Trans. Nonferrous Met. Soc. China 26(2016) 599-606
Hydrogen absorption characteristics and microstructural evolution of TC21 titanium alloy
Bao-guo YUAN, Yu-bin ZHENG, Yu-jie WANG, Long-qing GONG
School of Materials Science and Engineering, Hefei University of Technology, Hefei 230009, China
Received 28 March 2015; accepted 24 June 2015
Abstract:
The hydrogen absorption characteristics and microstructural evolution of TC21 titanium alloy were investigated by kinetic model analysis, optical microscopy (OM) and X-ray diffraction (XRD). The results show that the hydrogen absorption reaction occurred during the hydrogen absorption process of TC21 titanium alloy can be divided into two different stages according to the hydrogen absorption kinetics. After hydrogenation, the microstructure of TC21 titanium alloy changes obviously. Just a little hydrogen will change the contrast of transformed β phase. The contrast of α phase darkens when the hydrogen content in TC21 titanium alloy exceeds 0.5% (mass fraction). The phase/grain boundaries become ambiguous or even vanished, and β phase becomes the main phase instead of α phase when the hydrogen content reaches 0.625%. Moreover, α phase disappears when the hydrogen content reaches 1.065%. Additionally, the XRD analysis shows that α' martensite and FCC δ hydride appear in the hydrogenated alloy. According to the microstructures and XRD analysis, the schematic diagrams of hydrogen diffusion process in TC21 titanium alloy were established.
Key words:
TC21 titanium alloy; hydrogen absorption characteristics; kinetics; microstructure; diffusion;
1 Introduction
Titanium alloys, magnesium alloys and aluminum alloys have received much attention in engineering over the recent years because of their high specific strengths [1-3], which can save energy. Titanium alloys have low density, high specific strength, excellent corrosion resistance, good fatigue properties and biochemical compatibility, which are widely used as structural components in aerospace, chemical, marine and orthopedic industries [4,5]. TC21 titanium alloy, which is developed by Northwest Institute for Nonferrous Metal Research of China, is a new kind of α+β titanium alloy with high strength, high toughness and high damage tolerance property. However, similar to most high strength titanium alloys, TC21 titanium alloy suffers from high deformation resistance [6], low plasticity [7] and poor machining properties [8] which restricts its applications.
Thermohydrogen processing (THP) of titanium alloys is a kind of technology to use hydrogen as a temporary alloying element in titanium-based materials, which can refine microstructures, reduce β transus temperature, improve plasticity, machinability and deforming limits [9,10]. Since ZWICKER and SCHLEICHER [11] found that the addition of suitable content of hydrogen could improve the plasticity of titanium alloys, THP technology has been an important research topic. Lately, SHAN et al [12] established a model of eutectoid transformation to explain the mechanism of δ hydride formation according to Ti-H phase diagram. SHEN and WANG [9] found that βH treatment with hydrogen contents below 0.66 H/M at 600 °C was a satisfactory way to refine grain and enhance the mechanical properties of Ti-6Al-4V (mass fraction, %). Moreover, HUANG et al [13] succeeded in the application of THP to Ti-6Al-4V alloy blade isothermal forging. It was found that THP could reduce the forging temperature by 100 °C, and the mechanical properties of blade were improved.
The nominal composition of TC21 titanium alloy is Ti-6Al-2Mo-1.5Cr-2Zr-2Sn-2Nb (mass fraction, %), which has more stable elements (Mo, Cr and Nb) for β phase than Ti-6Al-4V alloy. H is a stable element for β phase. With the addition of hydrogen in TC21 titanium alloy, more amount of plastic β phase could be acquired at room temperature theoretically. Therefore, it is meaningful to study the microstructural evolution of TC21 titanium alloy after hydrogenation. Recent related works only focus on the microstructural evolution of titanium alloys containing different hydrogen contents [10]. The effect of holding time on the microstructural evolution of titanium alloys has not been studied systematically. In the present work, TC21 titanium alloys were hydrogenated at 750 °C for different holding time. The hydrogen absorption characteristics, kinetics, microstructural evolution and hydrogen diffusion were investigated.
