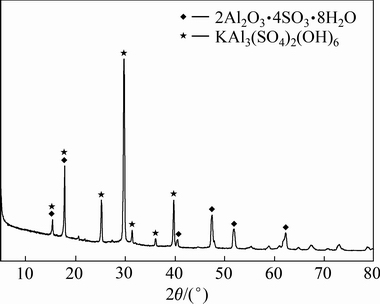
图1 镓精矿的XRD谱
Fig. 1 XRD pattern of gallium concentrate
1.2 实验主要设备
实验设备主要有电感耦合等离子体光谱分析仪,X射线衍射光谱仪,烘箱,恒温水浴搅拌器,电动搅拌机,蠕动泵,离子交换柱,直流电源等。
1.3 实验方法
镓精矿提取金属镓工艺流程如图2所示,主要分为镓精矿浸出、离子交换、溶液净化和镓电积4个工序,具体的实验方法如下。
1) 浸出实验。将称量的镓精矿与一定量的水进行混合,然后加入NaOH在不同的温度下搅拌浸出,矿浆采用定量滤纸过滤,分析浸出液中的铝、钾、钒和镓的离子浓度。
2) 离子交换实验。吸镓树脂先采用40 g/L的NaOH溶液活化24 h,将活化后的树脂装入离子交换柱(800 mm×10 mm)中,采用蠕动泵将浸出液以6.5 BV/h的流速淋入离子交换柱中进行吸附实验。在吸附过程中,每5 BV收集一次吸附后液进行成分分析,直至出现穿透点后,停止滴淋。随后,取40 g/L的NaOH溶液1 BV,以2.5 BV/h流速进行洗涤。最后,采用20 g/L的H2SO4溶液作为解吸液,解吸液流速为2 BV/h,解吸液体积为7 BV,定期收集解吸液进行成分分析。液体体积1 BV按式(1)计算:
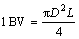 (1)
(1)
式中:π为圆周率,3.14;L为离子交换柱的高度,m;D为离子交换柱的内径,m。
3) 溶液净化。用NaOH将解析液的pH中和至 12,再加入一定质量的CaO,在50 ℃的条件下搅拌反应30min后固液分离,分析钒的脱除效果。
4) 取316L不锈钢电极2片,依次用800号和1200号砂纸分别打磨,再放入5%硫酸浸泡10 min,经超声清洗后作为阴极和阳极。将净化后的解析液,装入0.25 L的电积槽中,电极间距6 cm,接入直流电源进行电积。
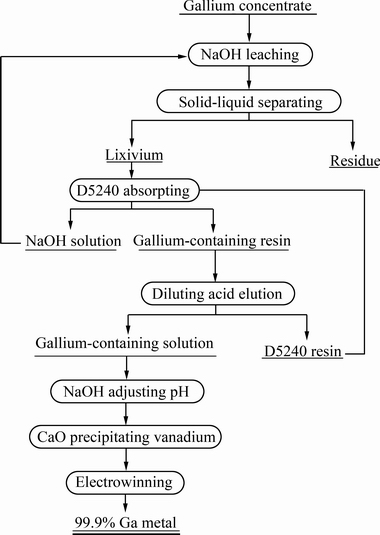
图2 镓精矿提取镓的工艺流程图
Fig. 2 Flowsheet of extraction of gallium from gallium concentrate
2 结果与讨论
2.1 镓精矿的浸出
2.1.1 初始碱度对浸出效果的影响
取200 g镓精矿置于烧杯中,在液固比5、30 ℃的条件下搅拌浸出2 h,考察不同初始NaOH浓度对浸出效果的影响,实验结果如图3所示。由图3可知,随着初始NaOH浓度的升高,铝、镓和钒的浸出率也迅速升高。当初始NaOH浓度为75 g/L时,65.31%,76.90%和84.37%;当初始NaOH浓度为150 g/L时,铝、镓和钒的浸出率分别提高至97.07%、99.08%和93.76%,继续提升初始碱度,铝、镓和钒的浸出率不再提高。此外,由图3可知,钾的浸出率受初始NaOH浓度的影响较小,当初始NaOH浓度由75增加到200 g/L时,钾的浸出率都保持在98%左右。由此可知,最佳的初始NaOH浓度为150 g/L。
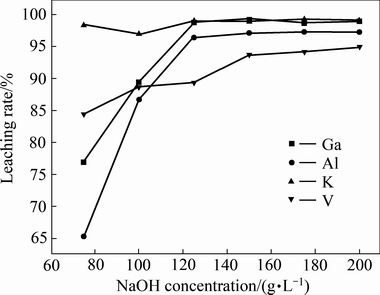
图3 初始NaOH浓度对浸出效果的影响
