
Synthesis of nanoscale zero-valent iron supported on exfoliated graphite for removal of nitrate
ZHANG Huan(张 环)1,2, JIN Zhao-hui(金朝晖)1, HAN Lu(韩 璐)1, QIN Cheng-hua(秦承华)1
1.Department of Environmental Science and Engineering, Nankai University, Tianjin 300071, China;
2.Department of Material Science and Chemical Engineering, Tianjin Polytechnic University, Tianjin 300160, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
Nano ZVI particles supported on micro-scale exfoliated graphite were prepared by using KBH4 as reducing agent in the H2O/ethanol system. The supported ZVI materials generally have higher activity and greater flexibility for environmental remediation applications. The exfoliated graphite as the support was treated beforehand to hydrophilic material. Nano iron particles are deposited onto the rough graphite surface while those were formed by borohydride reduction. The possible nitrate reduction pathways were proposed. The TEM image shows that iron particles are highly dispersed on the surface of graphite and several of iron particles are imbedded in the pit of support surface. In this synthesis, iron particles have a nearly spherical shape with a grain size of 50-100 nm. The surface areas of materials with different iron loadings of 3.5%, 7.0%, 10.0%, 15.0% and 20.0%(mass fraction) are 2.89, 9.55, 8.45, 23.8 and 6.18 m2·g-1 by BET surface analyzer. The chemical reduction of nitrate by supported nano ZVI in aqueous solution were tested in series batch experiments. Experiment results suggest that NO3- can be more rapidly reduced to NH4+ at neutral pH and anaerobic conditions by supported nano ZVI than unsupported nano ZVI or ZVI scraps. The 15% nano Fe/graphite shows the best reduction efficiency contrasted with other Fe loading particles.
Key words:
supported nano zero-valent iron; exfoliated graphite; borohydride kalium; nitrate;
1 Introduction
The utilization of nano-sized particles of zero-valent iron(ZVI) or bimetallic combinations of ZVI in environment remediation currently attracts the most attention. Nano-sized ZVI is an effective reductant and catalyst for a wide variety of reducible contaminants including chlorinated organic compounds, heavy metal ions(e.g., Cr6+, Hg2+, Cd2+) and oxo-anions(e.g., ![]() ). The nitrate has high chemical stability, especially at low concentrations, which can be transformed to harmful nitrite ions and lead to several health treats to human including cancers and methemoglobinemia[1]. Because of nitrate’s high standard reduction potential, it can be reduced by some reducing agents to the form of nitrogen gas or ammonia.
). The nitrate has high chemical stability, especially at low concentrations, which can be transformed to harmful nitrite ions and lead to several health treats to human including cancers and methemoglobinemia[1]. Because of nitrate’s high standard reduction potential, it can be reduced by some reducing agents to the form of nitrogen gas or ammonia.
While it is well known that the nano-scale iron is a powerful remediant, it is easy to agglomerate during the utilizing process. The supported zero-valent iron materials generally have higher activity and greater flexibility for environmental remediation applications than other forms of ZVI.
Because the iron is highly dispersed, the remediation is faster and more efficient. The supported material can also serve to pre-concentrate reactants, mediate electron transfer reactions, and nucleate the growth of product phases. Supported ZVI nanoparticles are soil-permeable remediants that may facilitate transport through soil and sand-packed columns and minimize the need for excavation. It can be more efficiently target remediants that are delivered by injecting into the soil or groundwater directly for in situ application.
In this study, an efficient method of synthesizing supported nanoscale iron particles was presented. Characteristics of synthesized nanoscale iron particles were studied in experiment. Chemical reduction of nitrate by supported ZVI in solution was investigated through series of batch experiments. The mechanism of nitrate reduction by supported ZVI was discussed according to the experiment results.