2 Experimental
The material used in the present work was an extruded and annealed TC21 titanium alloy bar with 30 mm in diameter. The chemical composition of alloy was as follows: 7.69% Al, 2.09% Mo, 1.43% Cr, 1.74% Zr, 2.17% Sn, 1.72% Nb and balance Ti (mass fraction). The specimens (d 8 mm × 12 mm) used for hydrogena- tion were obtained from the received bar by an electric discharging machine (EDM), and polished with 800 grit and 1000 grit sandpapers, then cleaned ultrasonically in acetone solution, and finally dried. The aim of these operation processes was to ensure that the surfaces of these specimens were clean and to reduce the effects of their surface oxide films on the hydrogen absorption process. The specimens were hydrogenated in a self-made tubular hydrogen processing equipment at 750 °C under a set hydrogen pressure for a set holding time, and then air cooled to room temperature. The hydrogen pressure was in the range of 40.7-61.2 kPa. The holding time was in the range of 0-120 min. The vacuum degree of furnace pipe system was lower than 2.0×10-3 Pa when the system was heated with a rate of 10 °C/min. The actual hydrogen content in specimens was measured by weighting the specimens before and after hydrogenation treatment using a high precision electronic balance (SHIMADZU AUW220D) with a measurement accuracy of 10-5 g.
All metallographic specimens were first mechanically polished with sandpaper, and then chemically etched using a solution of HF, HNO3 and H2O (volume ratio of 1:1:8). The microstructure was observed by optical microscopy (OM, Jiangnan MR2000). The phase analysis was identified by X-ray diffraction (XRD, Rigaku D/MAX2500V) with Cu Kα radiation under 40 kV and 40 mA and scanning parameter of 3 (°)/min.
3 Results and discussion
3.1 Hydrogen absorption characteristics of TC21 titanium alloy
3.1.1 Relationship between hydrogen pressure and holding time
The laws of hydrogen pressure changing with different holding time under the initial hydrogen pressure of (40.7±0.2) kPa at a hydrogenated temperature of 750 °C are shown in Fig. 1. It can be seen that the hydrogen absorption reaction can be divided into non-equilibrium area and equilibrium area. Initially, the hydrogen pressure drops very quickly, suggesting that the hydrogen absorption rate of the alloy is large and the hydrogen absorption reaction is in non-equilibrium area. The hydrogen absorption rate decreases gradually with the increase of holding time. The hydrogen pressure tends to be stable when the holding time reaches 60 min, showing that the hydrogen absorption reaction reaches equilibrium. From Fig. 1, it can be seen that the coincidence degrees of these curves are high, indicating that the results are accurate.

Fig. 1 Relation between hydrogen pressure and holding time during hydrogen absorption process of TC21 titanium alloy at initial hydrogen pressure of (40.7±0.2) kPa
3.1.2 Relationship between hydrogen content and holding time
As shown in Fig. 2, the relationship between hydrogen content and holding time is nonlinear. The hydrogen content of specimen is low when the holding time is short. The hydrogen content increases with the increase of holding time, and tends to be stable when the holding time reaches 60 min. The hydrogenated reaction tends to be balanced when the holding time reaches 60 min, which is in accordance with the phenomenon of hydrogen pressure changing with the holding time shown in Fig. 1.
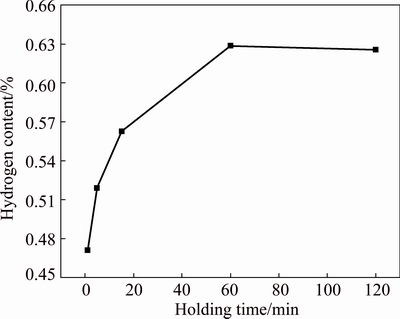
Fig. 2 Hydrogen content of TC21 titanium alloy hydrogenated for different holding time at 750 °C at initial hydrogen pressure of (40.7±0.2) kPa
3.1.3 Analysis of hydrogen absorption kinetics
The reaction fraction during the process of hydrogen absorption can be expressed as
α=(P0-Pt)/(P0-Peq) (1)
where α is the reaction fraction, P0 is the initial hydrogen pressure, Peq is the hydrogen absorption equilibrium pressure, Pt is the hydrogen pressure at time t.