Fig. 3 Effect of NaOH concentration on leaching rate
2.1.2 浸出温度对浸出效果的影响
取200 g镓精矿置于烧杯中,在液固比5、初始NaOH浓度为150 g/L的条件下搅拌浸出2 h,考察不同反应温度对浸出效果的影响,实验结果如图4所示。由图4可知,反应温度对浸出效果的影响较小。当温度由30 ℃升高到90 ℃时,镓、铝、钾和钒的浸出率仅增加1%~3%。这是由于浸出温度为30 ℃时,镓、铝、钾的浸出率已分别高达99.08%、97.07%和98.9%,继续升高温度浸出率的提升空间很小,且会提高杂质钒的浸出率,不利于镓的回收,因此最佳浸出温度为30 ℃。
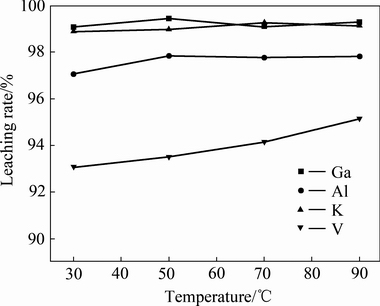
图4 反应温度对浸出效果的影响
Fig. 4 Effect of temperature on leaching rate
2.1.3 液固比对浸出效果的影响
取5组200 g的镓精矿分别置于个烧杯中,各加入150 g NaOH,再分别加入600、800、1000、1200和1400 mL去离子水,在30 ℃条件下搅拌浸出2 h,考察不同液固比对浸出效果的影响,实验结果如图5所示。由图5可知,液固比对浸出效果的影响不大。当液固比为3时,镓、铝、钾和钒的浸出率分别为98.46%,96.98%,98.89%和93.46%。当液固比增大至6和7时,浸出率几乎不增加。这是因为液固比变化主要影响浸出液中各元素和碱的浓度,而试验中碱添加量较高,浸出液中的碱度可维持在较高的范围,使得样品中主要的元素铝和钾在碱液中均具有极高的溶解度。因此,当液固比变化不大时,对浸出效果的影响较小。
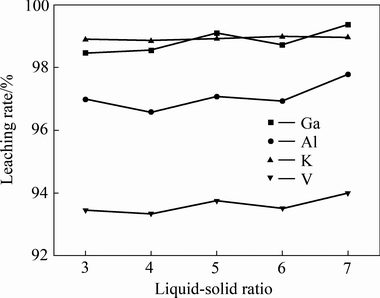
图5 液固比对浸出效果的影响
Fig. 5 Effect of liquid-solid ratio on leaching rate
2.2 镓的吸附与解吸
将镓精矿在浸出温度为30 ℃、NaOH浓度为150 g/L、液固比3的条件下搅拌浸出2 h,固液分离后送样分析,浸出液组成如表2所示。由表2可知,浸出液中铝、钾和镓的浓度分别为38.51 g/L、12.09 g/L和360 mg/L,还含有少量的铁、钒等杂质。当电解液中镓浓度很低时,采用汞齐电解法能有效地从含镓的NaAlO2液中电解提镓[21],但汞会污染NaAlO2溶液,而且生产1 kg镓需要2~3 t汞。采用电积法直接从低浓度溶液中提取镓,理论上是可行的,但电流效率太低[22]。因此,常采用萃取[23]或离子交换[24]的方法富集溶液中的镓,其中离子交换法具有流程短,回收率高,反应速度快等优点,被广泛应用于工业化生产[2]。
表2 浸出液中多元素分析结果
Table 2 Analysis results of leaching solution (g/L)

图6所示为浸出液中镓和钒的动态吸附实验结果。由图6可知,当进料溶液量小于10 BV时,吸附后液中镓和钒的浓度均较低,分别为86.51和14.35 mg/L。当进料液超过30 BV时,树脂接近饱和,吸附后液中镓和钒浓度分别为345.71 mg/L和59.62 mg/L。镓的吸附率为38.23%,原始浓度为360 mg /L,镓的总吸附量Qc约为302.57 mg;钒的吸附率为43.11%,钒的总吸附量Qc为61.60 mg。由此可知,Purolite D5240树脂对镓和钒的饱和吸附容量分别为4.81和1.02 g/L,具有较好的吸附效果,但Purolite D5240也存在镓和钒共吸附的问题[25]。
