
Technology of antique imitation for surface of nonmetal artworks
ZHAO Zi-yu(赵紫玉), ZHANG Su-zhi(张素芝), FANG Jian-cheng(方建成)
College of Mechanical Engineering and Automation, Huaqiao University, Quanzhou 362021, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Two different technologies, electro-brush plating and arc spraying, were employed to deposit copper film and brass coating on the surface of nonmetal artworks, respectively. The principles of the oxidizing corrosion and coloring were researched. The nonmetal artworks attain vivid and ancient bronze effect by the surface deposition and surface coloring processing. By using this technology, the problems of difficulty-to-plating copper and difficulty-to-archaizing for the large outdoor sculptures and other artworks can be solved, and it has prospective application due to low investment.
Key words:
electro-brush plating; arc spraying; copper film; brass coating; antique imitation technology;
1 Introduction
Copper and its alloys are the nonferrous metals which are the most firstly and widely used. With the increasing of requirements for the appearance color of copper and its alloy products, the study on antique imitation technology of surface has been paid more attention to[1-4]. The compound membrane of copper and its alloys, which is formed by surface coloring, not only can avoid oxidation but also has anticorrosion and decoration functions[5]. The patina with pure color and good artistic vision on ancient bronze ware is formed through long natural oxidation in air environment, which can protect copper substrate[6]. At present, the simple and unsophisticated hue exclusively owned by bronze-color is the reason for its wide application in square sculptures, craftworks, horologe, lamps and lanterns, furniture and building decorations. Coloring processing is the key technology in the archaizing process, and research on it mainly focuses on electrochemical coloring[7-9] and chemical coloring[10-11]. The former color membrane is stable but is difficult to use for large products, and the latter has the advantages of vivid archaizing effect, low cost and simple operation. However, the most difficult problem for antique imitation of copper and its alloy is that the material is too expensive, and the technique is relatively sophisticated. Therefore, to develop the antique imitation technology with quick surface processing and coloring at low-cost for copper and its alloy has a brilliant prospect.
In this work a prospective technology was advanced by using electro-brush plating and arc spraying to deposit copper film and brass coating on the surface of nonmetal artworks, respectively. The best chemical composition and coloring technique are chosen for the antique imitation so that bronze imitation can be achieved.
2 Coloring principle
The principle of chemical coloring on surface of copper and copper alloys is that the coloring solution acts with the copper and copper alloys, then oxide layer, sulfide layer and other compound layer formed[12]. Different chemical compositions and conditions will result in different coloring effects. The typical chemical coloring solutions are listed in Table 1.
2.1 Persulfide coloring
The typical chemical composition can be found in case 1 listed in Table 1. Potassium persulphate (K2S2O8) is, a kind of strong oxidant and can create active oxygen atoms when reacting with alkaline solution, oxidizing the copper surface to generate black cupric oxide protective film. It is uneasy to guarantee the evenness degree of the film, only partial copper surface can display blue-black color while the rest is hoar by using this solution as shown in Fig.1(a). To achieve better effect, the technique should be further improved.
Table 1 Compositions of coloring solutions and action conditions

2.2 Sulfide coloring
The typical chemical composition of case 2 is listed in Table 1. The coloring principle is that the sulphion decomposed by sulfide acts with copper and generates copper sulphide. Ammonium chloride in the solution can accelerate the forming of corrosion film and make it even and flat. With this coloring solution, the color of the copper film changes from red to purple then to black. This solution has strong corrosivity, so the concentration and temperature should be proper, and reaction time should be as short as possible. Otherwise, the relatively thinner copper film will become corrosive easily. The blue-black bronze-color was obtained by reaction between the brush plating film and the solution after 20 s (in the middle section in Fig.1(b)), but the film is almost completely corroded when the corrosion time is up to 40 s (in the right section in Fig.1(b)). Therefore, the time for solution corrosion should be strictly controlled.

Fig.1 Photos showing corrosive effect by different coloring solution: (a) Persulfide corrosion; (b) Sulfide corrosion
2.3 Thiosulfate coloring
The typical coloring solution is composed of two-component reagent as listed in case 3 in Table 1. The coloring principle is as follows: the S2- decomposed from sodium hyposulfite (Na2S2O3) combines with Pb2+ in the coloring solution or a few copper ions dissolved in the substrate to form the film of metal sulfide, and the color of the surface film is then affected by the solution concentration, component proportions and temperature. Generally, the reaction time is long[13].
2.4 Coloring by complex compound
As shown in case No.4 in Table 1, ammonia solution is most suitable for brass coloring. The ammonia solution dissolves basic cupric carbonate and generates two complex compounds, cuprammonia carbonate and basic cuprammonia as follows:
![]()
![]() (1)
(1)
In the process of oxidation treatment, the zinc in the brass is complexed to make oxide film generate rapidly.
![]()
![]() (2)
(2)
![]() (3)
(3)
The coloring solution acts on the surface of the brass coating (shown in Fig.2(a)), and then the color turns to be bronze-color gradually as shown in Fig.2(b).