2 Experimental
2.1 Synthesis of materials
Iron-on-graphite particle was made using exfoliatedgraphite(average 26 μm) as the support. 1.2 g FeSO4·7H2O was dissolved in 50 mL of 40%(volume fraction) ethanol, 60% deoxygenated deionized water. Polyethylene glycol with molecule mass of 4 000 as surfactant was added in above solutions. Then exfoliated graphite was added while stirring. 20 mL 0.5 g KBH4 alkaline solution was added slowly to the mixture while stirring. After addition of all of the KBH4, the mixture was stirred intensely for 30 min. Then the obtaining black solid was washed by 150 mL deionized water three times and by ethanol twice. The black solid was vacuum-dried overnight.
![]() (1)
(1)
Materials with different iron loadings of 3.5%, 7.0%, 10.0%, 15.0% and 20.0%(mass fraction) were prepared by adding various mass supports. Unsupported ZVI nanoparticles were also prepared only when the graphite was absent in the above procedure. To assay the effect of the support itself on contaminant removal, graphite was treated by omitting the iron salt in the same procedure[2].
Surface areas (BET area) of the supported ZVI nanoparticles were measured using the nitrogen adsorption method with a NOVA 2000 surface analyzer. Morphology of the particles was observed with a Phillips EM 400ST transmission electron microscope (TEM) to characterize the particle and distribution of the iron particles. Material structures were examined through X-ray diffractometer (XRD) by using a D/MAX-2500 automated powder with Cu Kα radiation at 40 kV and 100 mA.
2.2 Batch experiments for chemical reduction of nitrate by supported ZVI nanoparticles
Individual denitrification experiments were performed in anaerobic (sealed) batch systems prepared in a serum bottle. Unless specified, freshly prepared supported ZVI or unsupported ZVI at a concentration of Fe 1.67 g·L-1 was added to 150 mL nitrate solution. The solution was mixed at ambient conditions(25 ℃) by a rotary shaker at 150 r/min. Samples were withdrawn periodically using a glass syringe and filtered with a 0.45 μm membrane filter to remove the solid particles and other products. The filtrate was used to determine aqueous concentrations of ![]() ,
, ![]() ,
, ![]() , Fe3+ and Fe2+. In the course of the reaction, pH value was also monitored.
, Fe3+ and Fe2+. In the course of the reaction, pH value was also monitored.
![]() and
and![]() were measured by an ion chromatography system using conductivity detection (Dionex DX-120).
were measured by an ion chromatography system using conductivity detection (Dionex DX-120). ![]() was determined by NESSLER’s reagent colorimetric method using a UV-751N spectrophotometer (Lingguang, China). Fe3+ and Fe2+ were measured by 1,10-phenanthroline method using a UV-751N spectrophotometer. The pH value was measured using Delat-320 pH meter(Mettle-Toledo).
was determined by NESSLER’s reagent colorimetric method using a UV-751N spectrophotometer (Lingguang, China). Fe3+ and Fe2+ were measured by 1,10-phenanthroline method using a UV-751N spectrophotometer. The pH value was measured using Delat-320 pH meter(Mettle-Toledo).
3.1 Characterization of supported ZVI nanoparticles
The surface area of graphite itself, after being treated with kalium borohydride (but in the absence of iron) is 3.3 m2·g-1. The surface areas of material with different iron loadings of 3.5, 7.0, 10.0, 15.0 and 20.0%(mass fraction) are 2.89, 9.55, 8.45, 23.80 and 6.18 m2·g-1, respectively. It can be concluded from these results that the surface area of the material are derived primarily from the supported iron nanoparticles.
Fig.1 shows the images of supported ZVI nanoparticles observed by transmission electron microscopy. It indicates that iron particles are mainly
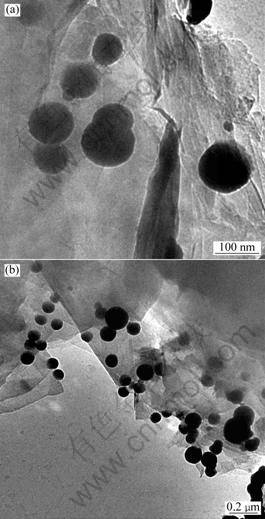
Fig.1 The TEM photograph of supported ZVI nanoparticles
dispersed on the surface of the graphite. The nano iron particles are deposited onto the rough graphite surface while those are formed by borohydride reduction and several of those are imbedded in the pit of support surface. In this synthesis, iron particles have a nearly spherical shape with a grain size range about 50-100 nm.