The kinetics of hydrogen absorption behavior of TC21 titanium alloy can be described by the nucleation and growth theory. Therefore, the hydrogen absorption data are analyzed by the Johnson-Mehl-Avrami (J-M-A) theory [14]. The reaction fraction α is a function of time t. The J-M-A equation is expressed as
α=1-exp(-ktn) (2)
where k=k(T) is a rate constant depending on temperature, n is the reaction order which represents the effect degree of reactant concentration on the reaction rate. The reaction order increases when the effect of reactant concentration on the reaction rate increases. Formula 2 can be transformed into formula 3.
ln[-ln(1-α)]=nln t+ln k (3)
Formula 3 indicates that ln[-ln(1-α)] has a linear relation with lnt at a given pressure and temperature. The slope is n and the vertical intercept is ln k in the relationship between ln[-ln(1-α)] and lnt.
As shown in Fig. 3, ln[-ln(1-α)] and lnt present a linear relationship within a certain area. The reaction occurred during hydrogen absorption process of TC21 titanium alloy can be divided into two different stages at the initial hydrogen pressure of 40.7 kPa and hydrogen absorption temperature of 750 °C. In the first stage, n equals 0.757, and then drops to 0.514 in the second stage, indicating that the effect of hydrogen pressure on the reaction rate decreases. Almost in all the kinetic plots, each one consists of different linear segments, which extends in the certain range rather than linear over the entire reaction range [14], and different linear segments represent different stages of reaction [15,16]. The hydrogen absorption reaction of titanium alloy is a complicated process which is comprehensively affected by physical adsorption, chemical decomposition, internal hydrogen diffusion, titanium-hydrogen reaction and phase transformation in the alloy [17], therefore, these two stages contain different reaction processes. In addition, the physical adsorption and chemical decomposition occurred during the hydrogen absorption process of titanium alloy are very fast at 750 °C, and the hydrogen diffusion in titanium alloy at 750 °C is also fast. Therefore, the first stage in Fig. 3 contains physical adsorption, chemical decomposition and internal hydrogen diffusion in the alloy. n is close to 0.5 in the second stage, indicating that the lamellar phases (hydride and β phase proved in the subsequent microstructure analysis) thicken, therefore, the second stage in Fig. 3 mainly includes hydrogen diffusion, titanium-hydrogen reaction and phase transformation.

Fig. 3 ln[-ln(1-α)] vs lnt plots of TC21 titanium alloy hydrogenated at 750 °C at initial hydrogen pressure of 40.7 kPa
3.2 Microstructural evolution of TC21 titanium alloy after hydrogenation
3.2.1 Metallographic microstructure analysis
As shown in Fig. 4(a), the as-received TC21 titanium alloy is a typical binary microstructure, including white discrete lath-like and equiaxed primary α phase and dark transformed β phase. The primary α phase uniformly distributes in the matrix of transformed β phase. In order to eliminate the effect of heat treatment on the microstructures and properties of TC21 titanium alloy, the experiment with the same heat treatment on the as-received TC21 titanium alloy but without hydrogenating was conducted (the specimen was heated from room temperature to 750 °C and holding for 2 h with a heating rate of 10 °C/min, and then air cooled to room temperature). As shown in Fig. 4(b), it can be seen that the microstructures of alloy after heat treatment have no obvious change compared with those of as-received alloy.