Fig.2 Photos showing corrosive effect by complex compound: (a) Sample of brass coating; (b) Coloring effect
3 Experimental
The deposited copper film and brass coating on the surface of the nonmetal artworks were prepared by using electro-brush plating and arc spraying, respectively.
3.1 Antique imitation technique of brush plating film
For the large-sized productions which could not be plated in the vessel, it has obvious advantages to prepare the copper film by electro-brush plating[14]. The key problem of this technique is how to metallize the surface of nonmetal materials, and the typical process for preparing copper film on the surface of ABS plastic artworks is shown as follows:
Deoiling—Roughening—Sensitization—Activation—Metallization—Electro-brush plating—Cleaning—Drying.
It is to be noted that the adequate water cleaning should be arranged after each process and the continuous working between processes is required to ensure the film quality of electro-brush plating. As silver mirror reaction has the advantages of simpleness and high efficiency, in this experiment it is adopted to carry on metallization processing to obtain the silver plating film[15]. The special nickel should be the bottom film with thickness of 2 μm before adopting electro-brush plating, and then plates copper film up to 0.12 mm in thickness with high speed in acid plating solution. The work voltage is 5-10 V and the relative movement velocity is 9-20 m/min. After plating, the piece shown in Fig.3(a) must be cleaned by water and then dried.

Fig.3 Comparison between before and after corrosion: (a) Before corrosion; (b) After corrosion
The next step is coloring process. The coloring solution (No.2) is used to spray and brush the surface of the piece, and the residence time should not be more than 3s. When the surface of copper film becomes aubergine color, it should be flushed with water immediately and toweled off with the towels. Thereafter, it can be seen that the color would gradually become bronze-similar color to chocolate-like color. The spraying and brushing time should be increased according to the coloring effect. From Fig.3(b), it is known that different colors display in the sagged part which is not easy to touch after the imitation processing. It has the characteristics of antique flavour and an obvious effect of imitation antique.
3.2 Antique imitation technique of spraying coating
Thermal spraying technology not only has wide applications in repairing the surface of mechanical parts and fabricating molds and dies[16], but also has prospective development space in the field of cultures and arts. In order to memorize the giants, famous persons and heroes, the statues were usually set up for looking with reverence and learning by the people. The bronze statues are preferred for their grandness and elegance. However, the technique to cast the bronze statues is complex with a long production period and a great number of raw materials. If the materials, such as plaster, clay and cement are adopted to fabricate the large-size statues or single statue, and an archaized coating on the surface is sprayed by thermal spraying technology, and a large number of labors and materials can be saved.
3.2.1 Plaster mould fabrication
The basic mould made up of the plaster mixed with water by 1?1 was formed in a special mould. After stripping, the mould surface was firstly carved by hand to keep in accord with the designed portrait under the wet condition, then the concavo-convex sections were roughened with sand paper to achieve the required dimension when completely dried, and finally the plaster mould can be obtained as shown in Fig.4(a). As a result, the combination strength of the mould surface and coating could be increased with spraying.
3.2.2 Arc spraying
For the bad conductivity of plaster mould, it is difficult to form coatings when spraying high-melting point metal and it is usually applicable to spray the materials of low-melting point[17]. For brass spraying, it is required to pre-spray the bottom materials such as zinc with 0.3 mm thickness on the plaster surface, and then to spray brass onto the surface of the zinc coating up to the 0.5 mm thickness. This method has been proved to be effective by experiments as shown in Fig.4. To ensure the combination strength of the zinc coating with the plaster mould, it is necessary to optimize the air pressure to obtain a reasonable velocity of the in-flight particles. The spraying parameters are listed in Table 2.
Table 2 Spraying parameters