Fig.2 shows the XRD spectrum of supported ZVI nanoparticles. The main reflection at 44.7? corresponding to Fe0 and reflection at 26.4? and 54.5? corresponding to graphite were presented in the spectrum as shown in Fig.2. The weakly diffract peak of Fe0 indicates that nano-scale ZVI synthesized by KBH4 in the aqueous solution is poorly crystalline.
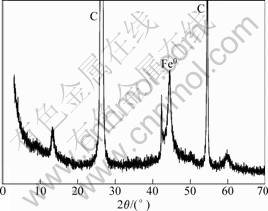
Fig.2 XRD spectrum of supported ZVI nanoparticles
3.2 Effect of supported ZVI nanoparticles on nitrate removal
Fig.3 shows the effect of nano ZVI and supported ZVI nanoparticles on nitrate removal in batch experiments. The initial pH value of nitrate solution was 6.7 and the dosages of both unsupported and supported nano iron were 1.67 g?L-1 in the closed experiment systems. Based on the experiment results shown in Fig. 3,
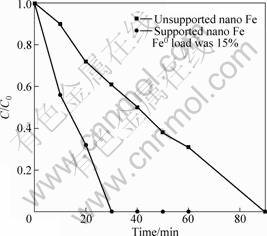
Fig.3 Comparison of nitrate reduction by supported nano iron and unsupported nano iron
the rate of nitrate removal by supported nano iron is higher than that by unsupported nano iron. The 80 mg?L-1 nitrate were totally removed in 30 min by supported nano iron at neutral pH value condition. In the experiments, graphite particles treated by kalium borohydride were also tested and shows no removal of nitrate over 2 h.
In this experimental nitrate reduction by supported ZVI is rapidly completed because nano-scale iron has high surface area, high surface energies and high reaction activity. The support effectively disperses the nanoparticles over its surface so that it can prevent the aggregation of the nano iron particles. Graphite is an excellent electric carbon and usually used for the cathode material. So the graphite particle is a congeries which includes large numbers of galvanic cell composed ofFe0/ graphite.
In the reaction system, there are some electrode reactions[3]:
![]() (2)
(2)
![]() (3)
(3)
In the reaction system of Fe0/graphite/![]() /H2O, ZVI particles as anode material are rapidly corroded and release the electrons.
/H2O, ZVI particles as anode material are rapidly corroded and release the electrons. ![]() is electron accepter due to its strong oxidation. Graphite as metal carrier also provides a conductive pathway for electrons transfer.
is electron accepter due to its strong oxidation. Graphite as metal carrier also provides a conductive pathway for electrons transfer.
3.3 Effect of different Fe0 loadings on nitrate removal
To test the effect of different Fe0 loadings material on nitrate removal, five different Fe0 loadings, i.e., 3.5, 7.0, 10.0, 15.0 and 20%(mass fraction) were prepared in the case of graphite. In every reactor, there were 1.67 g·L-1 Fe0 which was loaded on graphite support with different masses. The initial pH value of solution was controlled at 6.7.
Fig.4 shows that the nitrate degradation rates are different with the variation of Fe0 loadings. When Fe0 loading is low (3.5%) , the nitrate degradation rate is slower and the 80 mg·L-1 nitrate is not completely removed in 120 min. With the increase of the Fe0 loadings, the nitrate reduction rate becomes faster and especially the initial rate of nitrate reduction increases. After 30 min of reaction, about 70% of nitrate was removed as Fe0 loading is 7%, and nitrate removal percent is about 80% as Fe0 loading is 10%. A complete removal of nitrate by 15% Fe0 loading material is obtained after 30 min. However, when the Fe0 loading was 20%, the nitrate removal rate is decreased. When Fe0 loading is 15%, the volume ratios of iron and graphite are appropriate and iron can be averagely dispersed on the surface of graphite. Whereas the Fe0 loading exceeds 20%, the volume ratios of iron and graphite are increased and the nano iron might aggregates while depositing on surface layer of the graphite.