Fig. 4 Microstructures of TC21 titanium alloy
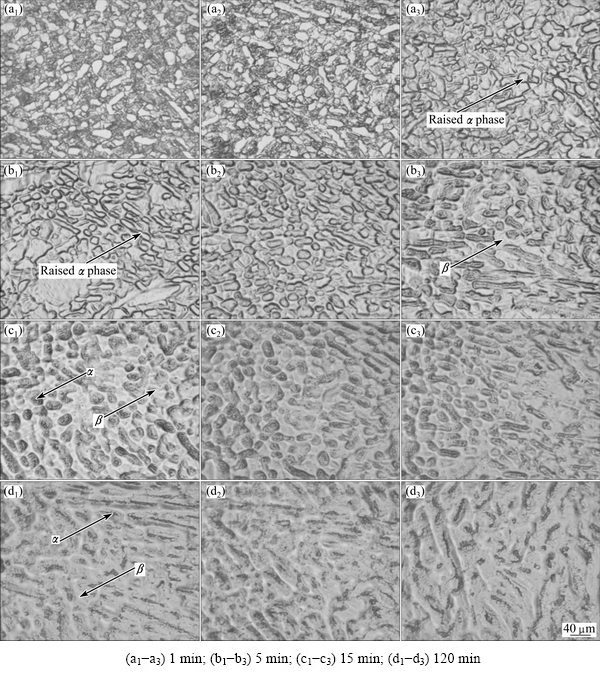
Fig. 5 Microstructures from center to edge of TC21 titanium alloy hydrogenated at 750 °C for different holding time at initial hydrogen pressure of (40.7±0.2) kPa
After hydrogenation, the specimens were cut along the cylindrical axis, which were used to observe the microstructural evolution. Figure 5 shows the microstructures from the center to the edge of TC21 titanium alloy hydrogenated at 750 °C for different holding time. It can be seen that the microstructures from the center to the edge of hydrogenated specimens change obviously. The microstructures in different regions tend to be uniform with the increase of holding time. When the holding time reaches 120 min, the microstructures from the center to the edge are identical, indicating that hydrogen diffuses uniformly in the alloy. Figure 5(a1) shows the microstructure in the center of TC21 titanium alloy hydrogenated for 1 min which is similar to that of the as-received/heat-treated TC21 titanium alloy, indicating that hydrogen does not enter into the center of alloy. Observing the microstructures from the center to the edge of specimen shown in Figs. 5(a1)-(a3), it can be seen that there are two obvious changes between the edge and the center of the specimen. On one hand, the original dark transformed β phase brightens after hydrogenation due to the interstitial solution effect of hydrogen which strengthens the chemical potential of transformed β phase. On the other hand, the interfaces between the primary and secondary α phases are still distinct but α phases raise above transformed β phase visually, which is more obviously in Fig. 5(b1). Actually, during the corrosion process of specimen, the edges of TC21 titanium alloy hydrogenated for 1 and 5 min are more easily corroded than the centers, showing that the transformed β phase becomes more vulnerable to corrosion after absorbing hydrogen. Accordingly, the diffusion coefficient of hydrogen in β phase is several to dozen orders of magnitude larger than that in αphase [18,19], therefore, hydrogen enters into the transformed β phase preferentially. From Figs. 5(b1)-(c3), it can be seen that the size of α phase becomes small, and the grain shape of α phase becomes mellow and the convex degree of α phase decreases, which is because that the temperature of α→β phase transformation decreases after hydrogenation and the edge of α phase is eroded by phase transformation. As shown in Figs. 5(c1)-(c3), the contrasts of α phase and β phase under optical microscopy in the hydrogenated TC21 titanium alloy are completely reversed compared with those of the as-received alloy, which is essentially due to the change of relative chemical potentials of α phase and β phase [20]. The chemical potential of α phase becomes weak as hydrides precipitate in α phase which causes elastic or plastic strains, internal stresses, dislocations and crystal defects. Therefore, the original simplex α phase changes to complex structures with precipitates, ordered textures and high strains. From Figs. 5(b1)-(d3), it can be seen that the phase and grain boundaries become ambiguous or even vanished, and the proportion of α phase decreases with the increase of holding time owing to the decrease of α→β phase transformation temperature and the lattice expansion of β phase. In addition, β phase becomes the main phase instead of α phase when the hydrogen content in the alloy reaches 0.625% (mass fraction) (Figs. 5(d1)-(d3).