饱和的载镓树脂经洗涤后,采用20 g/L的H2SO4溶液进行动态解吸,解吸结果如图7所示。由图7可知,当解吸液量为1.5 BV时,解吸液中镓和钒的浓度达到最大值,分别为2105.48和234.04 mg/L。当解吸液量大于2 BV时,镓和钒的浓度迅速下降。当流出液为6 BV时,镓和钒的浓度分别为95.77和23.4 mg/L。在整个解吸过程中,镓和钒的解吸率分别为84.9%和51.3%。由此可知,Purolite D5240树脂对镓具有较好的吸附和解吸性能,能够有效地将镓富集至2 g/L以上。
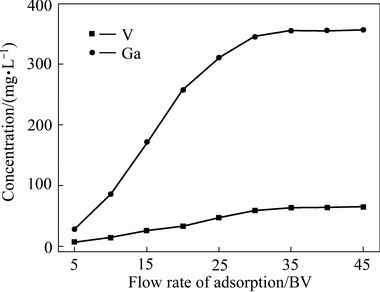
图6 镓和钒的吸附曲线
Fig. 6 Absorption curves of gallium and vanadium
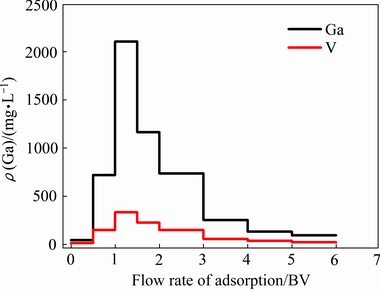
图7 镓和钒的解吸曲线
Fig .7 Elution curves of gallium and vanadium
2.3 溶液净化
由于镓与钒的共吸附作用,在富集镓过程中,钒也出现显著的富集现象。有研究表明[26],电积液中的钒离子会促进析氢反应,使金属镓表面的OH-浓度急剧升高,造成细颗粒金属镓返溶,降低电流效率。溶液中钒离子通常采用铁盐法、铵盐法、钙盐法或水解法脱除[27]。由于石灰价格低廉,钙盐法是最常用的。当pH为11~12.6时,钒将以Ca3(VO4)2的形式沉淀[28]。调节解吸液的pH值至12,考察不同Ca2+添加量对钒沉淀效果的影响,其结果如图8所示。由图8可知,当Ca2+添加量为1 mmol/L时,钒没有沉淀。随着Ca2+添加量的增加,钒的浓度降低;当Ca2+添加量为20 mmol/L时,钒浓度降至1.45 mg/L,对镓电积的影响不大[29]。
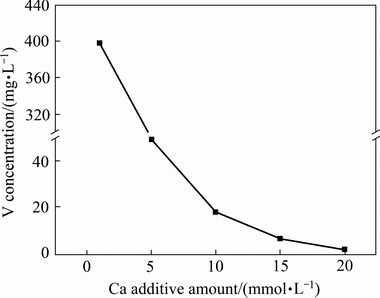
图8 不同Ca2+添加量条件下钒的沉淀效果
Fig. 8 Precipitation effect of vanadium at different Ca2+ additive amounts
2.4 镓电积
离子交换富集过程后,溶液中镓浓度达到1~2 g/L,满足电解沉积工艺中溶液中镓的提取要求,碱溶液中镓的电积总反应如下[30]:
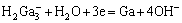 (2)
(2)
取0.25 L净化后液进行电积实验,当电流密度为0.25 A/cm2、42 ℃、NaOH浓度为100 g/L时,镓浓度与电流效率随时间的变化关系如图9所示。由图9可知,随着电积时间的延长,电解液中的镓浓度不断降低,但电流效率却呈现先升高后降低的趋势。这可能是由于在电积初期,金属镓不易在不锈钢阴极上形成镓晶核,随着电积时间的延长,阴极表明镓沉积的活性位点增加,促进了镓的型核过程,使电流效率升高。继续延长电积时间,由于电积液中镓的浓度不断降低,使浓差极化的影响更为突出,析氢反应增多,电流效率随之降低[31]。