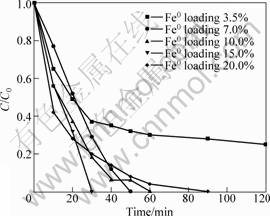
Fig.4 Effect of supported ZVI nanoparticles with different Fe0 loadings on nitrate removal
3.4 Effect of pH value
To investigate the effects of the initial pH value of solution on nitrate reduction by supported nano ZVI, batch experiments were conducted. The solution pH value was adjusted to the required value according to the experiment by 1 mol·L-1 HCl. Fig.5 shows the time courses of nitrate during its reduction by supported nano ZVI at various pH values. From Figs.3 and 4, it is shown that nitrate is removed rapidly at neutral pH by supported nano iron. Under acidic conditions the reduction rates are increased because of the H+ ion accelerating effect. In the solutions of an initial pH is 2 or 3, no ![]() was detected after 15 min reaction or after 30 min reaction.
was detected after 15 min reaction or after 30 min reaction.
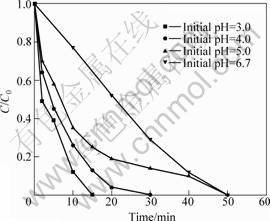
Fig.5 Effect of pH on reduction rate of nitrate by supported nano ZVI
In the reduction process of nitrate by Fe0, the solution pH value is an important parameter influencing the reaction rate and kinetics. CHENG et al[4] found that 325 mesh ZVI reduced the nitrate to ammonia under acidic solutions but there was no loss in nitrate under high pH solutions. HUANG et al[5] reported that nitrate could be rapidly reduced by ZVI at pH<4. CHOE et al[6, 7] also obtained the same conclusion that the reaction rates of nitrate reduction depended on not only the pH value but also the acids used for pH value control in their experiments. The process of reduction of nitrate by nano ZVI was the acidic-driven reaction process.
3.5 Proposed mechanism and reaction pathways
Fig.6 shows the three nitrogen species, i.e., ![]() ,
, ![]() and
and ![]() , and the total nitrogen mass of the three species at the test of 150 mL 80 mg·L-1
, and the total nitrogen mass of the three species at the test of 150 mL 80 mg·L-1 ![]() are reduced by 1.67 g·L-1 7% supported nano ZVI. From the results, the
are reduced by 1.67 g·L-1 7% supported nano ZVI. From the results, the ![]() is the main product of
is the main product of ![]() reduction. About 87% of nitrate are transformed to
reduction. About 87% of nitrate are transformed to ![]() at the end of reaction.
at the end of reaction. ![]() was also detected at every test although its mass was very low (nitrate mass fraction is lower than 4%) throughout the reduction. In every experiment the nitrite mass was gradually decreased till it disappeared with the time elapsed during the reduction. It is proposed that
was also detected at every test although its mass was very low (nitrate mass fraction is lower than 4%) throughout the reduction. In every experiment the nitrite mass was gradually decreased till it disappeared with the time elapsed during the reduction. It is proposed that ![]() is the middle product on the nitrate reduction and it can be continuously reduced to
is the middle product on the nitrate reduction and it can be continuously reduced to ![]() at the end. Nitrogen gas might be formed as one of the reduction products according to the total nitrogen elements balance.
at the end. Nitrogen gas might be formed as one of the reduction products according to the total nitrogen elements balance.
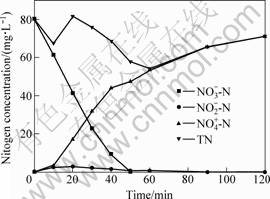
Fig.6 Nitrogen species on nitrate reduction by supported nano ZVI
In these experiments, the high reactive nano ZVI can be corroded rapidly by ![]() and H2O according to Eqn.(4). Fe2+ is the main product as Fe0 releases two electrons at the corrosion. At high pH value solution, Fe2+ will transform to ferrous hydroxide. Ferrous hydroxide is thermodynamically unstable and may be further oxidized to magnetite (Fe3O4) according to Eqn.(5) when pH is higher than 6-7[8, 9].
and H2O according to Eqn.(4). Fe2+ is the main product as Fe0 releases two electrons at the corrosion. At high pH value solution, Fe2+ will transform to ferrous hydroxide. Ferrous hydroxide is thermodynamically unstable and may be further oxidized to magnetite (Fe3O4) according to Eqn.(5) when pH is higher than 6-7[8, 9].