The microstructures of TC21 titanium alloy hydrogenated with 0.625% H and higher hydrogen contents are shown in Fig. 6. It can be seen that with the increase of hydrogen content, the amount of primary α phase decreases. The primary α phase is divided into a few small particles. The grain boundaries become more ambiguous and the phase surfaces become more flat.

Fig. 6 Microstructures of TC21 titanium alloy hydrogenated at 750 °C for 120 min and containing different hydrogen contents
3.2.2 XRD analysis
As shown in Fig. 7, the as-received TC21 titanium alloy consists of a large amount of α phase and a small amount of β phase. After hydrogenation, the relative intensity of α phase decreases while the relative intensity of β phase increases with the increase of hydrogen content, indicating that the quantity of β phase increases.
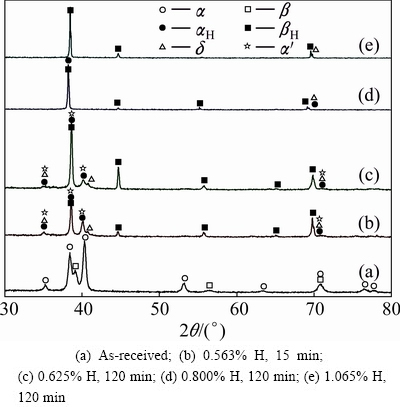
Fig. 7 XRD patterns of TC21 titanium alloy hydrogenated at 750 °C for different holding time and containing different hydrogen contents
The peaks of αH phase and βH phase move to low angle because the addition of hydrogen leads to the expansion of lattice parameter [12,20]. The expansion of lattice parameter is more obvious for βH phase because the solubility of hydrogen in β phase is much higher than that in α phase, which can be seen from Table 1. In addition, some peaks of face-centered-cubic (FCC) δ hydride appear which is recognized as TiHx (1.5≤x≤2) [21], and the calculated lattice parameter of δ hydride is 0.444 nm. Reportedly, three types of hydride have been found in titanium alloys [22-24], including face- centered-tetragonal (FCT, c/a>1) γ hydride, face- centered-cubic (FCC, c=a) δ hydride and face-centered- tetragonal (FCT, c/a<1) ε hydride, but only δ hydride is observed in Fig. 7. The diffraction peaks of a small amount of α' martensite with hexagonal structure appear after hydrogenation, but α'' martensite with rhombic structure is not found. The metastable β phase will transform to α' martensite during non-equilibrium cooling and α' martensite is not found in the as-received TC21 titanium alloy. Therefore, it can be speculated that alloying elements migrate because the α→β phase transformation and β→α' transformation occur during cooling. With the increase of hydrogen content in the alloy, the quantity of α' martensite decreases, which indicates that β phase becomes more stable when the hydrogen content increases.