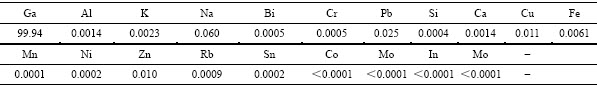
不同NaOH浓度条件下获得的阴极产物形貌如图10所示。由图10可知,随着电积液中NaOH浓度的升高,阴极产物逐渐由粗糙变得光滑,当NaOH浓度为200 g/L时,阴极产品的镓纯度达99.94%,如表3所示。

图9 镓浓度和电流效率随电积时间的变化
Fig. 9 Variation of gallium concentration and current efficiency with time of electrodeposition
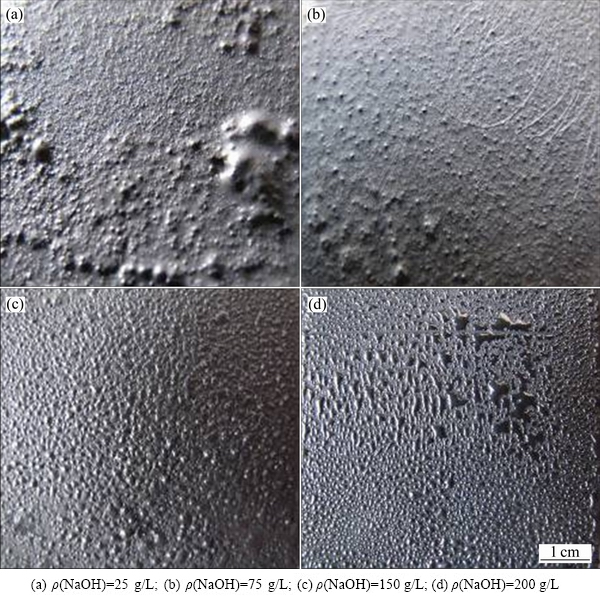
图10 不同NaOH浓度条件下阴极表面形貌
Fig. 10 Morphologies of electrowinning gallium at different NaOH concentrations
表3 阴极产品的多元素分析结果
Table 3 Analysis results of electrodeposited gallium (mass fraction, %)
3 结论
1) 镓精矿中镓含量为0.11%,NaOH浓度对镓的浸出率具有显著的影响,其最佳浸出工艺条件为NaOH浓度250 g/L,浸出温度30 ℃,液固比3:1,浸出时间2 h,此条件下镓的浸出率达98.46%。
2) Purolite D5240树脂吸附可有效富集镓,但也存在钒共吸附的现象,解吸液中镓浓度由原液的0.36 g/L提高至2.1 g/L,钒离子浓度高达0.23 g/L。采用钙盐法可有效脱除解吸液中的钒,当解析液中Ca2+添加量为0.02 mol/L时,钒浓度降至1.45 mg/L。
3) 镓电积过程中电流效率呈现先增大后减小的趋势,当电积时间为2 h时,电流效率达到峰值4.3%;随着电积液中NaOH浓度的升高,阴极表面形貌由粗糙向平整转变,当NaOH浓度为200 g/L时,阴极表面平整光滑,此时阴极产品镓纯度为99.94%。
RERERENCES
[1] 胡 亮, 刘大春, 陈秀敏, 杨 斌, 白平平, 段少飞. 砷化镓真空热分解的理论计算与实验[J]. 中国有色金属学报, 2014, 24(9): 2410-2417.
HU Liang, LIU Da-chun, CHEN Xiu-min, YANG Bin, BAI Ping-ping, DUAN Shao-fei. Thermal decomposition of gallium arsenide under vacuum: Theoretical calculation and experiment[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2410-2417.
[2] ZHAO Z, YANG Y, XIAO Y, FAN Y. Recovery of gallium from Bayer liquor: A review[J]. Hydrometallurgy, 2012, s125/126(8): 115-124.