3.2.3 Coloring technique
As spray coating has a rough surface, it’s necessary to burnish, polish and scrub it. When carrying on the coloring process at room temperature, No.4 coloring solution is selected. In order to avoid the solution permeating through the inside of substrate which would cause variedness and bad bond strength, discontinuous brushing is chosen. First, the coating surface was corroded up to 2 min and the coating color changed from golden to French gray, 5 min later, the color became dark and patina formed. Go on brushing until the color became bronze. Then scrubbing and drying the surface sufficiently, in addition, a layer of colorless transparent acrylic varnish is brushed to protect it and make it glossy, as shown in Fig.4(d).
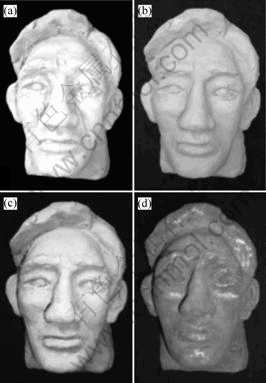
Fig. 4 Moulds prepared by antique imitation technique of brass coating: (a) Plaster mould; (b) Zn spraying; (c) Brass spraying; (d) Antique imitation
4 Conclusions
1) For the nonmetal artworks, it is an effective method to deposit copper film and brass coating using electro-brush plating and arc spraying, respectively. The processing of antique imitation on the nonmetal surface can greatly reduce the fabrication cost of bronze-imitated artworks which has a broad market prospect.
2) Due to the different thicknesses of the copper film and brass coating obtained by electro-brush plating and arc spraying respectively, it is necessary to control the corrosion time strictly in coloring solution, ensuring the conformity of corrosion thickness, and discoloration should be avoided.
3) As the nonmetal surface has the bad intensity and heat resistance, pre-spraying a layer of with low-melting point material can effectively guarantee the deposition efficiency and combination strength, and flaking off can be prevented.
References
[1] ZOU W Y, CAI Q, CUI F Z, Li H D. Electrochemical synthesis and characterization of mesoporous Cu/Cu2O films[J]. Materials Letters,2003, 57(13/14): 1934-1940.
[2] Kowalski, Arthur J. Brass plating[J]. Plating and Surface Finishing, 2004, 91(8): 9-10.
[3] Nathaniel Hall. Blackening and antiquing[J]. Metal Finishing,2000, 98(1): 471-479.
[4] XU Jin-lai, LUO Wei-yin. Chemical Coloring on Brass [J]. Surface Technology, 2004, 33(5): 55-56.
[5] RICHARDSON T J, SLACK J L, RUBIN M D. Electrochromism in copper oxide thin films [J]. Electrochimica Acta, 2001, 46: 2281-2284.
[6] ROBBIOLA L, PORTIER R. A global approach to the authentication of ancient bronzes based on the characterization of the alloy-patina-environment system[J]. Journal of Cultural Heritage, 2006, 7: 1-12.
[7] M?LLER B, SCHUBERT M. Corrosion inhibition of copper and brass pigments in aqueous alkaline media by copolymers[J]. Progress in Organic Coatings,1999, 37(3/4): 193-197.
[8] BUDKE E, KREMPEL-HESSE J, MAIDHOF H, SCH?SSLER H. Decorative hard coatings with improved corrosion resistance[J]. Surface and Coatings Technology,1999,112(1/3): 108-113.
[9] XU Bin-shi, LIU Shi-can. China materials engineering canon[M]. Beijing: Chemical Industry Press, 2006: 588-589.
[10] XIAO Li-na. Techniques of oxidization on copper surface[J]. Journal of Qinghai University, 1996, 14(1): 14-17.
[11] ZHANG Ya-fei. Research and application of a new chemical colouring technology for patina[J]. Plating and Finishing, 1995, 17(4): 11-13.
[12] ZHU Hong-fan. Mechanism of surface coloration of copper and alloy copper[J]. Sciences of Conservation and Archaeology, 2000, 12(1): 9-14.
[13] MANG Zi-dan, SUN Shu-yun, ZHAO Jing-min. The kinetics of colouring on brass surface treated in aqueous solution of Na2S2O3-Pb(AC)2 [J]. Sciences of Conservation and Archaeology, 1996, 8(2): 18-27.
[14] YE Xiao-zhao, HE Yan-yun. The research of bronze electroplating on nonmetallic statue with electric-brush[J]. Journal of Guangdong Industry Techical College, 2002, 1(1): 36-37.
[15] LIU Ren-zhi. Electroplating and finishing of nonmetal [M]. Beijing: Chemical Industry Press, 2006: 170-171.
[16] WU Zi-jian. Techniques and applications of thermal spraying[M]. Beijing: China Machine Press, 2006: 322-350.
[17] FANG Jian-cheng, XU Wen-ji, ZHAO Zi-yi. Arc spray forming[J]. Journal of Materials Processing Technology, 2005, 164/165: 1032-1037.
Foundation item: Project (50675072) supported by the National Natural Science Foundation of China; Project (2006F3084) supported by the Youth Innovation Found of Fujian Province, China; Project (06BS104) supported by the Science Research Found of Huaqiao University
Corresponding author: ZHAO Zi-yu; Tel: +86-13178010135; E-mail: zyzhao@hqu.edu.cn