Table 1 Lattice parameters of as-received TC21 titanium alloy and hydrogenated alloys

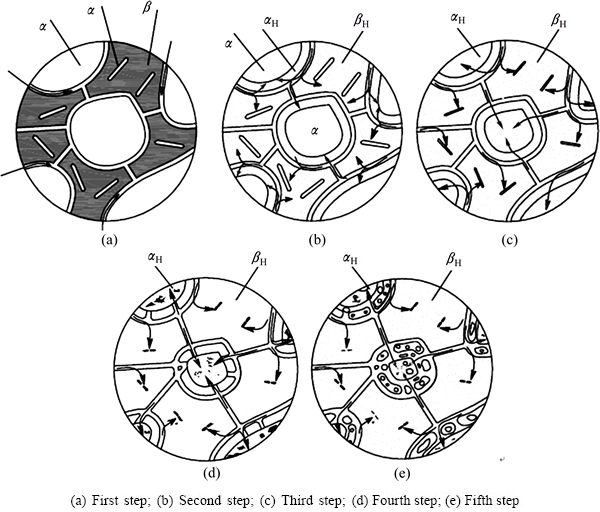
Fig. 8 Schematic diagrams of hydrogen diffusion process in TC21 titanium alloy
3.2.3 Analysis of hydrogen diffusion in TC21 titanium alloy
From the results above, the schematic diagrams of hydrogen diffusion process in TC21 titanium alloy are established, as shown in Fig. 8, which can describe the effect of hydrogen diffusion on the microstructural evolution of TC21 titanium alloy in detail. The hydrogen diffusion in TC21 titanium alloy can be divided into the following five steps when the hydrogen content in the alloy reaches 1.065% according to the microstructures and XRD analysis. Firstly, hydrogen enters into TC21 titanium alloy through grain and phase boundary, because the structures of grain and phase boundaries are loose [19]. Secondly, Hydrogen dissolves in β phase prior to α phase due to higher diffusion coefficient in β phase [17,18]. After hydrogenation, the chemical potential of transformed β phase strengthens, leading to the metallographic color of βH lightens. As hydrogen diffuses only to the edge of primary α grain and the corrosion resistance of primary α phase changes little, the optical contrast does not change temporarily. Therefore, the primary α phase and βH phase are all bright white in metallographic figure, and α phase rises visually compared with βH phase. Thirdly, when hydrogen diffuses into α phase further, the transformation of αH→βH occurs, making the edge of primary α phase corroded. The changes of microstructure morphology show that the phase boundaries migrate to the internal of α phase, thus the size of primary α phase significantly narrows and the grain direction disappears. Fourthly, the erosion effect of hydrogen on the primary α phase strengthens when hydrogen suffuses α phase, and the surface of α phase becomes mellow and the convex degree reduces, while the β phase becomes the main phase instead of α phase. Fifthly, when hydrogen continues diffusing into TC21 titanium alloy, the proportion of primary α phase continues to reduce. α phase is corroded and divided into a few small particles. The grain boundaries become more ambiguous, and the phase surfaces become more flat.
4 Conclusions
1) Hydrogen absorption reaction occurred during the hydrogen absorption process of TC21 titanium alloy is a complicated process, and can be divided into two different stages according to the hydrogen absorption kinetics.
2) After hydrogenation, the microstructures of TC21 titanium alloy change obviously. Hydrogen diffuses in β phase prior to α phase, and just a little hydrogen will change the contrast of transformed β phase. The transformed β phase becomes more vulnerable to corrosion after absorbing hydrogen. The contrast of α phase darkens when the hydrogen content in TC21 titanium alloy exceeds 0.5%. The phase/grain boundaries become ambiguous or even vanished, and β phase becomes the main phase instead of α phase when the hydrogen content reaches 0.625%. Moreover, the α phase disappears when the hydrogen content reaches 1.065%. Additionally, the XRD analysis shows that α' martensite and FCC δ hydride appear in the hydrogenated alloy.
3) According to the microstructures and XRD analysis, the schematic diagrams of hydrogen diffusion process in TC21 titanium alloy are established, and the hydrogen diffusion in TC21 titanium alloy can be divided into five steps when the hydrogen content in the alloy reaches 1.065%.
References
[1] QIAN Chen-hao, LI Ping, XUE Ke-min. Influence of deformation passes on interface of SiCp/Al composites consolidated by equal channel angular pressing and torsion [J]. Transactions of Nonferrous Metals Society of China, 2014, 25(5): 1376-1382.
[2] CHEN Qiang, ZHAO Zu-de, ZHAO Zhi-xiang, HU Chuan-kai, SHU Da-yu. Microstructure development and thixoextrusion of magnesium alloy prepared by repetitive upsetting-extrusion [J]. Journal of Alloys and Compounds, 2011, 509(26): 7303-7315.