[3] MOSKALYK R R. Gallium: The backbone of the electronics industry[J]. Minerals Engineering, 2003, 16(10): 921-929.
[4] FONT O, QUEROL X, JUAN R, RURZ C R, LOPEZ-SOLER A, COCAP, GARCIAPENAF. Recovery of gallium and vanadium from gasification fly ash[J]. Journal of Hazardous Materials, 2007, 139(3): 413-423.
[5] METZ S, TREFRY J H. Chemical and mineralogical influences on concentrations of trace metals in hydrothermal fluids[J]. Geochimica et Cosmochimica Acta, 2000, 64(13): 2267-2279.
[6] 许 可, 邓 彤, 陈家镛, 戴玉杰, 王 静. 黄磷电炉电尘浆提取镓的预处理[J]. 中国有色金属学报, 2004, 14(6): 1025-1030.
XU Ke, DENG Tong, CHEN Jia-yong, DAI Yu-jie, WANG Jing. Pretreatment of phosphorus industry flue dust for gallium extraction[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(6): 1025-1030.
[7] 周令治, 陈少纯. 稀散金属提取冶金[M]. 北京: 冶金工业出版社, 2008.
ZHOU Ling-ye, CHEN Shao-chun. The extraction metallurgy of dissipated metal[M]. Beijing: Metallurgical Industry Press, 2008.
[8] 张魁芳, 曹佐英, 肖连生, 曾 理, 张贵清, 李青刚. 采用HBL121从锌置换渣高浓度硫酸浸出液中萃取回收镓[J]. 中国有色金属学报, 2014, 24(9): 2400-2409.
ZHANG Kui-fang, CAO Zuo-ying, XIAO Lian-sheng, ZENG Li, ZHANG Gui-qing, LI Qing-gang. Extraction of gallium from high concentration sulfuric acid leaching solution of zinc replacing slag by HBL121[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2400-2409.
[9] 张俊杰, 李宏煦. 高铁铝土矿焙烧/高压碱浸提取镓的研究[J]. 有色金属(冶炼部分), 2017(12): 36-39.
ZHANG Jun-jie, LI Hong-xu. Study on gallium extraction from high iron-bearing bauxite by soda roasting and pressure alkali leaching[J]. Nonferrous Metals(Extractive Metallurgy), 2017(12): 36-39.
[10] 刘延红, 郭昭华, 池君洲, 王永旺, 陈 东. 镓回收方法与技术的研究与进展[J]. 稀有金属与硬质合金, 2016(1): 1-8.
LIU Yan-hong, GUO Zhao-hua, CHI Jun-zhou, WANG Yong-wang, CHEN Dong. Research and the latest development of gallium recovery process and technology[J]. Rare Metals and Cemented Carbides, 2016(1): 1-8.
[11] 冯 峰, 李鑫金, 于湘浩. 密实移动床离子交换法提取镓的工业应用[J]. 稀有金属, 2007(s1): 114-117.
FENG Feng, LI Xin-jin, YU Xiang-hao. Industry application of packed moving bed ion exchange in extraction of gallium[J]. Chinese Journal of Rare Metals, 2007(s1): 114-117.
[12] LI S, WU W, LI H, HOU X. The direct adsorption of low concentration gallium from fly ash[J]. Separationence & Technology, 2015, 51(3): 395-402.
[13] 刘兴芝, 刘长几. N2125萃取镓及其机理的研究[J]. 稀有金属, 1991(5): 327-329.
LIU Xing-zhi, LIU Chang-ji. The research of Ga extraction mechanism with N2125[J]. Chinese Journal of Rare Metals, 1991(5): 327-329.
[14] 熊 英, 张莹莹, 娄振宁, 单炜军, 宋玉林. 镓的萃取分离研究进展[J]. 辽宁大学学报(自然科学版), 2016, 43(4): 335-342.
XIONG Ying, ZHANG Ying-ying, LOU Zhen-ning, SHAN Wei-jun, SONG Yu-lin. Research progress of gallium by using solvent extraction[J]. Journal of Liaoning University(Material Science Edition), 2016, 43(4): 335-342.
[15] HART L R D. Alumina chemicals: Science and technology handbook[M]. Westerville, Ohio: American Ceramic Society, 1990.