![]() (4)
(4)
![]() (5)
(5)
This standpoint is consistent with the fact that Fe3O4 peaks appear at the XRD spectrum in this experiment. The XRD spectrum shown in Fig.7 displays several new broad peaks such as 30.0?, 35.4?, 62.5? assigned to magnetite (Fe3O4).
Some reports also indicated that Fe2+ played a specific role which was favorable for the nitrate reduction[10, 11]. Fe2+ might participate the nitrate reduction and provide a portion of electrons which are required for redox reaction (Eqn.6).
![]() (6)
(6)
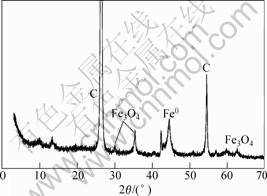
Fig.7 The XRD spectrum of supported ZVI nanoparticles after reducing nitrate
Based on the results in these experiments, the following possible reaction pathways are proposed for the nitrate reduction by supported nano ZVI.
![]() (7)
(7)
![]() (8)
(8)
![]() (9)
(9)
![]()
![]() (10)
(10)
4 Conclusions
1) Nanoscale zero-valent iron supported on exfoliated graphite synthesized in the experiments has high surface area and the nano irons of average 50-100 nm are dispersed on the surface of the support.
2) Supported nano ZVI can rapidly reduce nitrate in neutral pH reaction system. However, different Fe0 loading materials have different reduction effects. The reduction rate can be the most rapid when Fe0 loading was 0.15%. Low pH value of the reduction system is favable for the nitrate removal.
3) Most of nitrate is reduced by supported nano ZVI to ammonia and nitrite can be generated only at the reduction process and the end. The following two main possible pathways dominate:
![]()
![]()
![]()
[1] KAPOOR A, VIRARAGHAVAN T. Nitrate removal from drinking water-review[J]. J Environ Eng, 1997, 23 (5): 371-380.
[2] PONDER S M, DARAB J G, MALLOUK T E. Remediation of Cr(Ⅵ) and Pb(Ⅱ) aqueous solutions using supported nanoscale zero-valent iron[J]. Environ Sci Technol, 2000, 34 (12): 2564-2569.
[3] AGRAWAL A, TRATNYEK P G. Reduction of nitro aromatic compounds by zero-valent iron metal[J]. Environ Sci Technol, 1996, 30 (1): 153-160.
[4] CHENG I F. Reduction of nitrate to ammonia by zero-valent iron[J]. Chemosphere, 1997, 35(11): 2689-2695.
[5] HUANG Y H, ZHANG T C, Effects of low pH on nitrate reduction by iron powder[J]. Water Res, 2004, 38(11): 2631-2642.
[6] CHOE S, CHANG Y Y, HWANG K Y. Kinetics of reductive denitrification by nanoscale zero-valent iron[J]. Chemosphere, 2000, 41(8): 1307-1314.
[7] CHOE S, LILJESTRAND H M, KHIM J. Nitrate reduction by zero-valent iron under different pH regimes[J]. Applied Geochemistry, 2004, 19(2): 335-342.
[8] MACKENZIE P D, HORNEY D P, SIVAVEC T M. Mineral precipitation and porosity losses in granular iron columns[J]. J Hazard Materi, 1999, 68(1): 1-17.
[9] HUANG Y H, ZHANG T C. Effects of dissolved oxygen on formation of corrosion products and concomitant oxygen and nitrate reduction in zero-valent iron systems with or without aqueous Fe2+[J]. Water Res, 2005, 39(9): 1751-176.
(Edited by CHEN Can-hua)
Foundation item: Project(20477019) supported by the National Natural Science Foundation of China
Corresponding author: ZHANG Huan; Tel: +86-22-24528163; E-mail: yuhuan272@sina.com