[3] FAN Xiao-guang, YANG He, GAO Peng-fei, YAN Si-liang. Morphology development of elongated α phases in hot working of large-scale titanium alloy plate [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(12): 3508-3516.
[4] YANG Liu-qing, YANG Yan-qing. Deformed microstructure and texture of Ti6Al4V alloy [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(10): 3103-3110.
[5] CUI Chun-xiang, HU Bao-min, ZHAO Li-chen, LIU Shuang-jin. Titanium alloy production technology, market prospects and industry development [J]. Materials and Design, 2011, 32(3): 1684-1691.
[6] SHI Zhi-feng, GUO Hong-zhen, LIU Rui, WANG Xiao-chen, YAO Ze-kun. Microstructure and mechanical properties of TC21 titanium alloy by near-isothermal forging [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(1): 72-79.
[7] YUAN Bao-guo, YU Hai-ping, LI Chun-feng. Influence of hydrogen content on room temperature compressive properties of Ti-6Al-4V alloy at high strain rate [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(12): 2943-2951.
[8] PRAMANIK A. Problems and solutions in machining of titanium alloys [J]. The International Journal of Advanced Manufacturing Technology, 2014, 70(5-8): 919-928.
[9] SHEN Chia-chieh, WANG Chung-min. Effects of hydrogen loading and type of titanium hydride on grain refinement and mechanical properties of Ti-6Al-4V [J]. Journal of Alloys and Compounds, 2014, 601: 274-279.
[10] WANG Xiao-li, ZHAO Yong-qing, WEI Xiao-wei, HOU Hong-liang. Microstructures of TC21 alloys after hydrogenation and dehydrogenation [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(1): 82-88.
[11] ZWICKER U, SCHLEICHER H W. Process for improving the workability of titanium alloys: US, 2892742 [P]. 1959-06-03.
[12] SHAN De-bin, ZONG Ying-ying, LU Ting-fu,  Yan. Microstructural evolution and formation mechanism of FCC titanium hydride in Ti-6Al-4V-xH alloys [J]. Journal of Alloys and Compounds, 2007, 427(1-2): 229-234.
Yan. Microstructural evolution and formation mechanism of FCC titanium hydride in Ti-6Al-4V-xH alloys [J]. Journal of Alloys and Compounds, 2007, 427(1-2): 229-234.
[13] HUANG Shu-hui, ZONG Ying-ying, SHAN De-bin. Application of thermohydrogen processing to Ti6Al4V alloy blade isothermal forging [J]. Materials Science and Engineering A, 2013, 561: 17-25.
[14] SRINIVAS G, SANKARANARAYANAN V, RAMAPRABHU S. Hydrogen absorption and desorption properties of Ho1-xMmxCo2 alloys [J]. International Journal of Hydrogen Energy, 2007, 32(13): 2480-2487.
[15] MANI N, RAMAPRABHU S. Effect of substitutional elements on hydrogen absorption properties in ZrMnFe0.5Ni0.5 and ZrMnFe0.5Co0.5 [J]. International Journal of Hydrogen Energy, 2005, 30(1): 53-67.
[16] ZHANG W, CIMATO J, GOUDY A J. The hydriding and dehydriding kinetics of some LaNi5-xAlx alloys [J]. Journal of Alloys and Compounds, 1993, 201(1-2): 175-179.
[17] TAKASAKI A, FURUYA Y, OJIMA K, TANEDA Y. Hydrogen solubility of two-phase (Ti3Al+TiAl) titanium aluminides [J]. Scripta Metallugica et Materialia, 1995, 32(11): 1759-1764.
[18] CLARKE C F, HARDIE D, IKEDA B M. The effect of hydrogen content on the fracture of pre-cracked titanium specimens [J]. Corrosion Science, 1994, 36(3): 487-509.