[16] OZDEMIR M, CETISLI H. Extraction kinetics of alunite in sulfuric acid and hydrochloric acid[J]. Hydrometallurgy, 2005, 76(3): 217-224.
[17] HARTMAN G J, EWING V R. Process for recovering aluminum from alunite: US Patent, 4230678[P]. 1980-10-28.
[18] HERSHMAN P R. Process of producing alluminate from alunite: US Patent, 1191105[P]. 1916-07-11.
[19] MCCULLOUGH W E. Cyclic process for treating alunite: US Patent, 2120840[P]. 1938-06-14.
[20] NAZAROV S B, GULAKHMADOV K S, KHAKDODOV M M, et al. Processing of aluminum sulfates into alumina[J]. Russian Journal of Applied Chemistry, 2001, 74(8): 1392-1394.
[21] BERETEQUE P D L. Method of recovering gallium from an alkali aluminate lye: US Patent, 2793179A[P]. 1957-05-21.
[22] 武新宇. 酸性介质中镓的吸附和萃取性质及回收工艺研究[D]. 西安: 长安大学, 2014.
WU Xin-yu. Study on properties and recovery processes of gallium of adsorption and extraction in the acidic medium[D]. Xi’an: Chang’an University, 2014.
[23] PUVVAD G V K. Liquid-liquid extraction of gallium from Bayer process liquor using Kelex 100 in the presence of surfactants[J]. Hydrometallurgy, 1999, 52(1): 9-19.
[24] RIVEROS P A. Recovery of gallium from Bayer liquors with an amidoxime resin[J]. Hydrometallurgy, 1990, 25(1): 1-18.
[25] BAUTISTA R G. Processing to obtain high-purity gallium[J]. JOM, 2003, 55(3): 23-26.
[26] LIU L, WANG M, WANG Z, ZHANG Y. The influence of impurities on Ga electrowinning: Vanadium and iron[J]. Hydrometallurgy, 2014, 146(1): 76-81.
[27] DING Rui-feng, LIU Gui-hua. Advanced of Extraction of Vanadium from solution[J]. Hunan Nonferrous Metals, 2011, 27(3): 15-19.
[28] LI He, LIU Xu-heng, HE Li-hua. Thermodynamic study on vanadium precipitation with calcium salt[J]. Rare Metals and Cemented Carbides, 2014, 42(1): 15-19.
[29] DORIN R, FRAZER E J. The electrodeposition of gallium from synthetic Bayer-process liquors[J]. Journal of Applied Electrochemistry, 1988, 18(1): 134-141.
[30] BOCKRIS J O M, ENYO M. Electrodeposition of gallium on liquid and solid gallium electrodes in alkaline solutions[J]. Journal of the Electrochemical Society, 1962, 109(1): 48-54.
[31] 刘 玲. 高铝粉煤灰中金属镓电解提取技术基础与应用[D]. 北京: 中国科学院, 2016.
LIU Ling. The basic and applied research of Ga electrowinning from high aluminium fly ash[D]. Beijing: Chinese Academy of Sciences, 2016.
Extraction process of gallium from gallium concentrate
ZHU Mao-lan1, HUANG Zhong-sheng2, ZHONG Shui-ping3, CHEN Hang3 , HU Zhi-biao1
(1. School of Chemistry and Materials, Longyan University, Longyan 364012, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
3. College of Zijin Mining, Fuzhou University, Fuzhou 350108, China)
Abstract: A gallium concentrate with more than 0.1% Ga can be obtained by “roasting-sulfuric acid leaching-and precipitation” process from alunite concentrate. In order to realize the high value utilization of gallium concentrate, the technology of recovering metal gallium by “alkali leaching-ion exchange-solution purification-electrowinning” was proposed, and the optimum technological conditions were studied. Under the optimum conditions, the leaching rate of Ga in gallium concentrates reaches 98.46%. The ion exchange experiment shows that the D5240 resin can effectively enrich Ga, but the impurity vanadium is also enriched. The desorption solution contains gallium 2.1 g/L and vanadium 0.23 g/L. Vanadium can be removed by the calcium salt method under the condition of pH=12. When the Ca2+ additive amount is 0.02 mol/L, the vanadium concentration is reduced to 1.45 mg/L. The current efficiency in gallium electrodeposits increases firstly and then decreases. When the electrodeposition time is 2 h, the current efficiency reaches 4.3% of the peak value. With the increase of NaOH concentration in the electrodeposition electrolyte, the cathode surface is from coarse to smooth. Finally, the purity of 99.94% metallic gallium is obtained.