[19] NIINOMI M, GONG B, KOBAYASHI T, OHYABU Y, TORIYAMA O. Fracture characteristics of Ti-6Al-4V and Ti-5Al-2.5Fe with refined microstructure using hydrogen [J]. Metallurgical and Materials Transactions A, 1995, 26(5): 1141-1151.
[20] ZHU Tang-kui, LI Miao-quan. Effect of hydrogen addition on the microstructure of TC21 alloy [J]. Materials Science and Engineering A, 2010, 527(26): 7080-7085.
[21] ZHANG Y, ZHANG S Q. Hydrogenation characteristics of Ti-6Al-4V cast alloy and its microstructural modification by hydrogen treatment [J]. International Journal of Hydrogen Energy, 1997, 22(2-3): 161-168.
[22] TREFILOV V I, MOROZOV I A, MOROZOVA R A, DOBROVOLSKY V D, ZAULICHNY Y A, KOPYLOVA E I, KHYZHUN O Y. Peculiarities of interatomic interaction in titanium hydrides with different content of hydrogen [J]. International Journal of Hydrogen Energy, 1999, 24(2-3): 157-161.
[23] SINGH G, BAJARGAN G, DATTA R, RAMAMURTY U. Effect of hydrogen charging on tensile properties of B-modified Ti-6Al-4V alloy [J]. Materials Science and Engineering A, 2013, 576: 326-336.
[24] MATSUDA J, NAKAMURA Y, AKIBA E. Microstructure of Ti-V-Mn BCC alloys before and after hydrogen absorption- desorption [J]. Journal of Alloys and Compounds, 2011, 509(11): 4352-4356.
TC21钛合金吸氢特性和组织演变规律
袁宝国,郑育彬,王玉洁,龚龙清
合肥工业大学 材料科学与工程学院,合肥 230009
摘 要:通过动力学模型分析、光学显微镜(OM)和X射线衍射(XRD)研究TC21钛合金的吸氢特性和组织演变规律。结果表明:根据吸氢动力学,发生在TC21钛合金吸氢过程中的反应可分为两个阶段。氢化后,TC21钛合金的显微组织变化明显。仅少量氢就会改变β转变相的对比度。当氢含量超过0.5%(质量分数)时,α相的对比度变暗。当氢含量达到0.625%时,相界/晶界变得模糊甚至消失,并且β相代替α相成为主要相。当氢含量达到1.065%时,α相消失。此外,XRD分析表明,氢化后的合金中出现了α'马氏体和FCC δ氢化物。根据组织和XRD分析,创建了TC21钛合金氢扩散过程的示意图。
关键词:TC21钛合金;吸氢特性;动力学;组织;扩散
(Edited by Mu-lan QIN)
Foundation item: Project (51205102) supported by the National Natural Science Foundation of China; Project (2012M511401) supported by the Postdoctoral Science Foundation of China
Corresponding author: Bao-guo YUAN; Tel: +86-15155176765; E-mail: yuanbaoguo@163.com
DOI: 10.1016/S1003-6326(16)64147-X
Abstract: The hydrogen absorption characteristics and microstructural evolution of TC21 titanium alloy were investigated by kinetic model analysis, optical microscopy (OM) and X-ray diffraction (XRD). The results show that the hydrogen absorption reaction occurred during the hydrogen absorption process of TC21 titanium alloy can be divided into two different stages according to the hydrogen absorption kinetics. After hydrogenation, the microstructure of TC21 titanium alloy changes obviously. Just a little hydrogen will change the contrast of transformed β phase. The contrast of α phase darkens when the hydrogen content in TC21 titanium alloy exceeds 0.5% (mass fraction). The phase/grain boundaries become ambiguous or even vanished, and β phase becomes the main phase instead of α phase when the hydrogen content reaches 0.625%. Moreover, α phase disappears when the hydrogen content reaches 1.065%. Additionally, the XRD analysis shows that α' martensite and FCC δ hydride appear in the hydrogenated alloy. According to the microstructures and XRD analysis, the schematic diagrams of hydrogen diffusion process in TC21 titanium alloy were established.