Key words: gallium concentrate; alkaline leaching; ion exchange; calcium salt precipitation; gallium electrowinning
Foundation item: Projects(51474075, 51704153, 2016J01252) supported by the National Natural Science Foundation of China
Received date: 2018-02-26; Accepted date: 2018-06-20
Corresponding author: ZHONG Shui-ping; Tel: +86-15280385768; E-mail: zspcsu@163.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51474075,51704153,2016J01252)
收稿日期:2018-02-26;修订日期:2018-06-20
通信作者:衷水平,教授级高级工程师,博士;电话:0592-7765118;E-mail: zspcsu@163.com
摘 要:采用“焙烧-硫酸浸出-中和沉淀”工艺提取明矾石精矿中的镓,获得含Ga 0.1%以上的镓精矿。为了实现镓精矿的高值化利用,提出“碱浸-离子交换-溶液净化-电积”回收镓精矿中金属镓的工艺,对最佳工艺条件进行研究。在最佳工艺条件下,镓精矿中Ga的碱浸浸出率达98.46%;离子交换实验表明,采用D5240树脂可以有效地富集镓,但杂质钒也得到富集,树脂解吸液中含镓2.1g/L,钒0.23g/L;在pH=12的条件下,可通过钙盐法除钒,当解吸液中Ca2+添加量为0.02 mol/L时,钒浓度降至1.45 mg/L;镓电积过程中电流效率呈现先增大后减小的趋势,当电积时间为2h时,电流效率达到峰值4.3%;随着电积液中NaOH浓度的升高,阴极表面形貌由粗糙变得光滑。当NaOH浓度为200g/L时,阴极表面平整光滑,可获得99.94%金属镓。
[1] 胡 亮, 刘大春, 陈秀敏, 杨 斌, 白平平, 段少飞. 砷化镓真空热分解的理论计算与实验[J]. 中国有色金属学报, 2014, 24(9): 2410-2417.
[6] 许 可, 邓 彤, 陈家镛, 戴玉杰, 王 静. 黄磷电炉电尘浆提取镓的预处理[J]. 中国有色金属学报, 2004, 14(6): 1025-1030.
[7] 周令治, 陈少纯. 稀散金属提取冶金[M]. 北京: 冶金工业出版社, 2008.
[8] 张魁芳, 曹佐英, 肖连生, 曾 理, 张贵清, 李青刚. 采用HBL121从锌置换渣高浓度硫酸浸出液中萃取回收镓[J]. 中国有色金属学报, 2014, 24(9): 2400-2409.
[9] 张俊杰, 李宏煦. 高铁铝土矿焙烧/高压碱浸提取镓的研究[J]. 有色金属(冶炼部分), 2017(12): 36-39.
[10] 刘延红, 郭昭华, 池君洲, 王永旺, 陈 东. 镓回收方法与技术的研究与进展[J]. 稀有金属与硬质合金, 2016(1): 1-8.
[11] 冯 峰, 李鑫金, 于湘浩. 密实移动床离子交换法提取镓的工业应用[J]. 稀有金属, 2007(s1): 114-117.
[13] 刘兴芝, 刘长几. N2125萃取镓及其机理的研究[J]. 稀有金属, 1991(5): 327-329.
[14] 熊 英, 张莹莹, 娄振宁, 单炜军, 宋玉林. 镓的萃取分离研究进展[J]. 辽宁大学学报(自然科学版), 2016, 43(4): 335-342.
[18] HERSHMAN P R. Process of producing alluminate from alunite: US Patent, 1191105[P]. 1916-07-11.
[19] MCCULLOUGH W E. Cyclic process for treating alunite: US Patent, 2120840[P]. 1938-06-14.
[22] 武新宇. 酸性介质中镓的吸附和萃取性质及回收工艺研究[D]. 西安: 长安大学, 2014.
[25] BAUTISTA R G. Processing to obtain high-purity gallium[J]. JOM, 2003, 55(3): 23-26.


